Abstract
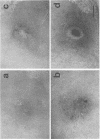
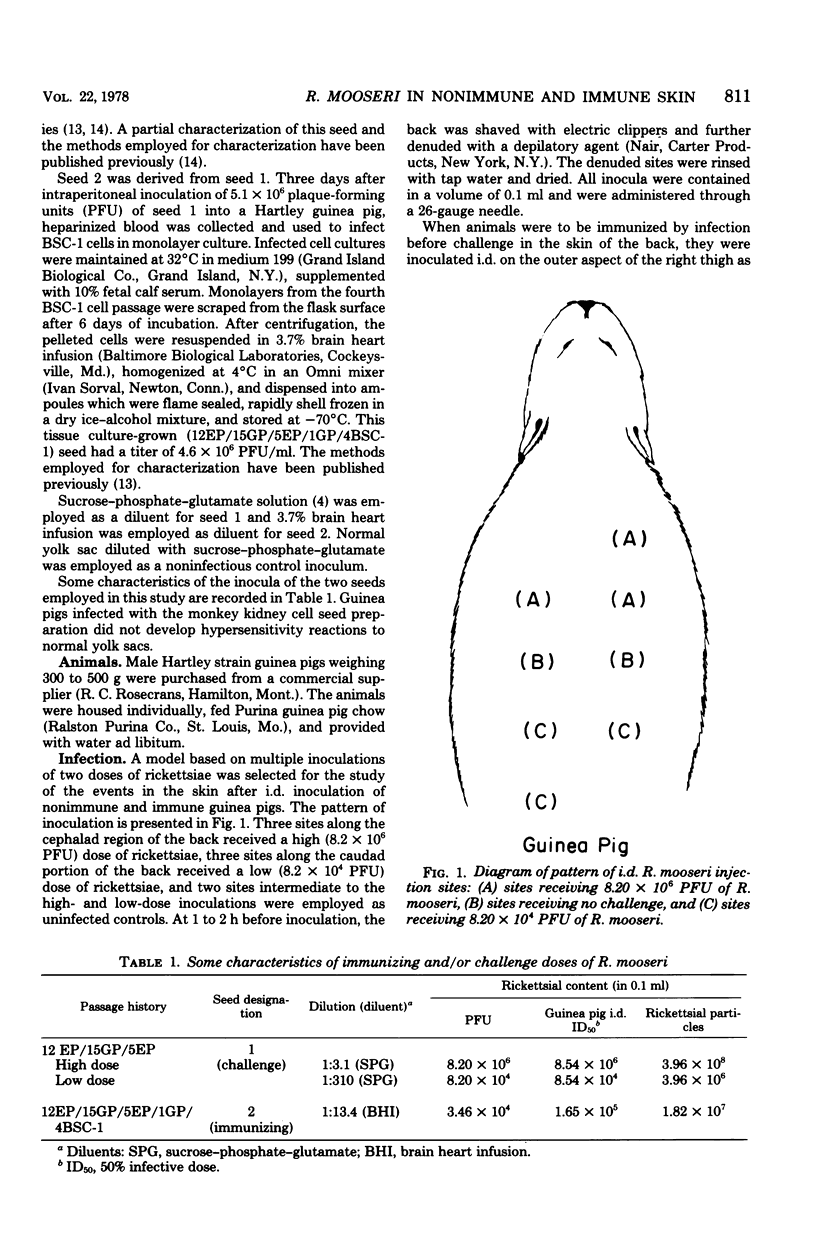
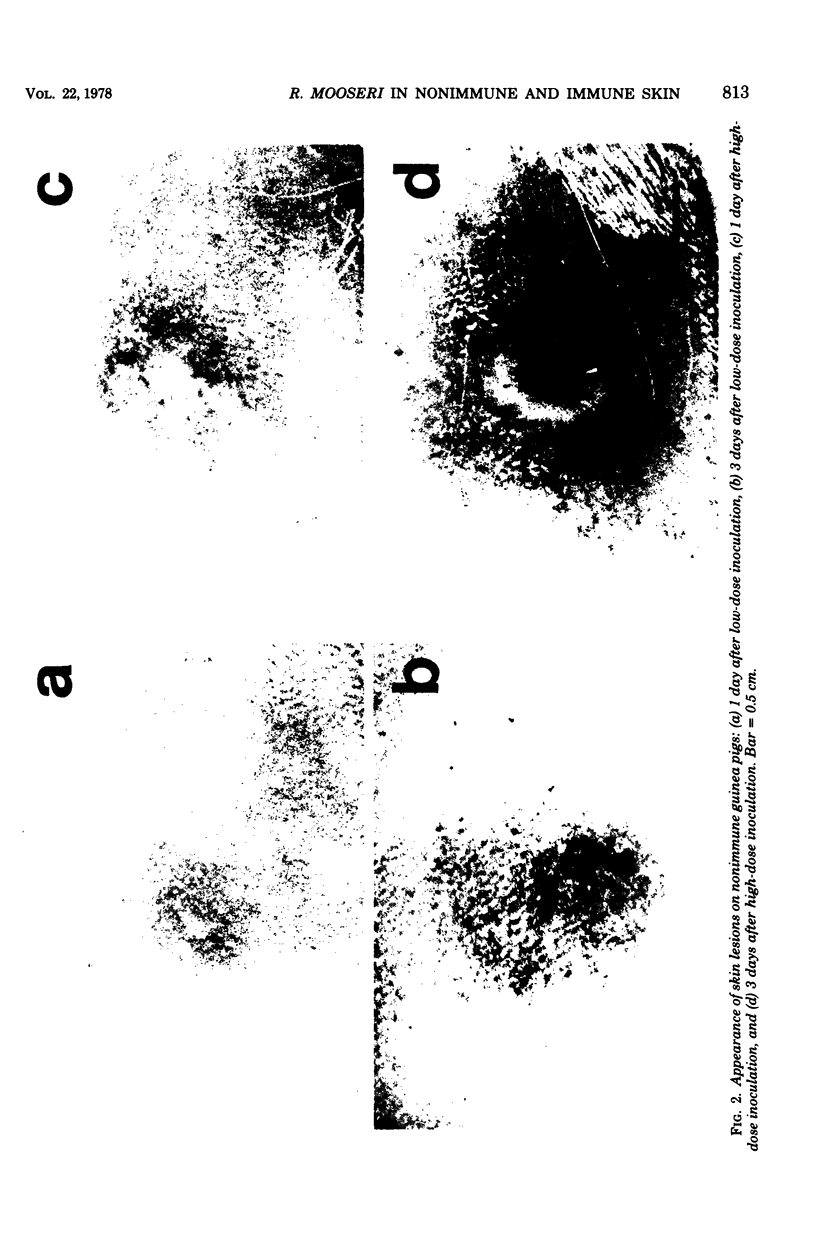
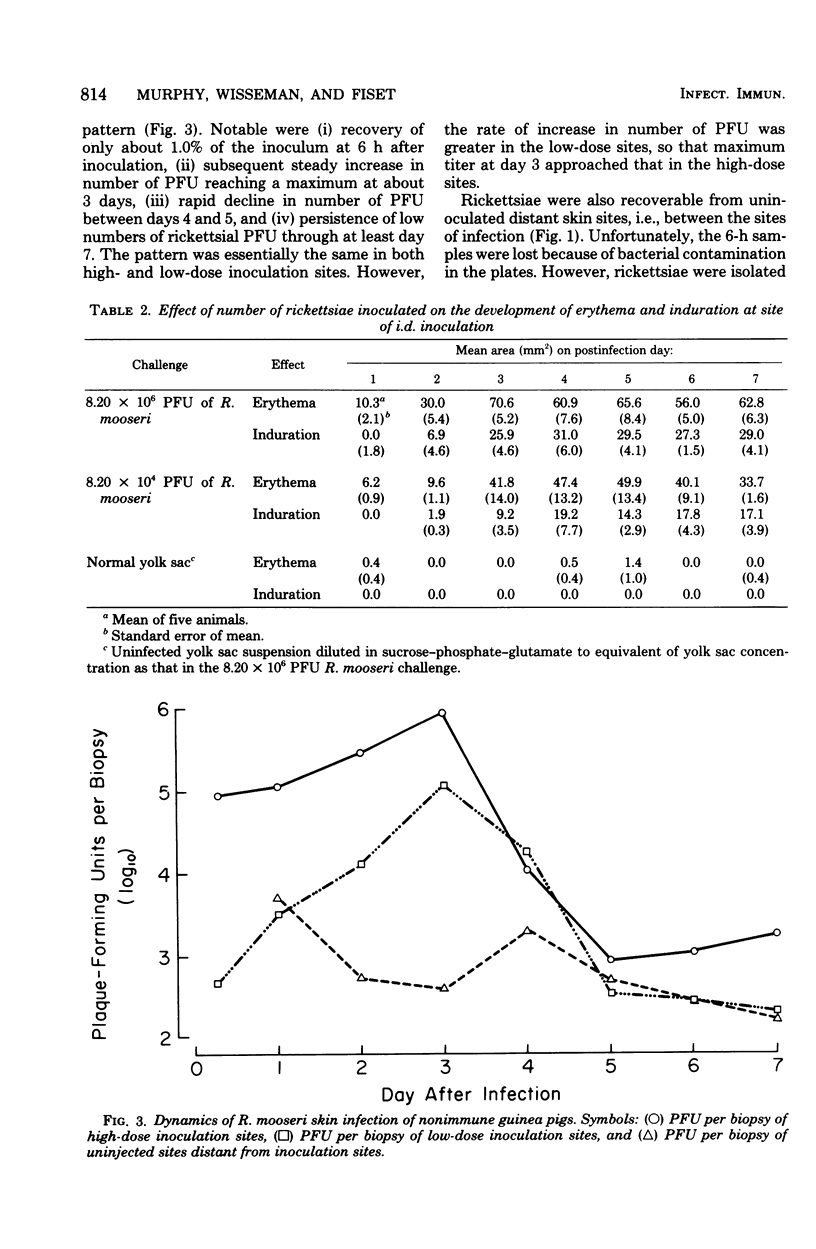
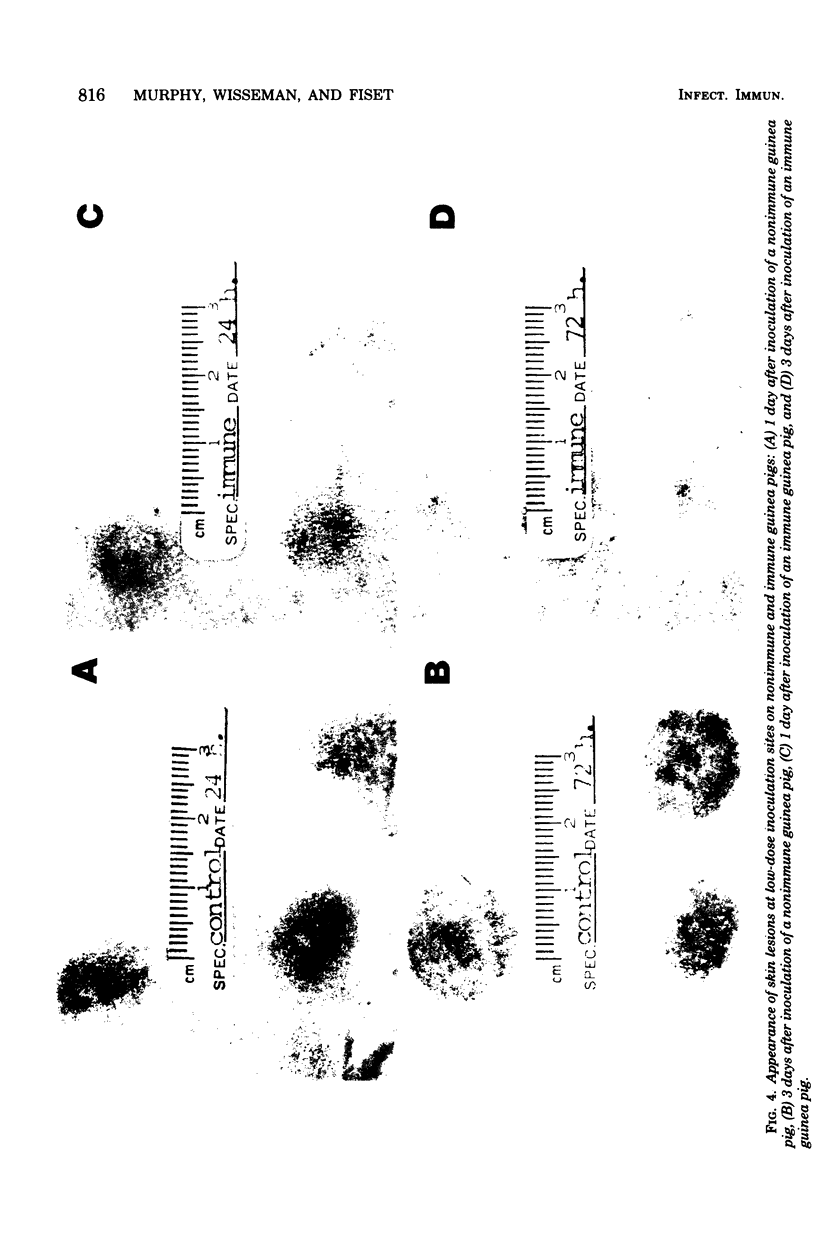
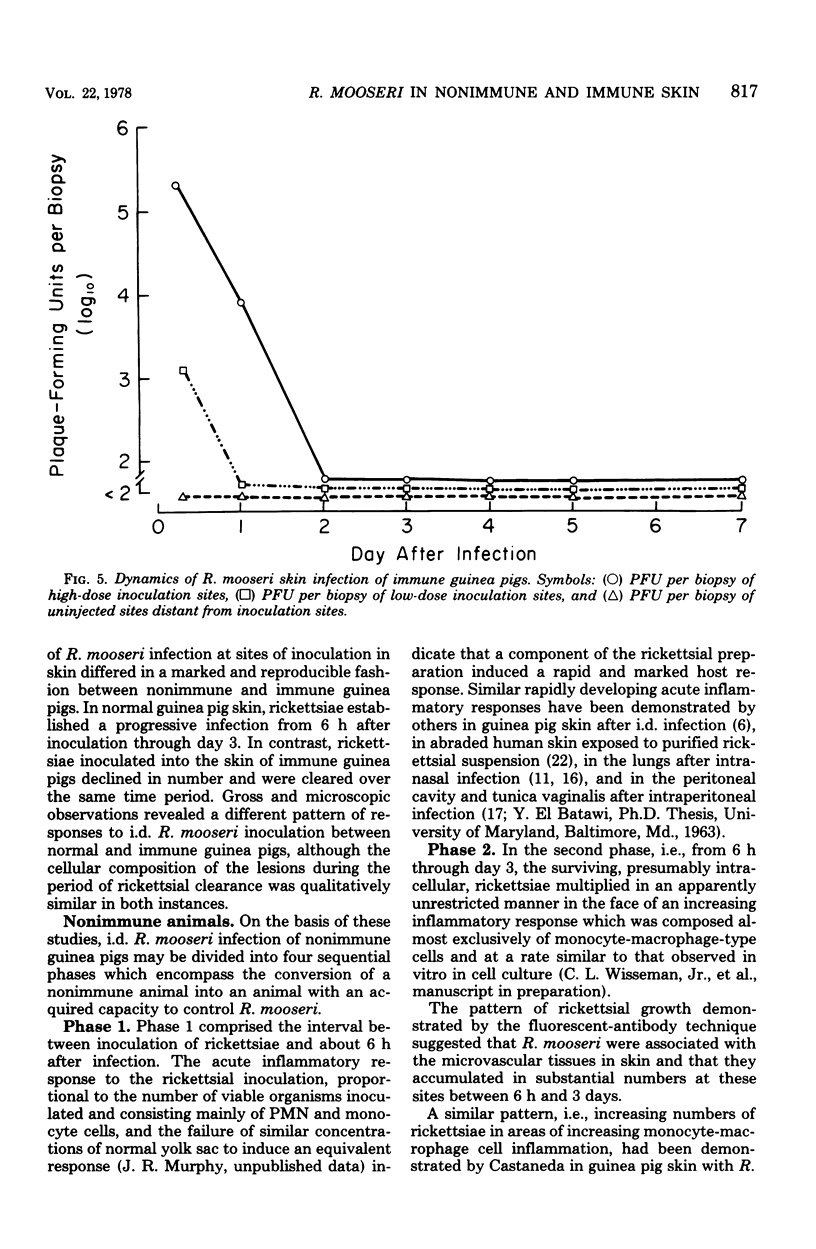
Rickettsia mooseri infection in skin at sites of intradermal inoculation was studied in nonimmune and immune guinea pigs with respect to dynamics of infection, localization of rickettsiae within tissues, and gross and microscopic pathology. Intradermal inoculation of R. mooseri into nonimmune guinea pigs resulted in gross lesions which, in magnitude, were directly related to the number of rickettsiae inoculated. The lesions progressively enlarged through 3 or 4 days and remained enlarged through at least 7 days. Histological examination revealed an early acute inflammation which progressed to a predominantly monocyte-macrophage inflammation and subsequently condensed into lymphocyte-containing