Abstract
Nonviable Mycobacterium tuberculosis strain Jamaica suspended in oil-droplet emulsions was used to enhance resistance of mice against encephalomyocarditis virus (EMCV). The mycobacteria-injected mice were significantly resistant to 50,000 50% lethal doses of EMCV. Similar concentrations of virus in plasma of normal and mycobacteria-injected mice from 1 to 120 min after injection of EMCV showed that resistance was not a result of rapid elimination of virus from the circulation. Furthermore, survival of viremic mice indicated protective mechanisms were operative after EMCV had escaped primary surveillance. Resistance did not appear to be associated with the mouse major histocompatibility gene complex. The spleen was intimately associated with protection, and the thymus was nonessential for enhanced resistance to EMCV. Protection was significantly diminished by cyclophosphamide injected intraperitoneally from 3 days before to the day of virus challenge. Finally, silica given intraperitoneally 24 h before virus completely abrogated resistance of mycobacteria-injected mice to EMCV. These results suggest that macrophages functioning independently of T-lymphocytes are important effector cells in resistance to EMCV of mice injected with nonviable mycobacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
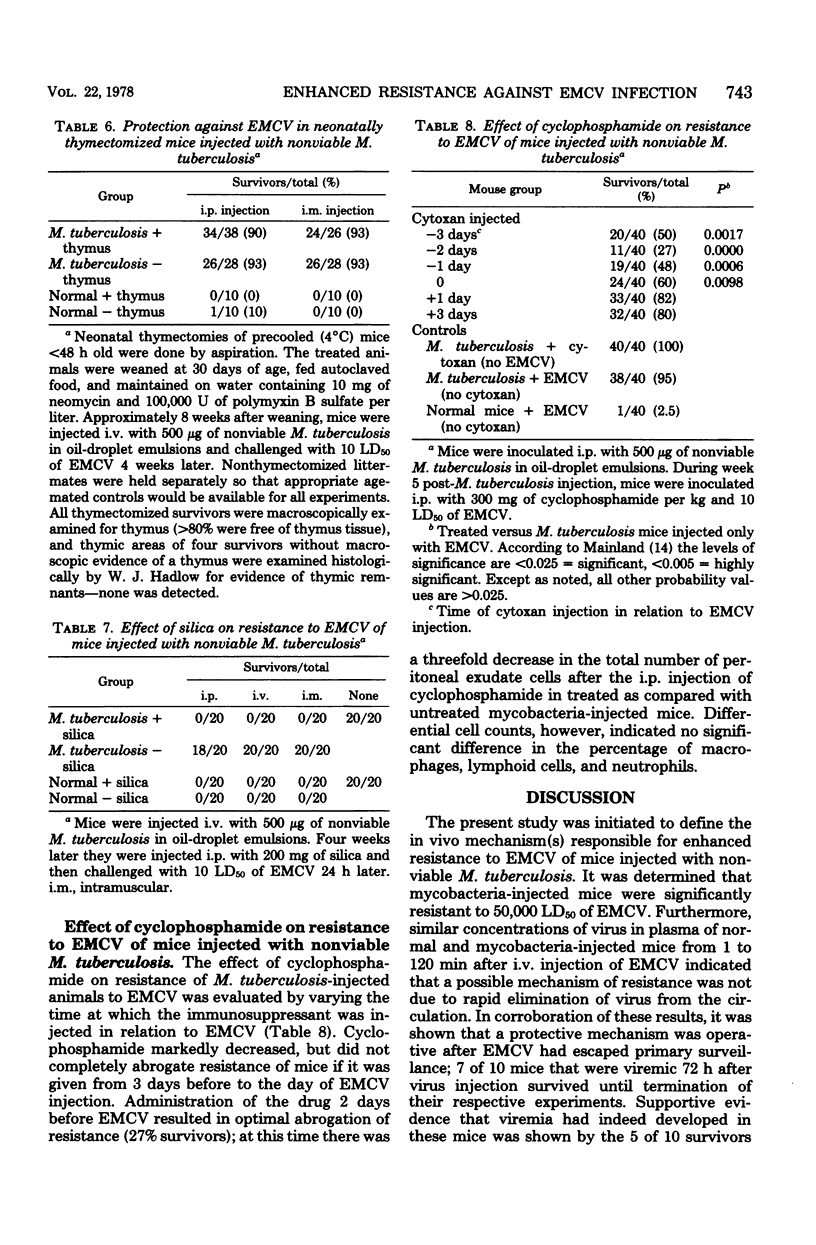
- Buhles W. C., Jr, Shifrine M. Effects of cyclophosphamide on macrophage numbers, functions and progenitor cells. J Reticuloendothel Soc. 1977 May;21(5):285–297. [PubMed] [Google Scholar]
- Civil R. H., Mahmoud A. A. Genetic differences in BCG-induced resistance to Schistosoma mansoni are not controlled by genes within the major histocompatibility complex of the mouse. J Immunol. 1978 Mar;120(3):1070–1072. [PubMed] [Google Scholar]
- Civil R. H., Warren K. S., Mahmoud A. A. Conditions for bacille Calmette-Guérin-induced resistance to infection with Schistosoma mansoni in mice. J Infect Dis. 1978 May;137(5):550–555. doi: 10.1093/infdis/137.5.550. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Wills E. J., Richmond J. E., Allison A. C. Suppression of babesiosis in BCG-infected mice and its correlation with tumor inhibition. Infect Immun. 1977 Aug;17(2):430–438. doi: 10.1128/iai.17.2.430-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci R. P., Barone J. J., Marrone J. C., Schour L. In vitro evidence of specific BCG-induced immunity to malignant melanoma in BALB-c mice. J Natl Cancer Inst. 1974 Jun;52(6):1913–1915. doi: 10.1093/jnci/52.6.1913. [DOI] [PubMed] [Google Scholar]
- Gadeberg O. V., Rhodes J. M., Larsen S. O. The effect of various immunosuppressive agents on mouse peritoneal macrophages and on the in vitro phagocytosis of Escherichia coli O4:K3:H5 and degradation of 125I-labelled HSA-antibody complexes by these cells. Immunology. 1975 Jan;28(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A., Cullen R. T., Woodruff M. A. Further analysis of the anti-tumour effect in vitro of peritoneal exudate cells from mice treated with Corynebacterium parvum. Br J Cancer. 1975 Jan;31(1):15–24. doi: 10.1038/bjc.1975.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T. Activation of guinea pig macrophages by cell walls of Mycobacterium bovis, strain BCG. Cell Immunol. 1976 Oct;26(2):254–263. doi: 10.1016/0008-8749(76)90369-5. [DOI] [PubMed] [Google Scholar]
- Laucius J. F., Bodurtha A. J., Mastrangelo M. J., Creech R. H. Bacillus Calmette-Guerin in the treatment of neoplastic disease. J Reticuloendothel Soc. 1974 Dec;16(6):347–373. [PubMed] [Google Scholar]
