Supplemental Digital Content is available in the text.
Abstract
Background:
Few previous studies of metabolic aberrations and prostate cancer risk have taken into account the fact that men with metabolic aberrations have an increased risk of death from causes other than prostate cancer. The aim of this study was to calculate, in a real-life scenario, the risk of prostate cancer diagnosis, prostate cancer death, and death from other causes.
Methods:
In the Metabolic Syndrome and Cancer Project, prospective data on body mass index, blood pressure, glucose, cholesterol, and triglycerides were collected from 285,040 men. Risks of prostate cancer diagnosis, prostate cancer death, and death from other causes were calculated by use of competing risk analysis for men with normal (bottom 84%) and high (top 16%) levels of each factor, and a composite score.
Results:
During a mean follow-up period of 12 years, 5,893 men were diagnosed with prostate cancer, 1,013 died of prostate cancer, and 26,328 died of other causes. After 1996, when prostate-specific antigen testing was introduced, men up to age 80 years with normal metabolic levels had 13% risk of prostate cancer, 2% risk of prostate cancer death, and 30% risk of death from other causes, whereas men with metabolic aberrations had corresponding risks of 11%, 2%, and 44%.
Conclusions:
In contrast to recent studies using conventional survival analysis, in a real-world scenario taking risk of competing events into account, men with metabolic aberrations had lower risk of prostate cancer diagnosis, similar risk of prostate cancer death, and substantially higher risk of death from other causes compared with men who had normal metabolic levels.
Prostate cancer incidence is up to 20-fold higher in industrialized countries compared with developing countries,1 and nutrition and other lifestyle factors have been suggested as a cause for this difference.2 To date, many studies have investigated the putative etiological association between metabolic aberrations and prostate cancer risk, with inconsistent results.3–8 We have previously investigated this within the Metabolic Syndrome and Cancer Project by use of Cox regression analysis, and we found no associations between metabolic aberrations and prostate cancer risk.9 In contrast, high levels of BMI, blood pressure, and a composite score of all metabolic factors were associated with increased risk of prostate cancer death.
However, men with metabolic aberrations have a higher risk of death from cardiovascular disease and other diseases,10 and such events are censored in studies of etiologic risk using conventional methods similar to the Cox model, despite the fact that they are considered competing events in analysis of prostate cancer. Few studies to date have taken risk of competing events into account when assessing a person’s risk of prostate cancer in a real-world scenario. This risk—previously denoted as actual risk,11 cumulative absolute risk,12 real-world probabilities,13 and crude probabilities14—can be calculated by cumulative incidence functions.
The aim of this study was to assess the risk of prostate cancer diagnosis, prostate cancer death, and death from other causes for men with normal metabolic levels and metabolic aberrations in a real-world scenario by use of data in a large prospective pooled European cohort.
METHODS
Study Population
This study was conducted within the Metabolic Syndrome and Cancer Project, which has previously been described in detail.15 In brief, the project consists of 7 sub-cohorts from Norway, Sweden, and Austria, with prospectively collected data on body mass index (BMI), systolic and diastolic blood pressure, and circulating levels of glucose, total cholesterol, and triglycerides from 578,700 men and women from 1972 to 2005.
The study was approved by The Research Review Board of Umeå, Sweden, the Regional Committee for Medical and Health Research Ethics, Southeast Norway and the Ethikommission of the Land Vorarlberg, Austria. Participants from Sweden and Austria provided written informed consent to participate in this study. In Norway, the participants were invited to come to the health survey and a questionnaire was sent together with the invitation. An attendance to the health examination where the participants delivered their filled in questionnaire, has been accepted by the Data Inspectorate as an informed consent, but not a written consent. Written consent was obtained from 1994 onwards.
