Abstract
Objective:
We sought to quantify and compare angular vestibulo-ocular reflex (aVOR) gain and compensatory saccade properties elicited by the head impulse test (HIT) in pontine-cerebellar stroke (PCS) and vestibular neuritis (VN).
Methods:
Horizontal HIT was recorded ≤7 days from vertigo onset with dual-search coils in 33 PCS involving the anterior inferior, posterior inferior, and superior cerebellar arteries (13 AICA, 17 PICA, 3 SCA) confirmed by MRI and 20 VN. We determined the aVOR gain and asymmetry, and compensatory overt saccade properties including amplitude asymmetry and cumulative amplitude (ipsilesional trials [I]; contralesional trials [C]).
Results:
The aVOR gain (normal: 0.96; asymmetry = 2%) was bilaterally reduced, greater in AICA (I = 0.39, C = 0.57; asymmetry = 20%) than in PICA/SCA strokes (I = 0.75, C = 0.74; asymmetry = 7%), in contrast to the unilateral deficit in VN (I = 0.22, C = 0.76; asymmetry = 54%). Cumulative amplitude (normal: 1.1°) was smaller in AICA (I = 4.2°, C = 3.0°) and PICA/SCA strokes (I = 2.1°, C = 3.0°) compared with VN (I = 8.5°, C = 1.3°). Amplitude asymmetry in AICA and PICA/SCA strokes was comparable, but favored the contralesional side in PICA/SCA strokes and the ipsilesional side in VN. Saccade asymmetry <61% was found in 97% of PCS and none of VN. Gain asymmetry <40% was found in 94% of PCS and 10% of VN.
Conclusion:
HIT gains and compensatory saccades differ between PCS and VN. VN was characterized by unilateral gain deficits with asymmetric large saccades, AICA stroke by more symmetric bilateral gain reduction with smaller saccades, and PICA stroke by contralesional gain bias with the smallest saccades. Saccade and gain asymmetry should be investigated further in future diagnostic accuracy studies.
Classification of evidence:
This study provides Class II evidence that aVOR testing accurately distinguishes patients with PCS from VN (sensitivity 94%–97%, specificity 90%–100%).
Acute vestibular syndrome (AVS), characterized by prolonged spontaneous vertigo,1 is frequently due to vestibular neuritis (VN) but may be caused by pontine-cerebellar stroke (PCS).2–4 A negative clinical head impulse test (HIT), or absence of compensatory saccade, plus assessment for skew deviation and direction-changing nystagmus predict PCS in the context of AVS5–8 better than the reference standard, diffusion-weighted imaging (DWI), which may be falsely negative.8,9
Because clinical HIT is subjective,10 quantitative assessment is desirable. A small video-oculographic (VOG) study has compared the angular vestibulo-ocular reflex (aVOR) gain, the ratio of eye velocity to head velocity, in a contemporaneous group of patients with AVS consisting of PCS and VN.11 However, the quantitative aspect of the pivotal sign of clinical HIT, the presence or absence of a compensatory saccade,12 remains to be investigated in AVS. We hypothesized that aVOR gain and compensatory saccade measures would differ between PCS subgroups and VN. In this study, we used the gold-standard search coil technique to record the HIT, analyzing aVOR gain and saccade characteristics in PCS subgroups as defined by vascular territories and lesional anatomy, and compared the findings with VN and normal subjects.
METHODS
Study protocol approvals, registrations, and patient consents.
Study protocol was approved by the Sydney Local Health District Ethics Review Committee (X11-0151) and University of Sydney (13076) with written informed consent from all subjects, in accordance with the Declaration of Helsinki.
Subjects.
We prospectively recruited 63 nonconsecutive patients from inpatient neurology at a quaternary hospital between 2011 and 2014, based on symptoms of acute prolonged spontaneous vertigo (>24 hours) and gait imbalance consistent with AVS. Patients with anterior circulation (n = 3) and lateral medullary stroke (n = 7) were excluded. Patients underwent targeted neuro-otological examination (HIT, skew deviation, and direction-changing nystagmus)6–8 and MRI; examiners were not explicitly masked to imaging or other test results.
Thirty-three patients with PCS (26 men; aged 24–80, 58.5 ± 15.8 years [mean ± SD]) were identified by abnormal DWI in the territories of posterior inferior cerebellar artery (PICA), anterior inferior cerebellar artery (AICA), or superior cerebellar artery (SCA). Subtypes of AICA strokes were defined: (1) AICAp with peripheral characteristics, indicated by positive clinical HIT; and (2) AICAc with central characteristics, indicated by negative clinical HIT. Twenty VN (10 men; aged 37–85, 59.2 ± 14.5 years) were diagnosed by normal DWI, a benign targeted examination (unidirectional nystagmus, positive clinical HIT, no skew deviation), and lack of neurologic deficits on follow-up (discharge and 6–8 weeks). HIT was recorded by L.C. ≤7 days from vertigo onset (PCS: 3.4 ± 2.0 days; VN: 3.4 ± 2.3 days). Normal subjects (n = 17, 14 men, aged 26–80, 50.5 ± 15.4 years) without vestibular or neurologic disorders consisted of ambulatory care staff or relatives of outpatients. All subjects completed the study protocol without complications. See appendix e-1-I and table e-A1 on the Neurology® Web site at Neurology.org for details of the clinico-radiologic findings.
MRI.
Stroke protocol MRI (1.5T, GE, Australia; 5-mm-thick axial slice, 0.5-mm gap) was performed in all patients with PCS (48–72 hours from vertigo onset) and VN (48–96 hours from vertigo onset). Acute stroke was defined by abnormal DWI13 and hypointense apparent diffusion coefficient map, supplemented by T2-weighted sequences. Anatomical localization and vascular territories were determined by LC using axial sections.14
Recording setup.