- Lodmell D. L., Ewalt L. C. Enhanced resistance against encephalomyocarditis virus infection in mice, induced by a nonviable Mycobacterium tuberculosis oil-droplet vaccine. Infect Immun. 1978 Jan;19(1):225–230. doi: 10.1128/iai.19.1.225-230.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Ewalt L. C., Notkins A. L. Inhibition of vaccinia virus replication in skin of tuberculin-sensitized animals challenged with PPD. J Immunol. 1976 Nov;117(5 PT2):1757–1761. [PubMed] [Google Scholar]
- Miller S. D., Zarkower A. Alterations of murine immunologic responses after silica dust inhalation. J Immunol. 1974 Nov;113(5):1533–1543. [PubMed] [Google Scholar]
- Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976 Jul 1;262(5563):77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., McCord R. S. Resistance to herpes simplex type 2 virus induced by an immunopotentiator (pyran) in immunosuppressed mice. J Immunol. 1975 Jul;115(1):311–313. [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Drapier J. C., Petit J. F., Wietzerbin J., Lederer Enhancement of nonspecific immunity to bacterial infection by cord factor (6,6'-trehalose dimycolate). J Infect Dis. 1977 May;135(5):771–777. doi: 10.1093/infdis/135.5.771. [DOI] [PubMed] [Google Scholar]
- Ruittenberg E. J., van Noorle Jansen L. M. Effect of Corynebacterium parvum on the course of a Listeria monocytogenes infection in normal and congenitally athymic (nude) mice. Zentralbl Bakteriol Orig A. 1975;231(1-3):197–205. [PubMed] [Google Scholar]
- Röllinghoff M., Starzinski-Powitz A., Pfizenmaier K., Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977 Feb 1;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J. C., Ganguly R., Waldman R. H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J Infect Dis. 1977 Aug;136(2):171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Stringfellow D. A., Overall J. C., Jr, Glasgow L. A. Interferon inducers in therapy of infection with encephalomyocarditis virus in mice. I. Effect of single doses of polyriboinosinic-polyribocytidylic acid and tilorone hydrochloride on viral pathogenesis. J Infect Dis. 1974 Nov;130(5):470–480. doi: 10.1093/infdis/130.5.470. [DOI] [PubMed] [Google Scholar]
- Suenaga T., Okuyama T., Yoshida I., Azuma M. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: participation of interferon in enhanced resistance. Infect Immun. 1978 Apr;20(1):312–314. doi: 10.1128/iai.20.1.312-314.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willers J. M., Sluis E. The influence of cyclophosphamide on antibody formation in the mouse. Ann Immunol (Paris) 1975 Apr;126(3):267–279. [PubMed] [Google Scholar]
- Winkelstein A. Mechanisms of immunosuppression: effects of cyclophosphamide on cellular immunity. Blood. 1973 Feb;41(2):273–284. [PubMed] [Google Scholar]
- Woodruff M. F., McBride W. H., Dunbar N. Tumour growth, phagocytic activity and antibody response in Corynebacterium parvum-treated mice. Clin Exp Immunol. 1974 Jul;17(3):509–518. [PMC free article] [PubMed] [Google Scholar]
- Yarkoni E., Bekierkunst A. Nonspecific resistance against infection with Salmonella typhi and Salmonella typhimurium induced in mice by cord factor (trehalose-6,6'-dimycolate) and its analogues. Infect Immun. 1976 Nov;14(5):1125–1129. doi: 10.1128/iai.14.5.1125-1129.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBuy H. Effect of silica on virus infections in mice and mouse tissue culture. Infect Immun. 1975 May;11(5):996–1002. doi: 10.1128/iai.11.5.996-1002.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


