Background: Cell-extracellular matrix (ECM) adhesion is critical for control of intracellular signaling and cell behavior.
Results: Kindlin-2 interacts with Src upon cell-ECM adhesion; disrupting this interaction inhibits paxillin tyrosine phosphorylation, cell migration, and proliferation.
Conclusion: Kindlin-2·Src interaction mediates cell-ECM adhesion-induced signaling.
Significance: Our findings reveal a novel kindlin-2·Src·paxillin signaling axis and suggest new strategies for controlling cell migration and proliferation.
Keywords: Cell Adhesion, Extracellular Matrix, Integrin, Signaling, Src, Kindlin-2, Paxillin, Tyrosine Phosphorylation
Abstract
Integrin-mediated cell-extracellular matrix (ECM) adhesion is critical for control of intracellular signaling; however, the mechanisms underlying this “outside-in” signaling are incompletely understood. Here we show that depletion of kindlin-2 impairs integrin outside-in signaling. Kindlin-2 is tyrosine-phosphorylated upon cell-ECM adhesion. Furthermore, kindlin-2 binds Src in a cell-ECM adhesion-regulatable fashion. At the molecular level, the kindlin-2·Src interaction is mediated by the kindlin-2 F0 and the Src SH2 and SH3 domains. Src activation increases kindlin-2 tyrosine phosphorylation and the kindlin-2·Src interaction. Conversely, inhibition of Src reduces kindlin-2 tyrosine phosphorylation and diminishes the kindlin-2·Src interaction. Finally, disruption of the kindlin-2·Src interaction, unlike depletion of kindlin-2, impairs neither cell-ECM adhesion nor cell-ECM adhesion-induced focal adhesion kinase Tyr-397 phosphorylation. However, it markedly inhibits cell-ECM adhesion-induced paxillin tyrosine phosphorylation, cell migration, and proliferation. These results suggest that kindlin-2 tyrosine phosphorylation and interaction with Src serve as a regulatable switch downstream of focal adhesion kinase in the integrin outside-in signaling circuit, relaying signals from cell-ECM adhesion to paxillin that control cell migration and proliferation.
Introduction
Integrin-mediated cell-ECM2 adhesion is essential for development and tissue homeostasis and represents one of the fundamental mechanisms through which cells communicate with the microenvironment (1–3). Cell adhesion to ECM elicits a cascade of intracellular signaling events that control cell behavior, including cell shape change, migration, and proliferation, among which the most prominent are tyrosine phosphorylation of FAK and paxillin (1, 4–17). Indeed, the discoveries that FAK and paxillin are tyrosine-phosphorylated in response to cell-ECM adhesion have contributed to the now widely accepted concept that cell-ECM adhesion not only provides physical anchorage but also transmits chemical signals into cells (“outside-in” signaling) and thereby controls cell behavior. Determination of the molecular mechanisms underlying integrin outside-in signaling is therefore one of the central questions in current cell biology.
Extensive studies over the past quarter century have elucidated to a large extent the mechanism by which integrin-mediated cell-ECM adhesion induces tyrosine phosphorylation of FAK, in which the binding of activators such as integrin β cytoplasmic domains or phosphatidylinositol 4,5-bisphosphate to FAK releases autoinhibition, resulting in FAK activation and autophosphorylation at Tyr-397 (18–22). The mechanism by which paxillin is tyrosine-phosphorylated in response to cell-ECM adhesion, however, is complex and incompletely understood. Both FAK and Src play important roles in tyrosine phosphorylation of paxillin (14, 16, 23–25). Overexpression of FAK promotes whereas inhibition of FAK reduces tyrosine phosphorylation of paxillin (26–29). Because FAK can bind directly to paxillin, FAK is believed to promote tyrosine phosphorylation of paxillin via, at least in part, directly phosphorylating it. A large body of evidence suggests that tyrosine phosphorylation of paxillin involves not only FAK but also Src (16, 25, 30). Indeed, paxillin was originally identified as a tyrosine-phosphorylated protein in Src-transformed cells (31). Furthermore, cells lacking Csk, a negative regulator of Src, exhibited enhanced Src activity and increased tyrosine phosphorylation of paxillin (32). Additionally, cell-ECM adhesion induced tyrosine phosphorylation of paxillin was diminished in cells lacking Src (33). However, despite the strong genetic evidence for a critical role of Src in tyrosine phosphorylation of paxillin, molecular mechanisms regulating Src-induced tyrosine phosphorylation of paxillin in response to cell-ECM adhesion remain to be defined.
Kindlin-2 is an integrin-binding focal adhesion protein that is emerging as a key regulator of integrin signaling (34–40). Extensive studies have demonstrated that kindlin-2 is critical for integrin activation (i.e. “inside-out” signaling) (35–41). Given the prominent role of kindlin-2 in integrin activation, however, it is not straightforward to determine the role of kindlin-2 in integrin outside-in signaling because removal of kindlin-2 inhibits integrin-mediated cell-ECM adhesion and consequently can indirectly impair outside-in signaling. In this study, we have designed and performed a series of experiments to assess the role of kindlin-2 in outside-in signaling. We report here our findings.
EXPERIMENTAL PROCEDURES
Antibodies and Other Reagents
Mouse anti-kindlin-2 monoclonal antibody (mAb 3A3) was described (42). Antibodies recognizing phosphotyrosine (PY-100 and PY-1000), Src, and phospho-Src (Tyr-416) were from Cell Signaling. Monoclonal anti-paxillin and anti-ILK antibodies were from Transduction Laboratories. Rabbit antibodies against paxillin Tyr(P)-118 and paxillin Tyr(P)-31 were from BIOSOURCE International, Inc. Antibodies recognizing FAK and phospho-FAK (Tyr-397) were from Santa Cruz Biotechnology, Inc. Anti-FLAG antibody M5- and anti-FLAG antibody M2-conjugated agarose beads were purchased from Sigma-Aldrich. Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was from Novus Biologicals. Horseradish peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories. Cell culture media were from Mediatech/Cellgro (Herndon, VA).