Head and binocular eye positions were recorded with precalibrated dual-search coils (Universal Trading Ventures, Cleveland, OH).15,16 Subjects wore a head coil secured to a dental impression bite bar. Search coils were placed after topical anesthesia, and recording was performed in a magnetic coil frame (CNC Engineering, Enfield, CT). Head and eye signals were recovered by phase detection, low-pass filtered (0–100 Hz), and sampled at 1 kHz with 16-bit resolution. The system had a resolution of 0.1 arcminute and <2% cross-coupling between orthogonal signals.
Recording procedure.
The subject was positioned with their head in the coil frame center, 91 cm from a target. An experimenter standing behind the subject manually delivered approximately 25 to 30 center-eccentric, unpredictable, passive head impulses in the plane of each horizontal canal.15,16 Impulses were matched to duration (onset to peak velocity approximately 100 milliseconds), amplitudes (15–20°), peak velocities (200–300°/s), and peak accelerations (2,500–4,500°/s2) aided by an LCD monitor (appendix e-1), which displayed individual impulse velocity profiles.
Data analysis.
MRI lesions were reported by neuroradiologists, corroborated by LC, mirrored as left, which was assigned as ipsilesional. For bilateral lesions, the side with qualitatively larger DWI lesion volume was assigned as left. AICA stroke was analyzed both as a group and as 2 divisions, AICAp and AICAc. Because ischemia in PICA and SCA affects the cerebellum but not the vestibular nuclei directly, we grouped them for analysis.
Head and eye data were analyzed offline with semiautomated LabView software (National Instruments, Austin, TX). Rotation vectors for head, gaze, and eye positions were determined.17,18 Head impulses were aligned to peak head acceleration. Trials with saccadic eye movements during the first 70 milliseconds after impulse onset were excluded and the remainder analyzed (table 1). Gain of the abducting eye (e.g., right eye for leftward impulses)19 was calculated based on peak head acceleration centered during a 40-millisecond window.20 Gain asymmetry (Gs) between ipsilesional (Gi) and contralesional (Gc) gains was determined as the absolute difference: Gs = (Gc − Gi) × 100%.
Table 1.
Head impulse gain and saccade characteristics in normal subjects, VN, and pontine-cerebellar stroke
Compensatory saccades were distinguished from fast phases of spontaneous and gaze-evoked nystagmus by criteria such as difference in intersaccadic interval (appendix e-1-II and figure e-1). Head impulse onset was defined as when peak head velocity reached 2%, and offset defined when it approached zero.17 Saccade onset latency was measured between impulse onset and peak saccade acceleration. Saccades were classified as overt if the onset occurred after impulse offset and covert if before.17,18 Peak velocity was used to detect covert saccades. Overt saccade amplitude was determined as cumulative (average amplitude of all saccades per trial per horizontal canal) and as mean (first, second, and third saccade). Overt saccade amplitude asymmetry (As) was calculated from the sum of ipsilesional (ΣAi) and contralesional (ΣAc) saccade amplitude using Jongkee's formula:
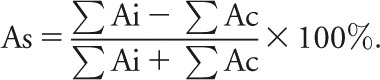 |
Statistical analysis.
Statistical analysis was performed using SPSS 21 (IBM Corp., Armonk, NY). Data were tested for normality with the Shapiro–Wilk test. Kruskal–Wallis test was used to compare difference in aVOR gain asymmetry, 1-way analysis of variance in overt saccade amplitude asymmetry, and linear mixed model between ipsilesional and contralesional gains and saccade amplitude among PCS, VN, and normal subjects. AICA stroke was considered as a group for analysis, and descriptive statistics are given for AICAp and AICAc. Gain and saccade asymmetry were analyzed as absolute values, and their direction (positive or negative) is separately described. Bonferroni correction was used for multiple comparisons to adjust the α level of 0.05 for all tests. Receiver operating characteristic curve analysis was used to identify potential quantitative cut-points for differentiating PCS from VN in future diagnostic accuracy studies, and to identify thresholds for detecting a positive clinical HIT based on cumulative saccade amplitude.
RESULTS
aVOR gains among subjects with PCS and VN.
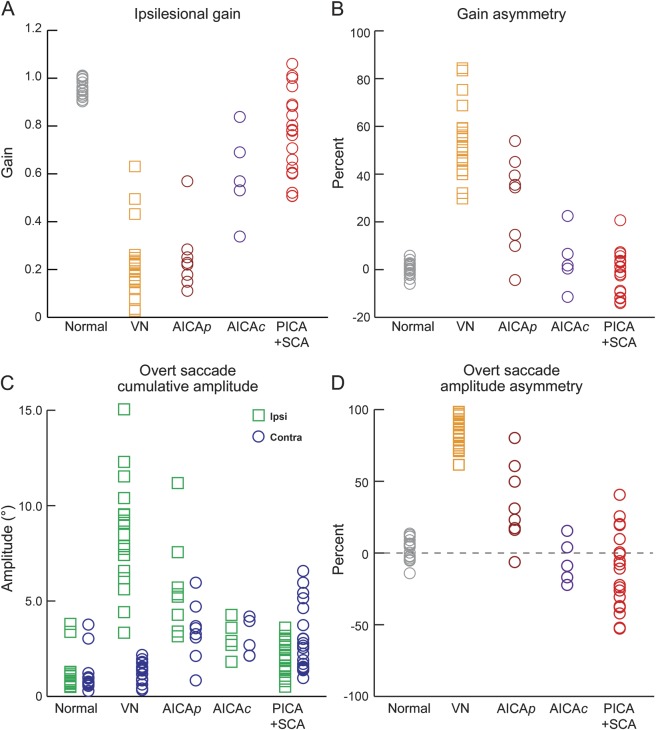
In normal subjects, aVOR gains were just below unity (0.96 ± 0.05, mean ± 95% confidence interval [CI]) and symmetric (2% ± 1%). Table 1 summarizes gains in PCS and VN. In VN (n = 20), gains were deficient ipsilesionally and reduced by approximately 20% contralesionally (figures 1A and 2A) leading to marked asymmetry (figure 3, A and B). AICA stroke (n = 13) was characterized by variable bilateral gain reduction. In AICAp stroke (n = 8), gains were bilaterally reduced (ipsilesional: 0.25 ± 0.10; contralesional: 0.53 ± 0.14) resulting in a spectrum of asymmetry (30% ± 13%) (figures 1B, 2B, and 3, A and B). In AICAc stroke (n = 5), gain reduction was moderate (ipsilesional: 0.59 ± 0.16; contralesional: 0.63 ± 0.22) and symmetric (9% ± 8%) (figures 1C, 2C, and 3, A and B). In PICA/SCA strokes (n = 20), gains were reduced by approximately 25% maintaining symmetry (figures 1D, 2D, and 3, A and B).