Endpoints
Prostate cancer cases were identified through linkage to each National Cancer Register, by use of code 177 in the International Statistical Classification of Diseases, revision 7 (ICD-7). All men diagnosed with prostate cancer were selected. Of these, approximately 5% were secondary or later cancer diagnoses for men previously diagnosed with cancer at another site. We excluded 78 men with prostate cancer diagnosed at the date of death. Causes of death were obtained by linkage to each National Cause of Death Register, with data available until the last date of follow up (31 December 2003 in Austria, and until 31 December 2004 in Norway and Sweden), and coded according to Eurostat European shortlist for causes of death.16 We used data only from the first health examination, and we excluded men with first health examination after last date of follow up (1,742 men) or without a recorded cause of death cause (3,084 men). Data were also linked to The Register of Total Population and Population Changes for migration status in Norway and Sweden.
Statistical Analysis
Follow-up started 1 year after the first health examination and continued until the first date of either the main event or the competing event, or until date of censoring due to emigration or end of follow-up. Incident prostate cancer and prostate cancer death were the main events in separate analyses, and death from all causes and death from causes other than prostate cancer were the competing events, respectively.
To investigate whether the risk of prostate cancer was related to diagnostic intensity—with a larger proportion of low-risk cancers after the introduction of prostate-specific antigen (PSA) testing—we performed sub-group analysis for 2 periods of follow up. The cut-point of 31 December 1996 was chosen because that time marked the beginning of an increase in the incidence of prostate cancer due to the introduction of PSA testing in Sweden,17 Norway,18 and Austria.19 We denoted the earlier period ending at 31 December 1996, as “pre-PSA era,” and the later period, beginning 1 January 1997, as “PSA era.” No sub-group analyses were performed for prostate cancer death.
Metabolic factors were transformed to a standardized score (z-score), with zero as mean and 1 as standard deviation in all analyses.9 BMI and blood pressure were transformed into z-scores separately for each sub-cohort, whereas glucose, cholesterol, and triglycerides were transformed into z-scores separately by sub-cohort and also according to fasting time (less than 1h, 1–2h, 2–4h, 4–8h, more than 8h). As the distributions of glucose and triglycerides were skewed, natural logarithm was applied before z-score transformation. Mid blood pressure was defined as (systolic + diastolic blood pressure)/2, and a composite score was defined as the sum of all single z-scores. The z-scores were dichotomized at z = 1, resulting in a nonexposed group comprising 84% of the participants who had measured levels of each metabolic factor and the composite score below this cut-point and the exposed group consisted of the remaining 16% with levels above this cut-point. We denoted men in the lower 84% of the composite score as “men with normal metabolic levels” and men in the top 16% of the composite score as “men with metabolic aberrations.”
For the main and competing event in each analysis, we calculated cumulative incidence functions,20 for men with normal and high levels of metabolic factors. Attained age was used as the time scale. All analyses also included smoking status (never, former, or current), 5 categories of birth year (before 1927, 1927–1929, 1930–1932, 1933–1938, 1939, and later), age at health examination, and sub-cohort. The cumulative incidence functions were calculated based on Cox regression models in which smoking and age at heath examination were allowed to have different effects on the main event and competing events, in addition to each metabolic factor. The assumption of proportional hazards in the Cox regression model was tested with Schoenfeld residuals and was found to be valid.
Calculations were performed with STATA MP/2 version 11.2 (StataCorp LP, College Station, TX).