Cell Culture and Treatment
Conditional immortalized human glomerular podocytes were propagated under permissive condition as we described (43). FAK+/+ and FAK−/− mouse embryonic fibroblasts were kindly provided by Dr. Jun-Lin Guan (University of Cincinnati College of Medicine) and cultured in Dulbecco modified Eagle's medium supplemented with 10% fetal bovine serum. SYF and SYF + c-Src mouse embryonic fibroblasts were purchased from ATCC and cultured in Dulbecco modified Eagle's medium supplemented with 10% fetal bovine serum. In some experiments, cells (as specified in each experiment) were treated with 10 μm PP2 for 1 h or 10 mm H2O2 in serum-free medium for 15 min prior to harvesting. Rat mesangial cells were cultured in RPMI 1640 medium containing 20% fetal bovine serum and 1× insulin-transferrin-selenium solution supplement. The cells were transfected with siRNAs or DNA constructs (as specified in each experiment) and cultured in RPMI 1640 medium for 2 days and then in RPMI 1640 medium supplemented with 20 ng/ml PDGF for 5 min. The cells were harvested and analyzed by Western blotting.
DNA Constructs, RNAi, and Transfection
cDNAs encoding wild type or mutant forms (as specified in each experiment) of kindlin-1, kindlin-2, or Src were generated by PCR and inserted into pFLAG-6c, pGEX-5x-1, or pMAL-c2 vectors. Sequences of the expression vectors containing kindlin-1, kindlin-2, or Src inserts were confirmed by DNA sequencing. siRNA that targets human kindlin-2 transcript (KD1) was described previously (42). siRNA that targets both human and rat kindlin-2 transcripts (KD2) (target sequence TCTTTAAGAGAGAAAGTTCTTCGGG) and siRNA that targets rat kindlin-2 transcript (KD3) (target sequence CCTGAGTTCGGCATCACACACTTCA) were obtained from Invitrogen. Cells were transfected with DNA expression vectors or siRNAs with Lipofectamine 2000 (Invitrogen) following the manufacturer's protocols. For re-expression of wild type or mutant forms of kindlin-2 in kindlin-2 siRNA transfectants, the cells were first transfected twice with a kindlin-2 siRNA. One day after the second siRNA transfection, the cells were then transfected with a DNA expression vector encoding FLAG-tagged wild type or mutant forms of kindlin-2. One day after the DNA transfection, the cells were analyzed as specified in each experiment. Knockdown or overexpression of proteins was confirmed by Western blotting with antibodies as specified in each experiment.
Cell Suspension and Adhesion
Human podocytes were transfected with DNA vectors or siRNAs as specified in each experiment. The cells were trypsinized, washed twice with serum-free medium, and rotated at 37 °C in serum-free conditions for 30 min. At the end of rotation, the cells were either harvested (suspension condition) or replated on fibronectin-coated dishes at 37 °C under a 5% CO2, 95% air atmosphere for various periods of time (adhesion condition). Cells cultured under the suspension and adhesion were harvested and analyzed by Western blotting and immunoprecipitation as specified in each experiment. The amounts of Src co-immunoprecipitated with kindlin-2 and that in the cell lysates were estimated by densitometry and the ImageJ program (National Institutes of Health). The percentages of Src co-immunoprecipitated with kindlin-2 were calculated as the amounts of Src co-immunoprecipitated with kindlin-2 divided by the amount of Src in the lysates.
Centrifugal Cell-ECM Adhesion Assay
A centrifugal cell-ECM adhesion assay was performed as we described previously (44). Briefly, cells were labeled with green fluorescent dye (Calcein-AM) for 30 min and seeded (2.5 × 104 cells/well) in triplicate in fibronectin (10 μg/ml)-coated 96-well plates (Greiner Bio-One) on ice. The plates were tightly sealed with sealing films (USA Scientific, Inc.) and centrifuged at 600 rpm for 10 min at 4 °C to facilitate cell settlement. The fluorescent signals from the total seeded cells were measured using a GENios Pro fluorescence microplate reader (Tecan) (at excitation wavelength = 485 nm and emission wavelength = 535 nm). The plates were then centrifuged upside down at 600 rpm for 1 min. After removing detached cells, the fluorescent signals from attached cells were measured again using the GENios Pro fluorescence microplate reader (Tecan). Cell adhesion was calculated as the fluorescence reading of attached cells divided by the fluorescence reading of the total seeded cells. The adhesion of kindlin-2 knockdown or FLAG-F0 (FLAG-wild-type or mutant forms of kindlin-2)-overexpressing cells was compared with that of control cells (normalized to 100).
Cell Migration Assay
The cell migration assay was based on a method described previously (45). Briefly, rat mesangial cells were transfected with siRNAs or DNA constructs (as specified in each experiment), suspended at a density of 2 × 105 cells/ml in RPMI 1640 supplemented with 0.4% bovine serum albumin (BSA), and seeded in 100 μl per chamber in collagen I-coated Transwell motility chambers (BD FlaconTM cell culture inserts with 8-μm pore size). To the lower chamber were added 850 μl serum-free RPMI 1640 with 20 ng/ml PDGF. The cells were incubated in a 37 °C incubator under a 5% CO2, 95% air atmosphere for 5 h. At the end of incubation, the cells remaining on the upside of the membrane were removed. Cells that migrated through the membrane were fixed, stained with hematoxylin, and photographed under an Olympus IX70 microscope equipped with a DVC-1310C Magnafire digital camera (Optronics). Cell migration was quantified by counting cells from five microscopic fields (means ± S.D.). The migration of kindlin-2 knockdown or overexpressing cells was compared with that of control cells (normalized to 100).
Cell Proliferation
Cell proliferation was analyzed using the CellTiter 96 AQueous One Solution cell proliferation assay (Promega) following the manufacturer's protocol. Briefly, rat mesangial cells were transfected with siRNAs or DNA constructs as specified in each experiment, suspended in serum-free RPMI 1640 medium, and seeded (5000 cells/well) in triplicates in 96-well plates (Greiner Bio-One) for 48 h. The cells were cultured in RPMI 1640 medium supplemented with 20 ng/ml PDGF for 22 h and then mixed with CellTiter One solution reagent. The absorbance (wavelength = 490 nm) signals from each well were measured using a GENios microplate reader (Tecan). The proliferation of kindlin-2 knockdown or overexpressing cells was compared with that of the control cells (normalized to 100).