Figure 1. HIT in PCS and VN.
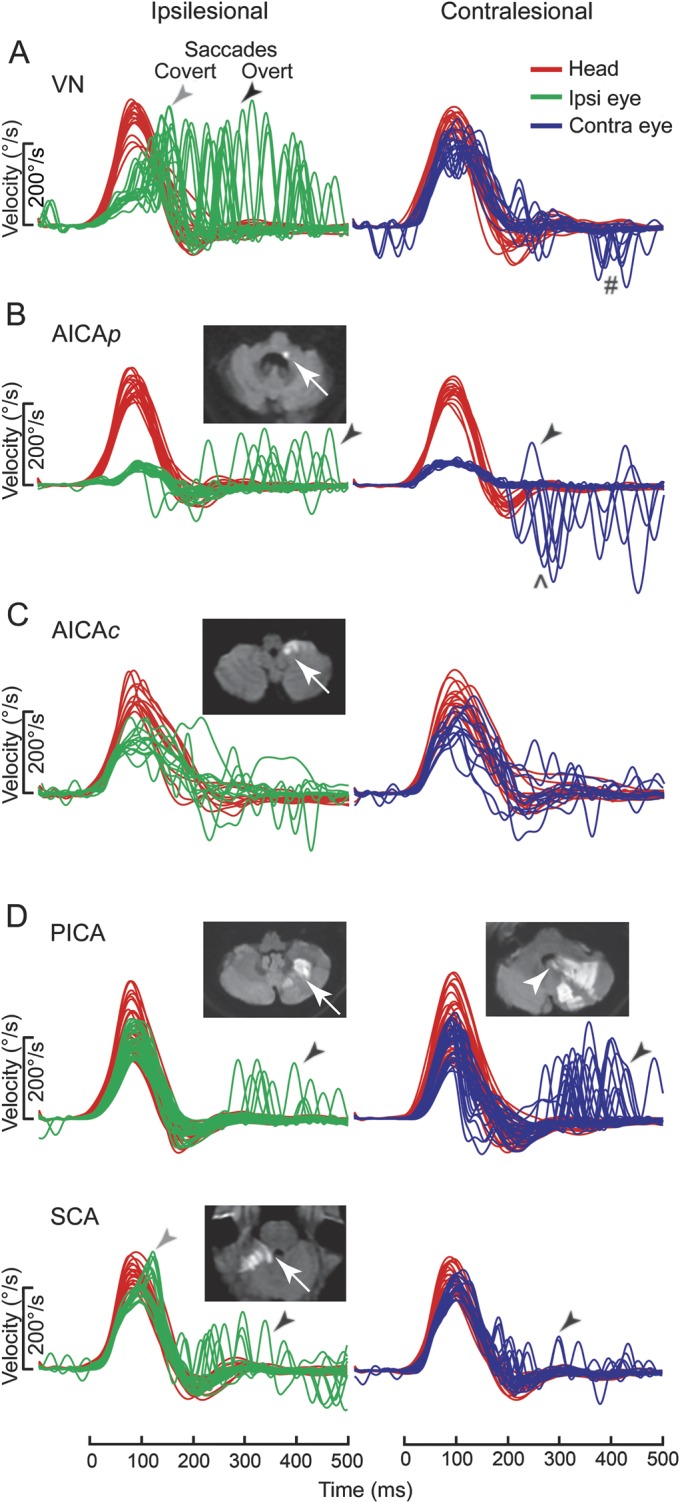
Typical examples of head impulse test (HIT) in pontine-cerebellar stroke (PCS) and vestibular neuritis (VN), shown as time series of inverted eye velocities to HIT trials. (A) Ipsilesional gain deficit (mean 0.16) in VN (table e-A1, VN 12) led to large overt (black arrow) saccades (first amplitude: 8.1°, mean) and covert (gray arrow) saccades (73% of trials). Contralesional gain was slightly reduced (0.72) with small overt saccades (1.8°). Saccades in the direction of contralesional impulses (#) represented the fast-phases of spontaneous nystagmus. (B) In anterior inferior cerebellar artery–peripheral (AICAp) stroke (AICAp 6) due to left vestibular nuclear infarction (white arrow), despite bilateral gain deficits (ipsilesional 0.11, contralesional 0.21) overt saccades were small (ipsilesional trials: 2.5°; contralesional trials: 2.9°) and occurred predominantly after ipsilesional trials. Anticompensatory saccades (^) were dominant after contralesional trials. (C) In anterior inferior cerebellar artery–central (AICAc) stroke (AICAc 4) due to isolated right floccular infarction, gains were asymmetrically reduced (ipsilesional: 0.55; contralesional: 0.75) with few small overt saccades. (D) Upper: In posterior inferior cerebellar artery (PICA) stroke (PICA 15) involving the left cerebellar hemisphere and nodulus (white arrowhead), gains were symmetric (ipsilesional: 0.85; contralesional: 0.82) with frequent overt saccades larger after contralesional (4.3°) than ipsilesional (2.8°) trials. Lower: In superior cerebellar artery (SCA) stroke (SCA 2) involving the superior vermis, gains were mildly reduced bilaterally (ipsilesional: 0.66; contralesional: 0.71) with small overt saccades (ipsilesional trials: 2.2°; contralesional trials: 1.2°).