granulomatous foci. Rickettsiae in the skin at sites of inoculation increased in numbers from 6 h through 3 days, in parallel with the increasing diffuse monocyte-macrophage inflammatory response, and then declined markedly on days 4 or 5 as ganulomatous foci appeared. Some rickettsiae, however, persisted through at least day 7. Fluorescent-antibody studies suggested that R. mooseri infected only a subset of cells available, i.e., cells associated with the microvascular system. Dissemination of infection was demonstrated by the presence of rickettsiae in the skin at sites distant from the point of inoculation. Immune guinea pigs, made immune by intradermal infection with R. mooseri 12 days before intradermal challenge, displayed an accelerated response. The lesions were maximal by 24 to 48 h and subsequently regressed. The inflammatory response of immune guinea pigs was a greater magnitude than the response of similarly challenged nonimmune guinea pigs, and the respose from acute inflammation through the formation of granulomatous lesions was accelerated. The number of rickettsiae in the skin of immune guinea pigs declined steadily from the time of inoculation, until no rickettsiae were recovered on or after day 3. Furthermore, dissemination of rickettsiae to sites in skin distant from the site of inoculation was not demonstrable. The results are discussed in terms of pathogenesis and of immunity to typhus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. VI. Differential opsonizing and neutralizing action of human typhus rickettsia-specific cytophilic antibodies in cultures of human macrophages. Infect Immun. 1976 Oct;14(4):1071–1076. doi: 10.1128/iai.14.4.1071-1076.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Shirai A., Hilderbrandt P. K., Osterman J. V. Host defenses in experimental scrub typhus: histopathological correlates. Infect Immun. 