Generation of Recombinant Proteins and GST Fusion Protein Pull-down Assays
Escherichia coli (BL21) cells were transformed with pGEX-5x-1 or pMAL-c2 vectors encoding wild type or mutant forms of kindlin-2, kindlin-1, or Src. The expression of recombinant GST or MBP fusion protein was induced with isopropyl 1-thio-β-d-galactopyranoside. Recombinant GST or MBP fusion proteins were purified with glutathione-Sepharose 4B and amylose-agarose, respectively, as we described previously (42). For GST fusion protein pull-down of mammalian cellular proteins, mammalian cells (as specified in each experiment) were lysed with 1% Triton X-100 in PBS (140 mm NaCl, 2 mm KH2PO4, 19 mm Na2HPO4, and 2.7 mm KCl) and protease inhibitors. The cell lysates were incubated with glutathione-Sepharose 4B beads that were precoated with GST fusion proteins or GST at 4 °C overnight. The precipitates were analyzed by Coomassie Blue staining or Western blotting with antibodies as specified in each experiment. For GST fusion protein pull-down of MBP-SrcY527F protein, purified MBP-SrcY527F protein was incubated with glutathione-Sepharose 4B beads that were precoated with GST fusion proteins or GST at room temperature for 30 min. MBP-SrcY527F that was pulled down by GST-tagged full-length or F0 domain of kindlin-2 was detected by Western blotting with an anti-Src antibody. GST and GST fusion proteins were detected by Coomassie Blue staining. For MBP-Src fusion protein pull-down of GST-kindlin-2 F0 fusion protein, purified GST-kindlin-2 F0 (residues 1–99) were incubated with amylose-agarose beads that were precoated with purified MBP-tagged Src SH3 (residues 1–149), SH2 (residues 150–248), or kinase domain (residues 249–536). GST-kindlin-2 F0 that was pulled down by MBP fusion proteins containing the Src SH2 or SH3 domain was detected by Western blotting with an anti-GST antibody.
Immunoprecipitation
Cells were lysed with lysis buffer (1% Triton X-100 in 50 mm Hepes, pH 7.1, containing 150 mm NaCl, 10 mm Na4P2O7, 1 mm Na3VO4, 100 mm NaF, 0.1 mm PMSF, 1 μm pepstatin, 5 μg/ml aprotinin, and 1 μg/ml leupeptin) on ice. To immunoprecipitate endogenous kindlin-2, the cell lysates were mixed with monoclonal anti-kindlin-2 antibody and incubated at 4 °C overnight. The samples were then incubated with UnitraLink immobilized Protein A/G PLUS-agarose (Santa Cruz Biotechnology) for 2 h. In some experiments (as specified), the cell lysates were mixed with agarose beads conjugated with anti-FLAG antibody M2 at 4 °C overnight. The beads were washed five times with lysis buffer, and the immunoprecipitates were analyzed by Western blotting with antibodies as specified.
Statistical Analysis
Student's t test was used for statistical analyses of the results. p values of <0.05 were considered statistically significant.
RESULTS
Kindlin-2 Is Critically Involved in Cell-ECM Adhesion-induced Tyrosine Phosphorylation of FAK and Paxillin
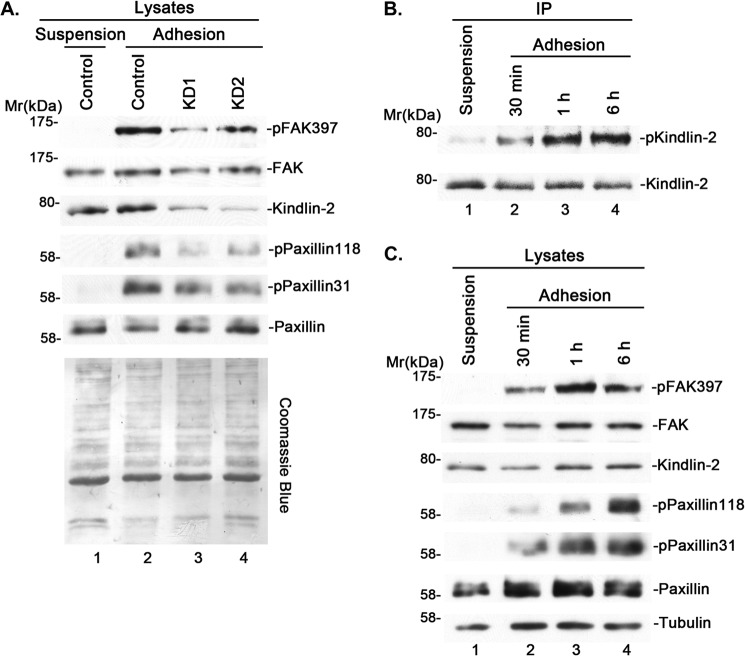
To begin to investigate the role of kindlin-2 in integrin outside-in signaling, we suppressed kindlin-2 expression in cells by RNAi (Fig. 1A, lanes 3 and 4) and tested the effect on cell-ECM adhesion-induced FAK and paxillin phosphorylation, two prominent events of integrin outside-in signaling. The results showed that depletion of kindlin-2 significantly inhibited cell-ECM adhesion-induced FAK Tyr-397 phosphorylation and paxillin Tyr-118 and -31 phosphorylation (Fig. 1A, compare lanes 3 and 4 with lane 2), suggesting that kindlin-2 is critically involved in these processes. These results, however, do not distinguish whether kindlin-2 is directly or indirectly involved in integrin outside-in signaling.
FIGURE 1.
Kindlin-2 is tyrosine-phosphorylated upon cell-ECM adhesion and is critical for cell-ECM adhesion-induced tyrosine phosphorylation of FAK and paxillin. A, human podocytes were transfected with kindlin-2 siRNA KD1 (lane 3), KD2 (lane 4), or a control RNA (lanes 1 and 2). Two days after siRNA transfection, the cells were starved and kept in suspension for 30 min (lane 1) or replated on fibronectin-coated dishes for 1 h (lanes 2–4). Cells were lysed and analyzed by Western blotting with antibodies as indicated in the figure or by Coomassie Blue staining. B and C, human podocytes were kept in suspension or replated on fibronectin-coated dishes for different periods of time, as indicated. Kindlin-2 was immunoprecipitated, and the samples were analyzed by Western blotting with antibodies recognizing phosphotyrosine or kindlin-2 (B). The cell lysates were analyzed by Western blotting with antibodies recognizing phospho-FAK (Tyr-397), FAK, kindlin-2, phospho-paxillin (Tyr-118), phospho-paxillin (Tyr-31), paxillin, or tubulin (C).