Figure 2. Angular vestibulo-ocular reflex gains in pontine-cerebellar stroke and VN.
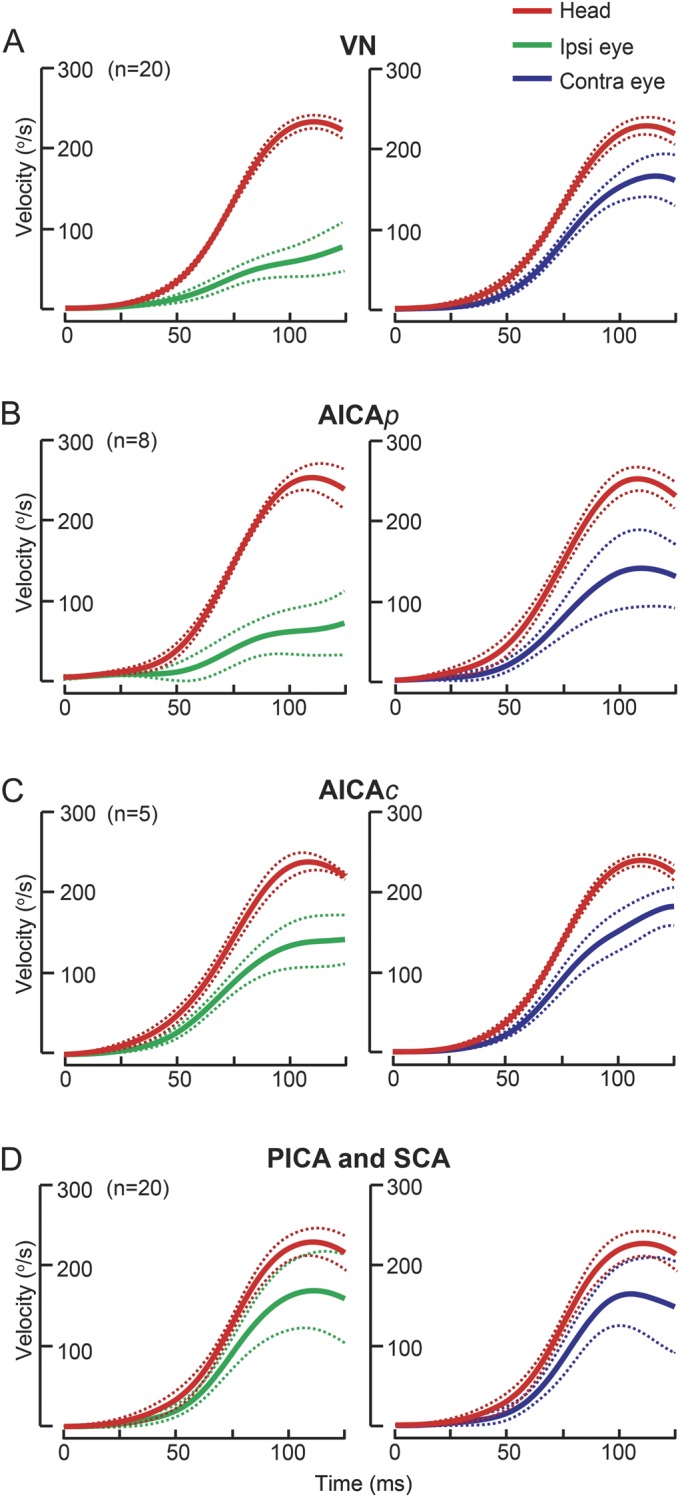
Group data (mean ± 95% confidence interval) displayed as time series of inverted eye and head velocities during the first 125 milliseconds of head impulses.16 (A) In vestibular neuritis (VN), ipsilesional gains were deficient while contralesional gains were reduced by approximately 20%. (B) In anterior inferior cerebellar artery–peripheral (AICAp) stroke, ipsilesional gains (0.25 ± 0.11, mean ± 95% confidence interval) were deficient like VN, but unexpectedly contralesional gains (0.52 ± 0.16) were also reduced. (C) In anterior inferior cerebellar artery–central (AICAc) stroke, compared with AICAp stroke, ipsilesional gains were less severely reduced (0.59 ± 0.16), while contralesional gains (0.63 ± 0.22) were similar. (D) In posterior inferior cerebellar artery/superior cerebellar artery (PICA/SCA) strokes, gains were mildly reduced bilaterally. Solid lines: means; dashed lines: 95% confidence intervals.
Figure 3. Gain and saccade properties in PCS and VN.
(A) Normal gains were just below unity and symmetric. Ipsilesional gains were lowest in vestibular neuritis (VN) and anterior inferior cerebellar–peripheral (AICAp) stroke, and ranged from 0.50 to 0.60 to unity in anterior inferior cerebellar artery–central (AICAc) and posterior inferior cerebellar/superior cerebellar artery (PICA/SCA) strokes. Gains in VN overlapped with pontine-cerebellar stroke (PCS). (B) Gain asymmetry was large with the ipsilesional gain lower in VN and, to a lesser extent, in AICAp stroke, but was relatively small in AICAc and PICA/SCA strokes. Some AICAc and PICA/SCA strokes had negative gain asymmetry. (C) Overt saccade amplitude asymmetry (As) compares ipsilesional and contralesional saccade amplitudes. Normal As was close to zero. In vestibular neuritis (VN), As was strongly positive indicating saccade dominance during ipsilesional head impulse. Asymmetry was less pronounced in AICAp stroke, and even less in AICAc stroke. In PICA/SCA strokes, As was comparable to AICA stroke. Negative As indicates more frequent or collectively larger saccades during contralesional trials. (D) Overt saccade cumulative amplitudes, the equivalent of clinical head impulse test, were very large ipsilesionally in VN compared with normal subjects. In PCS, amplitudes during ipsilesional trials were smaller than in VN, and comparable to normal subjects except in AICAp stroke. Contralesionally, amplitudes were larger than in VN and in normal subjects, particularly in PICA/SCA strokes.