1976 Mar;13(3):861–875. doi: 10.1128/iai.13.3.861-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khavkin T. N., Amosenkova N. I., Krasnik F. I. Infectious process in the lungs after intranasal challenge of white mice with Rickettsia prowazekii. A histologic immunoluminescent and electron microscopic study. Am J Pathol. 1974 Aug;76(2):213–224. [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. L., Jr, Fiset P. Mechanisms of immunity in typhus infection: some characteristics of Rickettsia mooseri infection of guinea pigs. Infect Immun. 1978 Aug;21(2):417–424. doi: 10.1128/iai.21.2.417-424.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. L., Jr, Snyder L. B. Plaque assay for Rickettsia mooseri in tissue samples. Proc Soc Exp Biol Med. 1976 Oct;153(1):151–155. doi: 10.3181/00379727-153-39499. [DOI] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman R., Fiset P. Method for counting Rickettsiae and Chlamydiae in purified suspensions. J Bacteriol. 1968 Jan;95(1):259–261. doi: 10.1128/jb.95.1.259-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, PAZOUREK L., BOCCUTI A., JAMES H., OLDSTONE M. B. Studies on rickettsial toxins. V. Dissimilarity between the action of rickettsial mouse lethal toxin and bacterial endotoxin in mice. J Immunol. 1961 Jun;86:613–617. [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Silverman D. J. In vitro studies on Rickettsia-host cell interactions: lag phase in intracellular growth cycle as a function of stage of growth of infecting Rickettsia prowazeki, with preliminary observations on inhibition of rickettsial uptake by host cell fragments. Infect Immun. 1976 Jun;13(6):1749–1760. doi: 10.1128/iai.13.6.1749-1760.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, el Batawi Y., Wood W. H., Jr, Noriega A. R. Gross and microscopic skin reactions to killed typhus Rickettsiae in human beings. J Immunol. 1967 Jan;98(1):194–209. [PubMed] [Google Scholar]