RESULTS
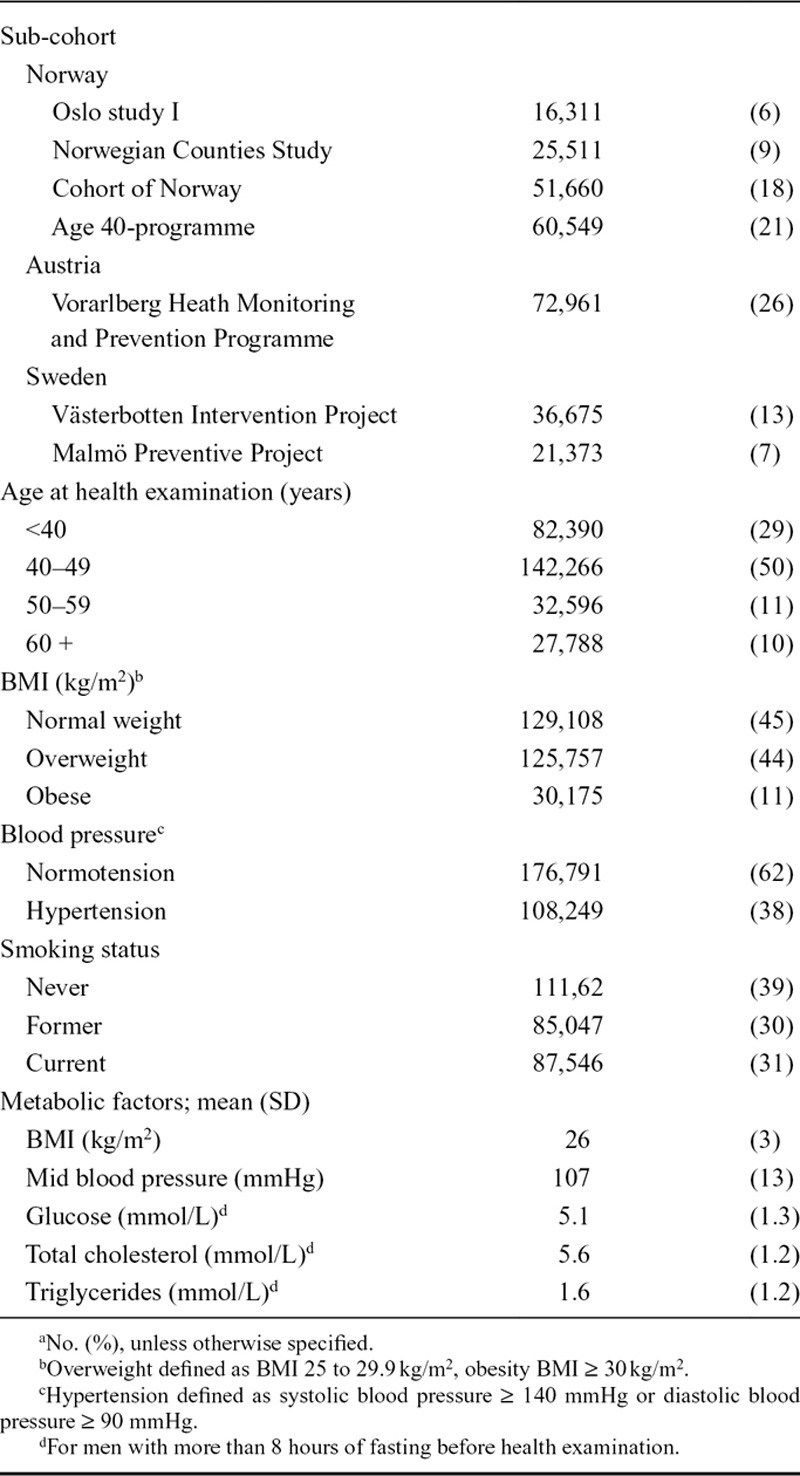
Mean baseline age among the 285,040 men in the study was 44 years (SD = 11), 44% of the men were overweight (BMI 25–29.9 kg/m2) and 10% were obese (BMI≥30 kg/m2) (Table).
TABLE.
Baseline Characteristicsa of Men in the Metabolic Syndrome and Cancer Project (n = 285,040)
A total of 5,893 men were diagnosed with prostate cancer, and 1,013 men died of prostate cancer during follow up, which was on average 12 years (SD = 8) (Figure 1). Of these men, 1,366 men had been diagnosed with prostate cancer in the pre-PSA era with end of follow up at December 31, 1996, and 4,527 men were diagnosed in the PSA era. The earliest prostate cancer case was diagnosed in 1974.
FIGURE 1.