Gains were different between the 2 sides in VN (p < 0.01; linear mixed model) and AICA stroke (p < 0.01), but not in PICA/SCA strokes (p = 0.60) or normal subjects (p = 0.96). Ipsilesional gains were lower in VN (p < 0.01) and AICA and PICA/SCA strokes (p < 0.01) compared with normal subjects, lower in VN than AICA stroke (p = 0.03), and lower in AICA stroke than PICA/SCA strokes (p < 0.01). Contralesional gains were lower in VN and AICA and PICA/SCA strokes (p < 0.01) compared with normal subjects, lower in VN than AICA stroke (p = 0.01) and in AICA than PICA/SCA strokes (p = 0.03), but not different between VN and PICA/SCA strokes (p = 1.00). There was a difference in gain asymmetry (Gs) (H3 = 47.31, p < 0.01; Kruskal–Wallis test) between VN and other groups (normal, PICA/SCA: p < 0.01; AICA: p = 0.02) and between AICA stroke and normal (p = 0.02), but not PCS and normal. Negative Gs, indicating contralesional gain bias, never occurred in VN, but was present in 20% of AICA and 50% of PICA/SCA strokes.
Overt and covert saccade characteristics.
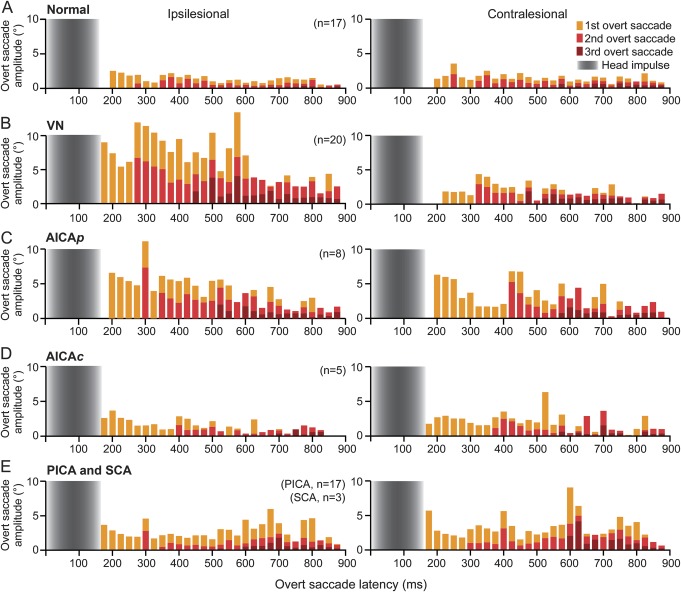
Table 1 summarizes the saccade characteristics across groups. In normal subjects, saccades were small and symmetric (figure 4A). In VN, mean overt saccade amplitude was about 4 times larger during ipsilesional than contralesional trials (figure 4B), but was similar between the 2 sides in PCS (figure 4, C–E). The first saccade contributed to two-thirds, the second saccade to one-third, while the third saccade occasionally to the total eye movements in all groups, except ipsilesionally in PICA/SCA strokes and in normal subjects (first 82%, second 15%, and third 3%). Saccade latency was similar between the 2 sides in all groups, with comparable intersaccadic interval. In VN of all saccades, spontaneous nystagmus fast phases comprised 2%, whereas 98% were compensatory saccades.
Figure 4. Compensatory overt saccade characteristics in pontine-cerebellar stroke and VN.
Bars represent stacked mean saccade amplitude in 25-millisecond bin. (A) Normal subjects generated small (1°–2°) symmetric saccades, not clinically detectable. (B) In vestibular neuritis (VN), large, dominant saccades after ipsilesional trials with latency >200 milliseconds can easily be detected, whereas after contralesional trials, saccades were small and undetectable. The first saccades (yellow bars) were largest, followed by smaller second (light red bars) and third (dark red bars) saccades. (C) In anterior inferior cerebellar artery–peripheral (AICAp) stroke, some saccades were sufficiently large bilaterally to be detected. Saccades after ipsilesional trials were smaller than in VN, but were larger after contralesional trials. (D) In anterior inferior cerebellar artery–central (AICAc) and (E) posterior inferior cerebellar artery/superior cerebellar artery (PICA/SCA) strokes, saccades were bilaterally larger than normal, smaller than in VN and in AICAp stroke after ipsilesional trials, but larger than in VN after contralesional trials. Gray gradients: mean head impulse duration (170–185 milliseconds).