Kindlin-2 Is Tyrosine-phosphorylated in Response to Cell-ECM Adhesion
We reasoned that if kindlin-2 is directly involved in integrin outside-in signaling, it should sense cell-ECM adhesion (i.e. its structure or activity should be altered in response to cell-ECM adhesion). Because increased tyrosine phosphorylation at cell-ECM adhesions is central to integrin outside-in signaling, we tested whether kindlin-2 is tyrosine-phosphorylated in response to cell-ECM adhesion. To do this, we plated cells on fibronectin for different periods of time, immunoprecipitated kindlin-2, and analyzed the levels of tyrosine phosphorylation. The result showed that tyrosine phosphorylation of kindlin-2 is significantly increased in response to cell-ECM adhesion (Fig. 1B, compare lanes 2–4 with lane 1). In control experiments, we analyzed FAK and paxillin phosphorylation in the same cells. Consistent with previous studies, paxillin Tyr-118 and -31 phosphorylation and FAK Tyr-397 phosphorylation were also increased in response to cell-ECM adhesion (Fig. 1C, compare lanes 2–4 with lane 1). Thus, kindlin-2 can sense and respond (i.e. become tyrosine-phosphorylated) to cell-ECM adhesion.
Kindlin-2 Forms a Complex with Src
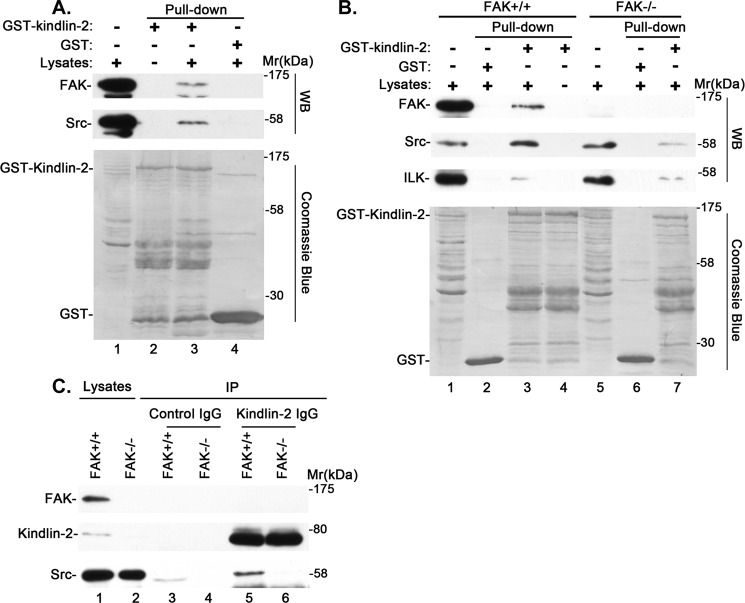
The finding that kindlin-2 is tyrosine-phosphorylated in response to cell-ECM adhesion prompted us to test whether kindlin-2 can form a complex with Src or FAK, tyrosine kinases that are known to localize at cell-ECM adhesions and are critical for integrin outside-in signaling. To do this, we generated GST-tagged kindlin-2 and performed pull-down experiments. The results showed that GST-kindlin-2 (Fig. 2A, lane 3), but not GST (Fig. 2A, lane 4), readily pulled down Src and FAK. Consistent with previous studies, GST-kindlin-2 (Fig. 2B, lane 3), but not GST (Fig. 2B, lane 2), pulled down ILK, which is known to interact with kindlin-2 (42, 46–48). To further analyze this, we performed GST-kindlin-2 pull-down experiments using FAK knock-out cells. The results showed that in the absence of FAK, GST-kindlin-2 pulled down Src, but the amount was much smaller than that in the presence of FAK (Fig. 2B, compare lane 7 with lane 3). By contrast, similar amounts of ILK were pulled down by GST-kindlin-2 irrespective of the presence or absence of FAK (Fig. 2B, compare lane 7 with lane 3).
FIGURE 2.
Kindlin-2 interacts with Src in a FAK-regulatable fashion. A and B, GST pull-down experiments were performed using lysates from human podocytes (A) or from FAK-positive (FAK+/+) or knock-out (FAK−/−) mouse embryonic fibroblasts (B) as described under “Experimental Procedures.” The cell lysates (A, lane 1; B, lanes 1 and 5), GST pull-downs (A, lane 4; B, lanes 2 and 6), and GST-kindlin-2 pull-downs (A, lane 3; B, lanes 3 and 7) were analyzed by Western blotting with antibodies recognizing FAK, Src, or ILK or Coomassie Blue staining. The samples in lane 2 of A and lane 4 of B were prepared as those in lane 3 of A and lane 3 of B except that cell lysates were omitted. C, cell lysates (lanes 1 and 2), anti-kindlin-2 immunoprecipitates (lanes 5 and 6), or control IgG immunoprecipitates (lanes 3 and 4) derived from FAK knock-out cells (lanes 2, 4, and 6) or FAK-positive cells (lanes 1, 3, and 5) were analyzed by Western blotting with antibodies recognizing FAK, kindlin-2, or Src.
To test whether endogenous kindlin-2 forms a complex with Src or FAK, we immunoprecipitated kindlin-2 from mouse embryonic fibroblasts and probed the kindlin-2 immunoprecipitates with Src and FAK antibodies, respectively. The results showed that Src was co-immunoprecipitated with kindlin-2 (Fig. 2C, lane 5), confirming that endogenous kindlin-2 forms a complex with Src. However, FAK was not detected in the kindlin-2 immunoprecipitates under the same experimental conditions (Fig. 2C, lane 5). Consistent with the results of the GST-kindlin-2 pull-down experiments (Fig. 2B), loss of FAK diminished the complex formation between endogenous kindlin-2 and Src (Fig. 2C, compare lane 6 with lane 5). Collectively, these results suggest that 1) kindlin-2 forms a complex with Src, and 2) FAK promotes the complex formation between kindlin-2 and Src.