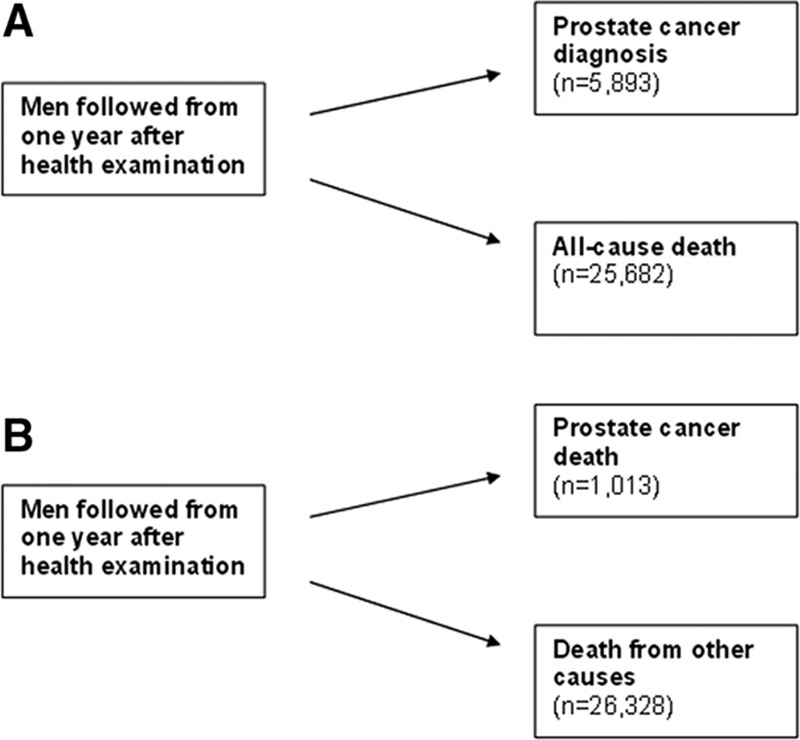
Main and competing events in analysis of risk of (A) prostate cancer diagnosis and (B) prostate cancer death in the Metabolic Syndrome and Cancer Project of 285,040 men. Men were followed until the first point in time of main or competing event, or until censoring due to migration or end of follow-up. The number of deaths from all causes in analysis (A) is smaller than the number of deaths from other causes in analysis (B) because many of men followed from date of prostate cancer diagnosis in (A) have died either from prostate cancer or other causes in (B).
The risk of prostate cancer diagnosis was lower for men with metabolic aberrations than for men with normal metabolic levels, in both the pre-PSA and PSA eras. In the pre-PSA era, the risk of prostate cancer for men up to age 80 years was 6% for men with normal metabolic levels and 5% for men with metabolic aberrations (Figure 2A). In the PSA era, the corresponding risk of prostate cancer diagnosis was 13% for men with normal levels and 11% for men with metabolic aberrations, and the risk of death from any cause was and 37% and 47%, respectively (Figures 2B and 3). Risk for men with normal and high levels of BMI, blood pressure, glucose, total cholesterol, and triglycerides were similar to the results for men with normal metabolic levels and metabolic aberrations. (See eFigure 1, http://links.lww.com/EDE/A831 which illustrates the risk of prostate cancer for separate metabolic factors in the PSA era.)
FIGURE 2.
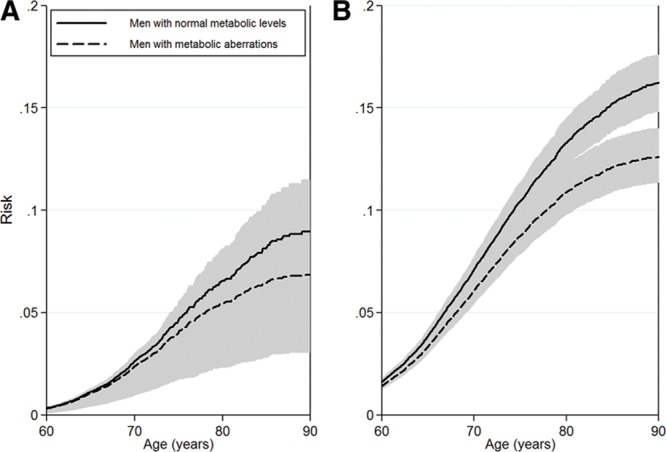
Risk of prostate cancer in (A) the pre-PSA era and (B) the PSA era in the Metabolic Syndrome and Cancer Project. Shaded areas are 95% confidence intervals. Smoking status, 5 categories of birth year, age at health examination, and sub-cohort were included in the analyses.