There was a spectrum of saccade abnormalities in PCS. Overt saccade amplitude asymmetry (As) compares ipsilesional and contralesional saccade amplitudes (figure 3D). In normal subjects, asymmetry was negligible (7% ± 2%, mean ± 95% CI). There was a difference in As (F3,65 = 78.84, p < 0.01; analysis of variance) between normal and VN (p < 0.01) or PCS (p < 0.01) and VN and PCS (p < 0.01) but not between AICA and PICA/SCA strokes (p = 1.00). It strongly favored the ipsilesional side in VN and became less asymmetric in AICA (AICAp = 36% ± 18%, AICAc = 14% ± 6%) and PICA/SCA strokes. Negative As, indicating more frequent or collectively larger saccades after contralesional trials, never occurred in VN, but was present in 33% of AICA and 70% of PICA/SCA strokes. Cumulative amplitudes (figure 3C) that measure the compensatory saccades seen during clinical HIT were small in normal subjects (1.1° ± 0.3°). Amplitudes were different between the 2 sides in VN (p < 0.01; linear mixed model) and in AICA (p = 0.03) and PICA/SCA (p = 0.02) strokes, and but not in normal subjects (p = 0.80). For ipsilesional trials, amplitudes were larger in VN (video 1) and AICA stroke (video 2; AICAp = 5.7° ± 1.9°, AICAc = 3.1° ± 0.8°) than in normal subjects (p < 0.01), but not different between PICA/SCA strokes and normal subjects (p = 0.86). However, amplitudes were larger in VN than in PCS (p < 0.01), and in AICA than in PICA/SCA strokes (p = 0.01). Contralesionally, amplitudes were larger in AICA (AICAp = 3.4° ± 1.1°, AICAc = 3.0° ± 0.9°) and PICA/SCA strokes (video 3) than VN (p < 0.01) or normal (p < 0.01), but not different between VN and normal (p = 1.00) and between AICA and PICA/SCA strokes (p = 1.00). In normal subjects, large saccades (>5°) were absent, while small saccades (1°–2°) were sometimes present (figure e-2A). In PCS, small saccades were prevalent bilaterally. In addition, in AICAp stroke, saccades of all sizes were more frequent after ipsilesional than contralesional trials. In VN, large saccades were frequent and dominant after ipsilesional but not contralesional trials. Covert saccades (figure e-2B) were present in <20% of trials in normal subjects. They were most frequent during ipsilesional trials in VN and AICAp stroke, while occurring in about every second trial contralesionally in AICAc and PICA/SCA strokes.
Aggregate aVOR gain and saccade properties in PCS vs VN.
When gain or saccade symmetry was abnormal (figure e-3A, table 1), VN accounted for about 50%, while AICA (20%) and PICA/SCA (30%) strokes combined for the other half. When gain or saccade symmetry was within normal limits, VN was never present; PICA/SCA strokes (60%–80%) was more frequent than AICA stroke (20%–40%). When gains were abnormal, VN accounted for 41% (n = 20), with AICA (26%, n = 13) and PICA/SCA (33%, n = 16) strokes combined for the majority. When cumulative amplitude was abnormal, VN was responsible for 53% (n = 20), AICA stroke 26% (n = 10), and PICA/SCA strokes 21% (n = 8, 7 PICA); of these, positive clinical HIT was observed in all VN, 80% of AICA strokes, and 50% of PICA/SCA strokes.
Receiver operating characteristic curve analysis identified cut-points for gain and saccade properties that maximized aggregate differences between patients with PCS and VN in our series (figure e-3B). The most robust was saccade asymmetry (<61%; sensitivity of 97%, specificity 100%; area under the curve [AUC] = 0.99, 95% CI = 0.97–1.00), followed by gain asymmetry (<38%; sensitivity 94%, specificity 90%; AUC = 0.97, 95% CI = 0.93–1.00) and smaller ipsilesional cumulative amplitude (<4.3°; sensitivity 94%, specificity 88%; AUC = 0.96, 95% CI = 0.92–1.00). Higher ipsilesional gain (>0.30; sensitivity 84%, specificity of 85%; AUC = 0.89, 95% CI = 0.80–0.98) was not as sensitive or specific.
Cut-points of cumulative amplitude for detecting a positive clinical HIT were also determined likewise (figure e-3C). The sensitivity was 100% and specificity 88% at 3.1°, 90% and 90% at 3.4°, and 84% and 99% at 4.3° (AUC = 0.98, 95% CI = 0.96–1.00).
DISCUSSION
We systematically investigated aVOR gain and compensatory saccade characteristics in prospectively recruited patients with PCS and VN using the gold-standard measure, scleral search coils, to record the HIT. Our data provide quantitative insights into the clinical sign previously shown to discriminate between PCS and VN in AVS—the presence or absence of a compensatory refixation saccade after the HIT.5–8 In our contemporaneous cohort of PCS defined by vascular territories and VN, we characterized HIT gain and saccade abnormalities to provide a framework for anatomical-physiologic correlation. Here, we elucidated gain and saccade parameters with implication for clinical HIT interpretation, and identified saccade amplitude for observing a positive clinical HIT.
We found gain symmetry in PICA/SCA strokes in contrast to VN, consistent with a VOG study.11 However, we showed symmetric 25% aVOR gain reduction supporting modulation of the human high-acceleration aVOR by focal cerebellar lesions, in contrast to diffuse processes, which affect gain variably21 or alter the rotation axis.22 PICA supplies the dorsal vermis, nodulus, and uvula,23 and ischemia can produce isolated vertigo.3 Approximately half of SCA stroke patients present with vertigo, probably related to efferent projections from the anterior lobe to the nodulus and uvula.24
In AICA stroke, in addition to ipsilesional gain deficit, contralesional gain was unexpectedly reduced, which might be explained by floccular involvement25 (figure 1C; table e-A1, AICAp 2 and 4). AICA supplies the labyrinth,26 lateral pons (including root entry zone and vestibular nucleus),27 and flocculus28; occlusion results in a spectrum of audio-vestibular loss.29 Our patient with isolated flocculus stroke had more severe gain reduction ipsilesionally, contrary to a case report25; this may reflect variable inhibition by the floccular target neurons on the ipsilesional vestibular nucleus, or adaptation of the contralateral flocculus. We speculate that gain asymmetry may be dependent on the relative involvement of the inner ear, vestibular nucleus, and flocculus, supported by the different gain and saccade characteristics between AICAp and AICAc strokes.