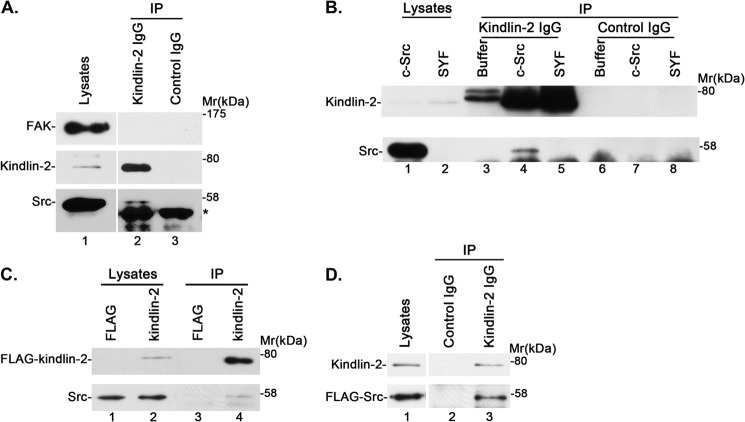
To confirm that kindlin-2 forms a complex with Src in other cell types, we immunoprecipitated kindlin-2 from human podocytes. Consistent with the results obtained from mouse fibroblasts (Fig. 2), Src was co-immunoprecipitated with kindlin-2 in these cells (Fig. 3A, lane 2). Again, FAK was not detected in the kindlin-2 immunoprecipitates (Fig. 3A, lane 2). We have also immunoprecipitated kindlin-2 from mouse Src-, Yes-, and Fyn-deficient SYF cells and SYF-c-Src cells, which were generated by re-expressing c-Src in SYF cells. As expected, Src was detected in kindlin-2 immunoprecipitates derived from SYF-c-Src (Fig. 3B, lane 4) but not SYF cells (Fig. 3B, lane 5). Additionally, we transfected human podocytes with FLAG-kindlin-2 expression vector or FLAG vector as a control and immunoprecipitated FLAG-kindlin-2 with an anti-FLAG antibody. Src was detected in FLAG-kindlin-2 immunoprecipitates (Fig. 3C, lane 4) but not the control FLAG immunoprecipitates (Fig. 3C, lane 3). Similarly, we expressed FLAG-Src in human podocytes and found that it was present in anti-kindlin-2 (Fig. 3D, lane 3) but not control (Fig. 3D, lane 2) immunoprecipitates. These results confirm that kindlin-2 forms a complex with Src in multiple types of cells.
FIGURE 3.
Kindlin-2 forms a complex with Src in multiple cell types. A, anti-kindlin-2 (lane 2) or control IgG (lane 3) immunoprecipitates derived from human podocytes were analyzed by Western blotting with antibodies recognizing FAK, kindlin-2, or Src. *, position of the immunoglobulin heavy chains derived from the antibodies used in the immunoprecipitation experiments. B, cell lysates (lanes 1 and 2), anti-kindlin-2 immunoprecipitates (lanes 3–5), or control IgG immunoprecipitates (lanes 6–8) derived from Src-deficient SYF cells (lanes 2, 5, and 8) or c-Src-expressing SYF + c-Src cells (lanes 1, 4, and 7) were analyzed by Western blotting with antibodies recognizing kindlin-2 and Src. The samples in lanes 3 and 6 were prepared as those in lanes 4 and 7 except that cell lysates were omitted. C, lysates of human podocytes transfected with FLAG-kindlin-2 or FLAG control vector were mixed with anti-FLAG antibody M2-conjugated agarose beads. The immunoprecipitates were analyzed by Western blotting with anti-FLAG or anti-Src antibodies. D, lysates of human podocytes transfected with FLAG-Src vector were mixed with mouse monoclonal anti-kindlin-2 antibody (lane 3) or irrelevant mouse IgG as a control (lane 2). The lysates (lane 1) and immunoprecipitates (lanes 2 and 3) were analyzed by Western blotting with anti-FLAG or anti-kindlin-2 antibodies.
Cell-ECM Adhesion Enhances the Complex Formation between Kindlin-2 and Src
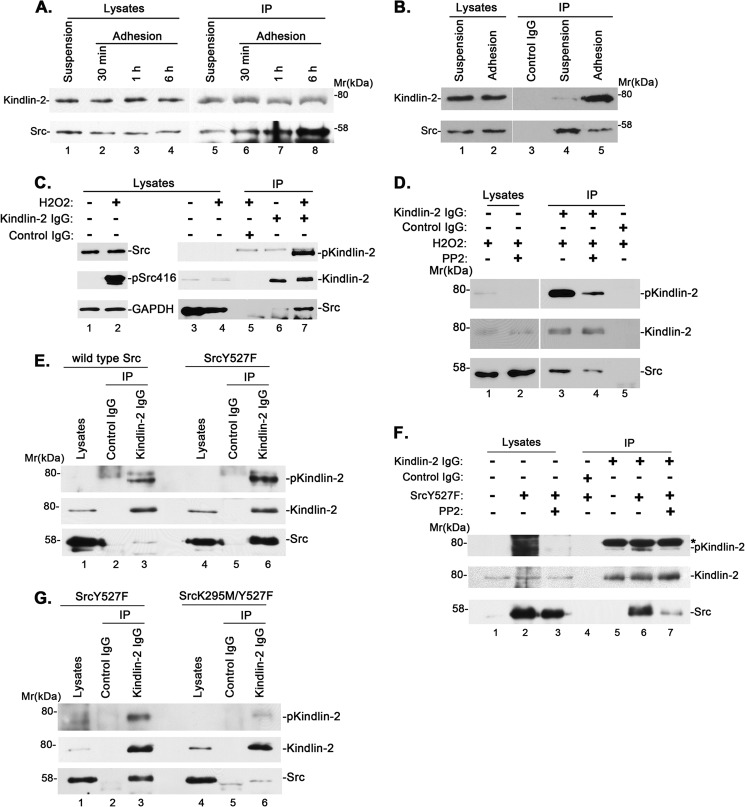
We next sought to test whether the complex formation between kindlin-2 and Src is influenced by cell-ECM adhesion. To do this, we plated cells on fibronectin for different periods of time and then immunoprecipitated kindlin-2. Probing the samples with an anti-kindlin-2 antibody showed that similar amounts of kindlin-2 were immunoprecipitated (Fig. 4A, lanes 5–8). In cells that were kept in suspension (i.e. in the absence of cell-ECM adhesion), a relatively small amount of Src (∼0.05% of Src in the cell lysates) was co-immunoprecipitated with kindlin-2 (Fig. 4A, lane 5). The amount of Src that was co-immunoprecipitated with kindlin-2 was significantly increased in response to cell-ECM adhesion (Fig. 4A, compare lanes 6–8 with lane 5), which correlates with the increases of kindlin-2 tyrosine phosphorylation (Fig. 1B). Specifically, the amount of Src associated with kindlin-2 increased by ∼4-fold (to ∼0.2% of Src in the cell lysates) within 30 min of cell-ECM adhesion (Fig. 4A, lane 6) and by ∼6- and 10-fold (to ∼0.3 and 0.5% of Src in the cell lysates) after 1 and 6 h of cell-ECM adhesion (Fig. 4A, lanes 7 and 8). To confirm this, we immunoprecipitated Src from the cells with an anti-Src antibody. Consistent with the results of the anti-kindlin-2 immunoprecipitation experiments, a small amount of kindlin-2 was co-immunoprecipitated with Src in cells that were kept in suspension, and the amount of kindlin-2 co-immunoprecipitated with Src increased significantly in cells that were adhered to fibronectin (Fig. 4B, lanes 4 and 5).