FIGURE 3.
Risk of prostate cancer diagnosis and of the competing event, death, in the PSA era in the Metabolic Syndrome and Cancer Project. The curves are stacked for each level of exposure, and the remaining area above the curves corresponds to the risk of no event. Smoking status, 5 categories of birth year, age at health examination, and sub-cohort were included in the analyses.
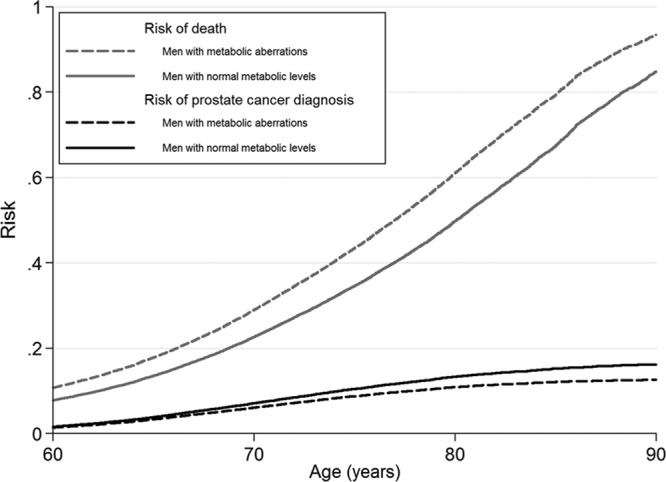
All men in the study up to age 80 had approximately 2% risk of prostate cancer death regardless of metabolic status. Men with normal metabolic levels had 30% risk of death from other causes and men with metabolic aberrations had 44% risk (Figure 4). Risk for men with normal and high levels of BMI, blood pressure, glucose, total cholesterol, and triglycerides was also around 2%. (See eFigure 2, http://links.lww.com/EDE/A831 which illustrates the risk of prostate cancer death for separate metabolic factors.)
FIGURE 4.
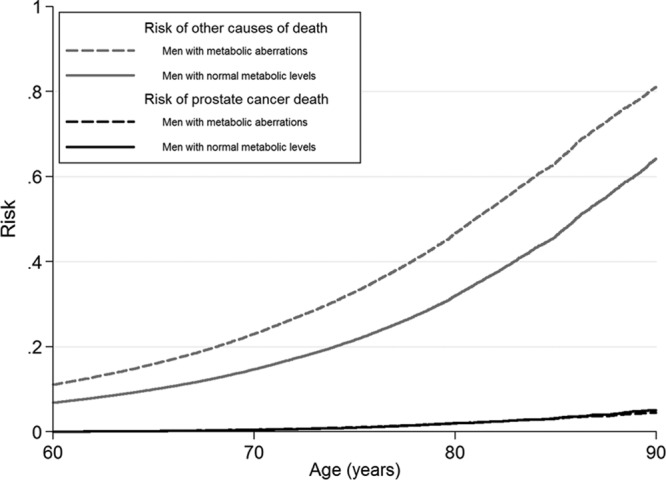
Risk of prostate cancer death and of the competing event, other causes of death, Metabolic Syndrome and Cancer Project. The curves are stacked for each level of exposure and the remaining area above the curves corresponds to the risk of no event. Smoking status, 5 categories of birth year, age at health examination, and sub-cohort were included in the analyses.
DISCUSSION
Etiologic studies with data in the PSA era have found no increased risk of prostate cancer for men with metabolic aberrations using conventional survival analysis.9,21,22 In our study of a real-world scenario with competing events taken into account, men with metabolic aberrations had a lower risk of prostate cancer diagnosis, similar risk of prostate cancer death, and substantially higher risk of death from other causes, compared with men who had normal metabolic levels.