Our gain and saccade analysis explains why clinical HIT is “falsely” positive in AICAp stroke and negative in AICAc/PICA/SCA strokes.6,7 Mild gain reduction with bilateral 2°–3° saccades, often larger contralesionally, was the hallmark of PICA/SCA strokes. Variable gain reduction with bilateral 3°–4° saccades, mostly larger ipsilesionally, characterized AICA stroke. In contrast, ipsilesional gain deficit accompanied by large (8°), unilaterally dominant saccades was typical of VN. We found the optimal clinical detection threshold to be approximately 3°–4°, larger than the previously reported 1°–2° for eye movements in general,30 probably because it is more difficult to discern smaller eye movements immediately after head rotation. Consequently, there was concordance in VN but discordance in AICAc/PCA/SCA strokes between frequency of those with abnormal cumulative amplitude and positive clinical HIT. A negative clinical HIT has high sensitivity and specificity,6–8 paradoxically because it misses small saccades, not because the implied aVOR is “normal” in PCS.6
The severity of gain reduction likely accounts for the difference in saccade amplitude between PCS and VN. However, in AICAp stroke, despite comparable ipsilesional gain deficit to VN, saccades were smaller. The flocculus might be implicated because it modulates saccades,31 although an experimental lesion causes postsaccadic drift and does not affect saccade velocity or accuracy.32 We found negative saccade asymmetry in 70% of PICA/SCA strokes implying that saccades toward the ipsilesional side were more frequent and/or collectively larger after contralesional trials. Because an experimental lesion of dorsal vermis causes ipsilesional hypometria,33,34 these saccades might represent refoveating eye movements in the presence of saccadic undershooting. Fast phases of direction-changing nystagmus were unlikely to explain saccade occurrence, because they were excluded from analysis and present in only 30% of PICA strokes and absent in SCA stroke.35
Defining potential cut-points for overt saccade amplitude asymmetry enabled accurate retrospective classification of PCS and VN in our series, while cut-points for gain asymmetry and ipsilesional cumulative amplitude were slightly less sensitive and specific. We found that in those with abnormal gain/saccade asymmetry or cumulative amplitude, VN and PCS accounted for about half each, suggesting that this approach of dichotomizing quantitative measures into normal or abnormal, based on comparison to normal, healthy controls, is unhelpful. Although the search coil technique has limited availability and is technically demanding in the hyperacute setting, it is the gold-standard method for gain and saccade analysis. Future studies could potentially utilize modern VOG, which is portable, rapidly deployable,36 and has sufficient spatial (0.1°) and temporal (>250 Hz) resolution. With VOG, it remains to be determined, however, whether saccade analysis is more or less reliable than gain measurement. VOG saccade analyses will likely be influenced by the effectiveness of algorithms that filter eye blinks and other pseudo-saccade artifacts.37 VOG gain measures depend on the computational algorithm used38 to factor for goggle slippage from inertia.39
Our study has several limitations. This is not a diagnostic accuracy study in unselected AVS, so the sensitivity, specificity, or predictive value of our findings in clinical practice is unknown. Nevertheless, we sought to generate hypotheses regarding quantitative parameters that might be used for such future studies. Our study included only those selected for neurology admission and imaging, so our results may not generalize to unselected AVS. We proposed criteria for differentiating compensatory overt saccades from fast phases of nystagmus, but this has not been formally validated; therefore, some misclassification might have occurred and affected our results. Our saccade analysis does not apply to lateral medullary stroke presenting with isolated vertigo, because these patients were excluded. Caution is required when correlating aVOR gain and saccade findings with a specific region of the cerebellum, nonvisualized lesions (e.g., labyrinthine infarction, adaptation in noninfarcted parts of cerebellum), and the relatively small number of patients with lesions in each particular functional region. Our results could have been influenced by the approximately 35% of patients who underwent recording between 4 and 7 days after vertigo, and ocular motor and vestibular findings might evolve over the first several days. It is unknown the extent to which our findings, measured by search coil technique, will generalize to the more clinically applicable VOG.
Our results have practical implications for clinical care and future research in patients with AVS. When performing the clinical HIT, clinicians should compare the left–right difference in size of compensatory saccades, and be cognizant that bilateral saccades suggest AICA stroke, small or no saccade PICA/SCA strokes, and unilaterally dominant large saccades VN.5,6 Future VOG-based studies should expressly seek to compare the diagnostic accuracy of various computerized algorithms (using saccade analysis, gain measures, or both) for quantitatively differentiating PCS from VN in unselected patients with AVS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Mr. Leigh A. McGarvie for assisting with patient testing.
GLOSSARY
- AICA
anterior inferior cerebellar artery
- AUC
area under the curve
- aVOR
angular vestibulo-ocular reflex
- AVS
acute vestibular syndrome
- CI
confidence interval
- DWI
diffusion-weighted imaging
- HIT
head impulse test
- PCS
pontine-cerebellar stroke
- PICA
posterior inferior cerebellar artery
- SCA
superior cerebellar artery
- VN
vestibular neuritis
- VOG
video-oculography
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. L. Chen designed the study, examined and tested the patients, analyzed their imaging and eye movement data, and prepared and interpreted all the research data and figures. He was principally in charge of drafting, revising for intellectual content, and submitting the manuscript to Neurology. Mr. M.J. Todd designed and implemented the hardware and software for data acquisition and analysis, and assisted in revising the manuscript for intellectual content. Prof. G.M. Halmagyi recruited the patients from Royal Prince Alfred Hospital, Australia, clinically examined the patients, and assisted in imaging analysis and in revising the manuscript for intellectual content. Dr. S.T. Aw designed the study, assisted in eye movement data analysis, interpreted all the research data and figures, and prepared and revised the manuscript for intellectual content.
STUDY FUNDING
This study was sponsored by the National Health and Medical Research Council (NHMRC) Australia, University of Sydney, Garnett Passe and Rodney Williams Memorial Foundation, and Royal Prince Alfred Hospital Neurology Trustees.