FIGURE 4.
Cell-ECM adhesion and Src activation regulate kindlin-2 tyrosine phosphorylation and kindlin-2·Src complex formation. A and B, cell-ECM adhesion regulates kindlin-2·Src complex formation. Human podocytes were kept in suspension or replated on fibronectin-coated dishes for different periods of time. Kindlin-2 (A) or Src (B) was immunoprecipitated with anti-kindlin-2 or anti-Src antibodies and analyzed by Western blotting with antibodies recognizing kindlin-2 or Src. C, human podocytes were treated with or without H2O2 for 15 min, and the cell lysates (lanes 1 and 2) were analyzed by Western blotting with antibodies recognizing Src, phospho-Src (Tyr-416), or GAPDH. Kindlin-2 tyrosine phosphorylation and complex formation with Src were analyzed by co-immunoprecipitation (lanes 5–7). The cell lysates (lanes 3 and 4), anti-kindlin-2 immunoprecipitates (lanes 6 and 7), or control IgG immunoprecipitates (lane 5) were analyzed by Western blotting with antibodies recognizing phosphotyrosine, kindlin-2, or Src as indicated. D, human podocytes were pretreated with (lanes 2 and 4) or without (lanes 1, 3, and 5) PP2 and then treated with H2O2 for 15 min. The cell lysates (lanes 1 and 2), anti-kindlin-2 immunoprecipitates (lanes 3 and 4), or control IgG immunoprecipitates (lane 5) were analyzed by Western blotting with antibodies recognizing phosphotyrosine, kindlin-2, or Src. E, the lysates (lanes 1 and 4), anti-kindlin-2 immunoprecipitates (lanes 3 and 6), or control IgG immunoprecipitates (lanes 2 and 5) derived from human podocytes expressing wild type Src (lanes 1–3) or SrcY527F (lanes 4–6) were analyzed by Western blotting with antibodies recognizing phosphotyrosine, kindlin-2, or Src. F, human podocytes were transfected with vectors encoding SrcY527F or control vectors and treated with or without PP2, as indicated. The cell lysates (lanes 1–3), anti-kindlin-2 immunoprecipitates (lanes 5–7), or control IgG immunoprecipitates (lane 4) were analyzed by Western blotting with antibodies recognizing phosphotyrosine (P-Tyr-100), kindlin-2, and Src. *, position of a nonspecific band derived from the anti-kindlin-2 antibody recognized by P-Tyr-100. G, the lysates (lanes 1 and 4), anti-kindlin-2 immunoprecipitates (lanes 3 and 6), or control IgG immunoprecipitates (lanes 2 and 5) derived from human podocytes expressing SrcY527F (lanes 1–3) or SrcK295M/Y527F (lanes 4–6) were analyzed by Western blotting with antibodies recognizing phosphotyrosine, kindlin-2, or Src.
Src Activity Is Critical for Control of Kindlin-2 Tyrosine Phosphorylation and Kindlin-2·Src Complex Formation
The close correlation between the complex formation of kindlin-2 with Src and kindlin-2 tyrosine phosphorylation, together with the fact that active Src is highly concentrated at cell-ECM adhesions, suggests that Src may function in tyrosine phosphorylation of kindlin-2. To test this, we sought to activate Src experimentally and determine whether this promotes tyrosine phosphorylation of kindlin-2. It has been shown that reactive oxygen species such as H2O2 can activate Src (49). Thus, we treated cells with H2O2 and found that it indeed, as expected, increased activating phosphorylation of Src (Fig. 4C, lane 2). Importantly, concomitantly, it increased tyrosine phosphorylation of kindlin-2 and promoted the formation of the kindlin-2·Src complex (Fig. 4C, compare lane 7 with lane 6). The extents of the increases of kindlin-2 tyrosine phosphorylation and complex formation with Src were substantially reduced when the cells were treated with Src inhibitor PP2 (Fig. 4D, compare lane 4 with lane 3). These results provide initial evidence suggesting that Src activation promotes kindlin-2 tyrosine phosphorylation and the formation of the kindlin-2·Src complex.
To further test this, we overexpressed a constitutively active form of Src (SrcY527F) and wild type Src as a control in cells and immunoprecipitated kindlin-2. Analyses of the kindlin-2 immunoprecipitates showed that the level of kindlin-2 tyrosine phosphorylation was higher in cells overexpressing the constitutively active form of Src compared with those overexpressing wild type Src (Fig. 4E, compare lane 6 with lane 3). Furthermore, the amount of Src that was co-immunoprecipitated with kindlin-2 was increased in cells overexpressing the constitutively active form of Src compared with those overexpressing wild type Src (Fig. 4E, compare lane 6 with lane 3). Again, treatment with Src inhibitor PP2 markedly inhibited kindlin-2 tyrosine phosphorylation and the formation of the kindlin-2·Src complex promoted by the overexpression of SrcY527F (Fig. 4F, lanes 5–7).
As a third approach to test the role of Src activity in regulation of kindlin-2 tyrosine phosphorylation and the formation of the kindlin-2·Src complex, we introduced a point mutation (K295M) that inactivates Src kinase activity (50) into SrcY527F. We overexpressed the inactive form of Src (SrcK295M/Y527F) and the constitutively active form of Src (SrcY527F) as a control in cells and analyzed the levels of kindlin-2 tyrosine phosphorylation and the kindlin-2·Src complex as described above. The results showed that inactivating Src kinase activity significantly reduced kindlin-2 tyrosine phosphorylation and diminished the formation of the kindlin-2·Src complex (Fig. 4G, compare lane 6 with lane 3). Collectively, these results provide strong evidence for a critical role of Src activity in control of kindlin-2 tyrosine phosphorylation and the formation of the kindlin-2·Src complex.