Strengths of our study include the large sample size from 7 European sub-cohorts and almost complete follow-up of cancer diagnoses.23–25 The main limitation was the lack of information on tumor characteristics and on covariates that affect the risk of a prostate cancer diagnosis, such as family history of prostate cancer26 and socioeconomic status.27
We previously found no association of these metabolic factors with prostate cancer diagnosis, but we did find an association of high BMI, blood pressure and the composite score with increased risk of prostate cancer death,9 in accordance with several other large studies.7,28,29 In the current study we considered whether men with metabolic aberrations have a different risk of prostate cancer diagnosis and prostate cancer death, compared with men with normal metabolic levels, when competing events are taken into account. There are 2 reasons why the present results differ from those in our earlier analyses. First, prostate cancer tends to be diagnosed at older ages, when many other men have already died (mean age at prostate cancer diagnosis in Scandinavia is 70–75 years);30 therefore, conventional survival analysis may overestimate the absolute risk.31 Second, high levels of metabolic factors are related to increased risk of death from all causes,10 which may affect calculations of relative risk,11 since men with high levels of metabolic factors have a shorter life expectancy, and are therefore more likely to be censored in conventional survival analysis. In other words, among men who are censored due to death from other causes there is a larger proportion of men with high levels of metabolic factors, than among men censored because of migration or end of follow-up. This difference is similar to reports on smoking as risk factor for Alzheimer disease32 and melanoma,33 in which a decreased risk of the disease was found among smokers. This is most likely a spurious association due to selection bias, since smokers have a shorter life expectancy compared with nonsmokers and therefore have lower risks of diseases occurring later in life. Accordingly, we hypothesize that a similar selection bias occurs in studies of metabolic factors and prostate cancer, since men with high levels of metabolic factors have a shorter life expectancy than men with normal levels.
Only a few studies on metabolic factors and prostate cancer have used methods of competing risk analysis, and results have been inconsistent.28,29,34 In our study, men with metabolic aberrations had lower risk of prostate cancer, in accordance with results from Fine and Gray regression analysis of a Swedish cohort study of almost 37,000 men, in which men with high levels of BMI at age 60 years had a lower risk of localized prostate cancer, slightly higher risk of advanced cancer, and an higher risk of prostate cancer death.29 However, our findings are in contrast with 2 studies28,34 based on conditional probability of prostate cancer—a method that yields results that are difficult to interpret.35 The first of these studies used data from 200,000 men with 5,000 prostate cancer cases and found an increased risk of prostate cancer for high levels of triglycerides and glucose at age 75.28 The second study of 2,322 men found that men with the metabolic syndrome had an increased risk of prostate cancer at age 80.34
A high proportion of men with high socioeconomic status undergo PSA testing,36 and therefore have an increased risk of prostate cancer, in particular of low-risk cancer.27 In contrast, men with high socioeconomic status have a lower prevalence of the metabolic syndrome,37 obesity,38 and diabetes mellitus type 2.39 Consistent with these associations, the metabolic syndrome, obesity, diabetes, and other metabolic aberrations have been linked to lower risk of prostate cancer diagnosis during the PSA era.9,21,22
Taking competing events into account, the risk of the event of interest will depend on the all-cause mortality. Our calculated risks of prostate cancer are in accordance with prostate cancer incidence in the Nordic countries during the same calendar period,30 and our results can be generalized to populations with similar all-cause mortality, e.g. Western countries.
In conlusion, conventional studies by use of Cox regression analysis from the PSA era have found a similar risk of prostate cancer for men with or without metabolic aberrations. In this study, taking risk of competing events into account in a real world scenario, men with metabolic aberrations had a lower risk of prostate cancer diagnosis, similar risk of prostate cancer death, and a higher risk of death from other causes, compared with men who had normal metabolic levels.