DISCLOSURE
L. Chen is funded by NHMRC grant 500200 and Garnett Passe and Rodney Williams Memorial Foundation. M. Todd reports no disclosures relevant to the manuscript. G. Halmagyi receives consultancy fees from GN Otometrics. S. Aw is funded by NHMRC grants 511900 and 500200 and receives support from University of Sydney and Royal Prince Alfred Hospital Neurology Trustees. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Baloh RW. Clinical practice: vestibular neuritis. N Engl J Med 2003;348:1027–1032. [DOI] [PubMed] [Google Scholar]
- 2.Huang CY, Yu YL. Small cerebellar strokes may mimic labyrinthine lesions. J Neurol Neurosurg Psychiatry 1985;48:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H, Sohn SI, Cho YW, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology 2006;67:1178–1183. [DOI] [PubMed] [Google Scholar]
- 4.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011;183:E571–E592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M. Bedside differentiation of vestibular neuritis from central “vestibular pseudoneuritis”. J Neurol Neurosurg Psychiatry 2008;79:458–460. [DOI] [PubMed] [Google Scholar]
- 6.Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology 2008;70:2378–2385. [DOI] [PubMed] [Google Scholar]
- 7.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009;40:3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med 2013;20:986–996. [DOI] [PubMed] [Google Scholar]
- 9.Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol 2000;21:1434–1440. [PMC free article] [PubMed] [Google Scholar]
- 10.Jorns-Haderli M, Straumann D, Palla A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatry 2007;78:1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman-Toker DE, Saber Tehrani AS, Mantokoudis G, et al. Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: toward an ECG for the eyes. Stroke 2013;44:1158–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol 1988;45:737–739. [DOI] [PubMed] [Google Scholar]
- 13.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain: brainstem and cerebellum. Neurology 1996;47:1125–1135. [DOI] [PubMed] [Google Scholar]
- 15.Aw ST, Haslwanter T, Halmagyi GM, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations: I: responses in normal subjects. J Neurophysiol 1996;76:4009–4020. [DOI] [PubMed] [Google Scholar]
- 16.Aw ST, Fetter M, Cremer PD, Karlberg M, Halmagyi GM. Individual semicircular canal function in superior and inferior vestibular neuritis. Neurology 2001;57:768–774. [DOI] [PubMed] [Google Scholar]
- 17.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology 2008;70:454–463. [DOI] [PubMed] [Google Scholar]
- 18.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology 2009;72:1417–1424. [DOI] [PubMed] [Google Scholar]
- 19.Weber KP, Aw ST, Todd MJ, et al. Inter-ocular differences of the horizontal vestibulo-ocular reflex during impulsive testing. Prog Brain Res 2008;171:195–198. [DOI] [PubMed] [Google Scholar]
- 20.Lehnen N, Aw ST, Todd MJ, Halmagyi GM. Head impulse test reveals residual semicircular canal function after vestibular neurectomy. Neurology 2004;62:2294–2296. [DOI] [PubMed] [Google Scholar]
- 21.Crane BT, Tian JR, Demer JL. Initial vestibulo-ocular reflex during transient angular and linear acceleration in human cerebellar dysfunction. Exp Brain Res 2000;130:486–496. [DOI] [PubMed] [Google Scholar]
- 22.Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol 2005;94:3417–3429. [DOI] [PubMed] [Google Scholar]
- 23.Amarenco P, Roullet E, Hommel M, Chaine P, Marteau R. Infarction in the territory of the medial branch of the posterior inferior cerebellar artery. J Neurol Neurosurg Psychiatry 1990;53:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Kim HA. Nystagmus in SCA territory cerebellar infarction: pattern and a possible mechanism. J Neurol Neurosurg Psychiatry 2013;84:446–451. [DOI] [PubMed] [Google Scholar]
- 25.Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol 2013;260:1576–1582. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Lopez I, DiPatre PL, Liu F, Ishiyama A, Baloh RW. Internal auditory artery infarction: clinicopathologic correlation. Neurology 1999;52:40–44. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Lee SH, Park JH, Choi JY, Kim JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol 2014;261:121–129. [DOI] [PubMed] [Google Scholar]
- 28.Amarenco P, Hauw JJ. Cerebellar infarction in the territory of the anterior and inferior cerebellar artery: a clinicopathological study of 20 cases. Brain 1990;113:139–155. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Kim JS, Chung EJ, et al. Infarction in the territory of anterior inferior cerebellar artery: spectrum of audiovestibular loss. Stroke 2009;40:3745–3751. [DOI] [PubMed] [Google Scholar]
- 30.Ludvigh E. Amount of eye movement objectively perceptible to the unaided eye. Am J Ophthalmol 1949;32:649. [DOI] [PubMed] [Google Scholar]
- 31.Noda H, Suzuki DA. The role of the flocculus of the monkey in saccadic eye movements. J Physiol 1979;294:317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zee DS, Yamazaki A, Butler PH, Gucer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol 1981;46:878–899. [DOI] [PubMed] [Google Scholar]
- 33.Sato H, Noda H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis [corrected]. Exp Brain Res 1992;88:455–458. [DOI] [PubMed] [Google Scholar]
- 34.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 1998;80:1911–1931. [DOI] [PubMed] [Google Scholar]
- 35.Huh YE, Kim JS. Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: imaging correlations. Brain 2011;134:3662–3671. [DOI] [PubMed] [Google Scholar]
- 36.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology 2009;73:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantokoudis G, Saber Tehrani AS, Kattah JC, et al. Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurootol (in press 2014). [DOI] [PubMed]
- 38.Macdougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS One 2013;8:e61488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal Y, Schubert MC, Migliaccio AA, et al. Evaluation of quantitative head impulse testing using search coils versus video-oculography in older individuals. Otol Neurotol 2014;35:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.