Kindlin-2 F0 and Src SH2 and SH3 Domains Are Critical for the Formation of the Kindlin-2·Src Complex
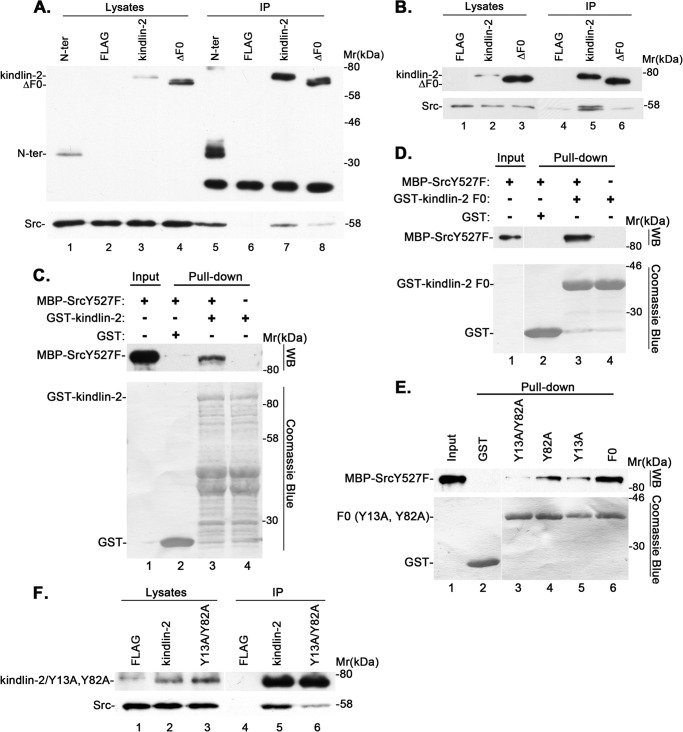
We next sought to identify the kindlin-2 domain that is critical for the formation of the kindlin-2·Src complex. To do this, we expressed FLAG-tagged wild type kindlin-2, an N-terminal fragment (N-ter) encompassing the F0 and part of the F1 domain (residues 1–256), or ΔF0 fragment, in which the F0 domain (residues 1–93) was deleted, in podocytes. We activated Src by treating the cells with H2O2 and analyzed the complex formation of Src with FLAG-tagged wild type or mutant forms of kindlin-2 by co-immunoprecipitation. The results showed that FLAG-tagged N-ter, like FLAG-kindlin-2, readily co-immunoprecipitated Src (Fig. 5A, lanes 5 and 7). By contrast, the amount of Src co-immunoprecipitated with FLAG-ΔF0 was markedly reduced compared with that co-immunoprecipitated with wild type kindlin-2 or the N-ter (Fig. 5A, compare lane 8 with lanes 5 and 7). These results suggest that the F0 domain of kindlin-2 is critically involved in the formation of the kindlin-2·Src complex. To confirm this, we expressed FLAG-ΔF0 and FLAG-kindlin-2, respectively, in podocytes and analyzed the complex formation with Src by co-immunoprecipitation. Again, the deletion of F0 significantly reduced the amount of Src co-immunoprecipitated with kindlin-2 (Fig. 5B, compare lanes 5 and 6), confirming that the kindlin-2 F0 domain is critical for the complex formation between kindlin-2 and Src. To further study this, we generated recombinant GST fusion proteins containing the full-length kindlin-2 or the kindlin-2 F0 domain alone and tested whether they could interact with purified MBP-tagged recombinant SrcY527F protein. The results showed that both GST-kindlin-2 (Fig. 5C, lane 3) and GST-F0 (Fig. 5D, lane 3), but not GST (Fig. 5, C and D, lane 2), interacted with MBP-SrcY527F. These results suggest that 1) kindlin-2 can directly interact with Src, and 2) the kindlin-2 F0 fragment is sufficient for mediating the interaction with Src. The kindlin-2 F0 domain contains two tyrosine residues (Tyr-13 and Tyr-82). Because kindlin-2 interaction with Src is mediated by the F0 domain and this interaction is increased upon kindlin-2 tyrosine phosphorylation, we tested whether the tyrosine residues located within kindlin-2 F0 are critical for Src binding. To do this, we generated GST-F0 fusion proteins in which Tyr-13 or Tyr-82 or both were substituted with Ala and tested their Src binding activities as described above. The results showed that substitution of either Tyr-13 or Tyr-82 with Ala partially reduced the interaction with Src (Fig. 5E, compare lanes 4 and 5 with lane 6). Substitution of both Tyr-13 and Tyr-82 with Ala further reduced, albeit it did not completely eliminate, the interaction with Src (Fig. 5E, lane 3). To confirm this, we expressed FLAG-tagged wild type or a mutant form of kindlin-2 in which Tyr-13 and Tyr-82 were substituted with Ala (Y13A/Y82A) in cells and immunoprecipitated FLAG-kindlin-2 and FLAG-Y13A/Y82A, respectively, with an anti-FLAG antibody. Western blotting analyses of the immunoprecipitates showed that Y13A/Y82A mutations markedly reduced, albeit they did not eliminate, the ability of kindlin-2 to associate with Src (Fig. 5F, compare lane 6 with lane 5), confirming an important role of Tyr-13 and Tyr-82 in the complex formation between kindlin-2 and Src.
FIGURE 5.
Kindlin-2 F0 domain mediates Src interaction. A and B, human podocytes were transfected with expression vectors encoding FLAG-tagged wild type or mutant forms of kindlin-2 or FLAG vector lacking kindlin-2 sequence, as indicated in the figure. The cells were treated with (A) or without (B) H2O2 for 15 min. FLAG-tagged wild type or mutant forms of kindlin-2 were immunoprecipitated with anti-FLAG antibody M2-conjugated agarose beads. The cell lysates and anti-FLAG immunoprecipitates were analyzed by Western blotting with anti-FLAG or anti-Src antibodies, as indicated in the figure. C–E, purified MBP-SrcY527F was incubated with GST (as a control), GST-tagged full-length kindlin-2 (C), kindlin-2 F0 fragment (D), or kindlin-2 F0 fragment bearing Y13A and/or Y82A mutations (E), as indicated in the figure. MBP-SrcY527F that was pulled down by GST-kindlin-2 (C, lane 3), GST-F0 (D, lane 3; E, lane 6), GST-Y13A (E, lane 5), GST-Y82A (E, lane 4), or GST-Y13A/Y82A (E, lane 3) was detected by Western blotting with an anti-Src antibody. No MBP-SrcY527F was pulled down by GST (C–E, lane 2). GST, GST-kindlin-2, and GST-F0 fusion proteins were detected by Coomassie Blue staining. F, human podocytes were transfected with expression vectors encoding FLAG-kindlin-2 (lanes 2 and 5), FLAG-Y13A/Y82A (lanes 3 and 6), or FLAG vector lacking kindlin-2 sequence (lanes 1 and 4). The cells were treated with H2O2 for 15 min and immunoprecipitated with anti-FLAG antibody M2-conjugated agarose beads. The cell lysates and anti-FLAG immunoprecipitates were analyzed by Western blotting with anti-FLAG or anti-Src antibodies.