ACKNOWLEDGMENTS
We thank the following: in Norway, the screening team at the former National Health Screening Service of Norway, now the Norwegian Institute of Public Health, the services of CONOR and the contributing research centres delivering data to CONOR; in the VHM&PP, Elmar Stimpfl, data base manager, Karin Parschalk at the cancer registry, and Markus Wallner, Christian Bernhard, Andrea Kaufmann and Gabriela Dür at the Vorarlberg State Government; in the VIP, Åsa Ågren, database manager at the Medical Biobank, Umeå University, Sweden; and in the MPP, Anders Dahlin, data base manager.
Footnotes
The authors report no conflicts of interest.
This work was supported by the World Cancer Research Fund (2007/09); Wereld Kanker Onderzoek Fonds (R2010/247) and the Swedish Cancer Society (2010/628).
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
REFERENCES
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Barnard RJ. Prostate cancer prevention by nutritional means to alleviate metabolic syndrome. Am J Clin Nutr. 2007;86:s889–s893. doi: 10.1093/ajcn/86.3.889S. [DOI] [PubMed] [Google Scholar]
- 3.Lund Håheim L, Wisløff TF, Holme I, Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am J Epidemiol. 2006;164:769–774. doi: 10.1093/aje/kwj284. [DOI] [PubMed] [Google Scholar]
- 4.Tande AJ, Platz EA, Folsom AR. The metabolic syndrome is associated with reduced risk of prostate cancer. Am J Epidemiol. 2006;164:1094–1102. doi: 10.1093/aje/kwj320. [DOI] [PubMed] [Google Scholar]
- 5.Engeland A, Tretli S, Bjørge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 7.Stocks T, Hergens MP, Englund A, Ye W, Stattin P. Blood pressure, body size and prostate cancer risk in the Swedish Construction Workers cohort. Int J Cancer. 2010;127:1660–1668. doi: 10.1002/ijc.25171. [DOI] [PubMed] [Google Scholar]
- 8.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Häggström C, Stocks T, Ulmert D, et al. Prospective study on metabolic factors and risk of prostate cancer. Cancer. 2012;118:6199–6206. doi: 10.1002/cncr.27677. [DOI] [PubMed] [Google Scholar]
- 10.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 11.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 12.Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97:1428–1437. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 13.Hinchliffe SR, Abrams KR, Lambert PC. The impact of under and over-recording of cancer on death certificates in a competing risks analysis: a simulation study. Cancer Epidemiol. 2013;37:11–19. doi: 10.1016/j.canep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Eloranta S, Adolfsson J, Lambert PC, et al. How can we make cancer survival statistics more useful for patients and clinicians: an illustration using localized prostate cancer in Sweden. Cancer Causes Control. 2013;24:505–515. doi: 10.1007/s10552-012-0141-5. [DOI] [PubMed] [Google Scholar]
- 15.Stocks T, Borena W, Strohmaier S, et al. Cohort profile: The Metabolic syndrome and Cancer project (Me-Can). Int J Epidemiol. 2010;39:660–667. doi: 10.1093/ije/dyp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eurostat. European Shortlist for Causes of Death. 1998. Available at: http://ec.europa.eu/eurostat/ramon/nomenclatures/index.cfm?TargetUrl=LST_NOM_DTL&StrNom=COD_1998&StrLanguageCode=EN&IntPcKey=&StrLayoutCode=HIERARCHIC. Accessed 1 November 2013. [Google Scholar]
- 17.Jonsson H, Holmström B, Duffy SW, Stattin P. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2011;129:1881–1888. doi: 10.1002/ijc.25846. [DOI] [PubMed] [Google Scholar]
- 18.Kvåle R, Auvinen A, Adami HO, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99:1881–1887. doi: 10.1093/jnci/djm249. [DOI] [PubMed] [Google Scholar]
- 19.Vutuc C, Schernhammer ES, Haidinger G, Waldhör T. Prostate cancer and prostate-specific antigen (PSA) screening in Austria. Wien Klin Wochenschr. 2005;117:457–461. doi: 10.1007/s00508-005-0395-y. [DOI] [PubMed] [Google Scholar]
- 20.Coviello E. STCOMPADJ: Stata Module to Estimate the Covariate-adjusted Cumulative Incidence Function in the Presence of Competing Risks. Statistical Software Components, Boston College Department of Economics. 2009. Available at: http://econpapers.repec.org/RePEc:boc:bocode:s457063. Accessed 17 March 2014.
- 21.Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control. 2009;20:1181–1192. doi: 10.1007/s10552-009-9319-x. [DOI] [PubMed] [Google Scholar]
- 22.Fall K, Garmo H, Gudbjörnsdottir S, Stattin P, Zethelius B. Diabetes mellitus and prostate cancer risk; a nationwide case-control study within PCBaSe Sweden. Cancer Epidemiol Biomarkers Prev. 2013;22:1102–1109. doi: 10.1158/1055-9965.EPI-12-1046. [DOI] [PubMed] [Google Scholar]
- 23.Sandblom G, Dufmats M, Olsson M, Varenhorst E. Validity of a population-based cancer register in Sweden–an assessment of data reproducibility in the South-East Region Prostate Cancer Register. Scand J Urol Nephrol. 2003;37:112–119. doi: 10.1080/00365590310008839. [DOI] [PubMed] [Google Scholar]
- 24.Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Rapp K, Schroeder J, Klenk J, et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945–952. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 26.Frank C, Fallah M, Ji J, Sundquist J, Hemminki K. The population impact of familial cancer, a major cause of cancer. Int J Cancer. 2014;134:1899–1906. doi: 10.1002/ijc.28510. [DOI] [PubMed] [Google Scholar]
- 27.Wirén SM, Drevin LI, Carlsson SV, et al. Fatherhood status and risk of prostate cancer: nationwide, population-based case-control study. Int J Cancer. 2013;133:937–943. doi: 10.1002/ijc.28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Hemelrijck M, Garmo H, Holmberg L, et al. Prostate cancer risk in the Swedish AMORIS study: the interplay among triglycerides, total cholesterol, and glucose. Cancer. 2011;117:2086–2095. doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 29.Discacciati A, Orsini N, Andersson SO, Andrén O, Johansson JE, Wolk A. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: a population-based prospective study. Br J Cancer. 2011;105:1061–1068. doi: 10.1038/bjc.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engholm G, Ferlay J, Christensen N, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 5.3 (25.04. 2013). Association of the Nordic Cancer Registries. Danish Cancer Society. Available at: http://www-dep.iarc.fr/NORDCAN/SW/frame.asp. Accessed 1 November 2013.
- 31.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernán MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19:448–450. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 33.Thompson CA, Zhang ZF, Arah OA. Competing risk bias to explain the inverse relationship between smoking and malignant melanoma. Eur J Epidemiol. 2013;28:557–567. doi: 10.1007/s10654-013-9812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundmark B, Garmo H, Loda M, Busch C, Holmberg L, Zethelius B. The metabolic syndrome and the risk of prostate cancer under competing risks of death from other causes. Cancer Epidemiol Biomarkers Prev. 2010;19:2088–2096. doi: 10.1158/1055-9965.EPI-10-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen PK, Keiding N. Interpretability and importance of functionals in competing risks and multistate models. Stat Med. 2012;31:1074–1088. doi: 10.1002/sim.4385. [DOI] [PubMed] [Google Scholar]
- 36.Karlsen RV, Larsen SB, Christensen J, et al. PSA testing without clinical indication for prostate cancer in relation to socio-demographic and clinical characteristics in the Danish Diet, Cancer and Health Study. Acta Oncol. 2013;52:1609–1614. doi: 10.3109/0284186X.2013.831474. [DOI] [PubMed] [Google Scholar]
- 37.Perel P, Langenberg C, Ferrie J, Moser K, Brunner E, Marmot M. Household wealth and the metabolic syndrome in the Whitehall II study. Diabetes Care. 2006;29:2694–2700. doi: 10.2337/dc06-0022. [DOI] [PubMed] [Google Scholar]
- 38.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 39.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40:804–818. doi: 10.1093/ije/dyr029. [DOI] [PubMed] [Google Scholar]