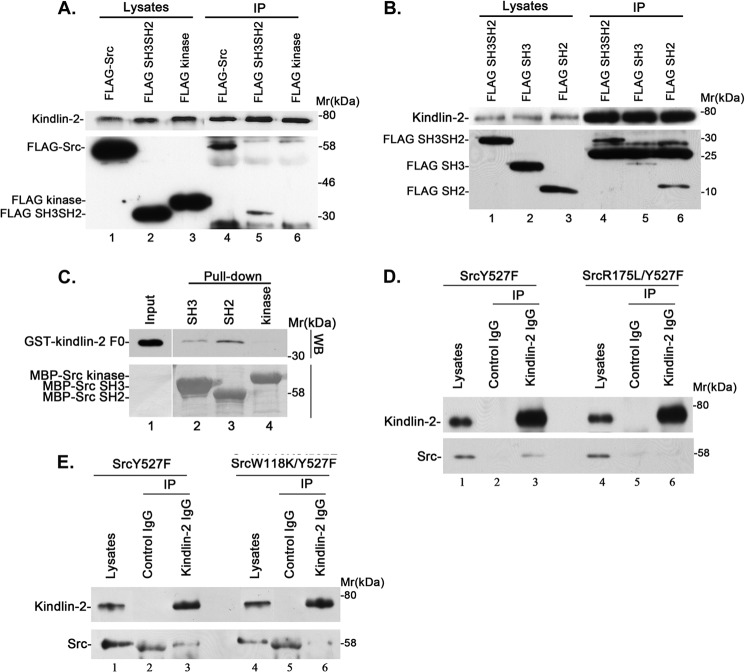
To identify the Src domains that are involved in the complex formation, we expressed FLAG-tagged Src or its fragments encompassing the SH2 and SH3 domains (residues 1–248) or the kinase domain (residues 249–536) in podocytes (Fig. 6A, lanes 1–3) and analyzed kindlin-2 binding by co-immunoprecipitation. The results showed that both FLAG-Src (Fig. 6A, lane 4) and FLAG-SH3SH2 (Fig. 6A, lane 5), but not FLAG-kinase (Fig. 6A, lane 6), was co-immunoprecipitated with kindlin-2. To further analyze this, we expressed a FLAG-tagged Src fragment encompassing the SH3 domain (residues 1–149), the SH2 fragment (residues 150–248), or FLAG-SH3SH2 as a control in cells (Fig. 6B, lanes 1–3) and immunoprecipitated kindlin-2. Western blotting analyses showed that the amount of FLAG-SH2 (Fig. 6B, lane 6) co-immunoprecipitated with kindlin-2 was similar to that of FLAG-SH3SH2 (Fig. 6B, lane 4), whereas the amount of FLAG-SH3 that was co-immunoprecipitated with kindlin-2 was much smaller than that of FLAG-SH2 or FLAG-SH3SH2 (Fig. 6B, compare lane 5 with lanes 4 and 6) despite the presence of abundant FLAG-SH3 in the lysates (Fig. 6B, lane 2). To further analyze this, we tested the ability of MBP-tagged Src SH2, SH3, or kinase domain to interact with GST-tagged kindlin-2 F0 fragment in a pull-down assay. The results showed that GST-F0 was pulled down by MBP-SH2 and to a lesser extent by MBP-SH3 but not by MBP-kinase (Fig. 6C). Collectively, these results suggest that the complex formation with kindlin-2 is mediated primarily by the Src SH2 domain, albeit the Src SH3 domain may also contribute to the complex formation with kindlin-2. To confirm this, we expressed SrcY527F or SrcY527F bearing an R175L point mutation (which disrupts the SH2 domain-mediated phosphotyrosine binding (51)) or W118K point mutation (which disrupts the SH3 domain-mediated binding to proline-rich sequences (52)) in cells and tested their abilities to form complexes with kindlin-2 by co-immunoprecipitation. The results showed that the R175L point mutation in SH2 nearly completely inhibited the complex formation with Src (Fig. 6D, compare lane 6 with lane 3), whereas the W118K point mutation reduced, albeit it did not eliminate, the complex formation with Src (Fig. 6E, compare lane 6 with lane 3). Thus, consistent with the finding that the formation of the kindlin-2·Src complex is markedly enhanced upon tyrosine phosphorylation of kindlin-2, the Src SH2 domain plays a predominant role in mediating the formation of a complex between kindlin-2 and Src.
FIGURE 6.
Src SH2 and SH3 domains mediate the complex formation with kindlin-2. A and B, human podocytes were transfected with vectors encoding FLAG-tagged full-length Src, Src SH3 and SH2 domains, Src kinase domain, Src SH3 domain, or Src SH2 domain, as indicated, and treated with H2O2 for 15 min. Kindlin-2 was immunoprecipitated with mouse monoclonal anti-kindlin-2 antibody 3A3. The cell lysates and anti-kindlin-2 immunoprecipitates were analyzed by Western blotting with anti-kindlin-2 or anti-FLAG antibodies, as indicated in the figure. C, purified GST-tagged kindlin-2 F0 fragment was incubated with MBP-Src SH3 domain (lane 2), MBP-Src SH2 domain (lane 3), or MBP-Src kinase domain (lane 4), as indicated in the figure. The input (lane 1) and GST-kindlin-2 F0 that was pulled down by MBP-Src fragments were detected by Western blotting with an anti-GST antibody. MBP-Src fragment fusion proteins were detected by Coomassie Blue staining. D and E, the cell lysates (lanes 1 and 4), anti-kindlin-2 immunoprecipitates (lanes 3 and 6), and control IgG immunoprecipitates (lanes 2 and 5) derived from human podocytes expressing SrcY527F (D and E, lanes 1–3), SrcR175L/Y527F (D, lanes 4–6), or SrcW118K/Y527F (E, lanes 4–6) were analyzed by Western blotting with antibodies recognizing kindlin-2 or Src.
The Kindlin-2·Src Interaction Is Dispensable for Integrin-mediated Cell-ECM Adhesion and Cell-ECM Adhesion-induced Tyrosine Phosphorylation of FAK but Is Critical for cell-ECM Adhesion-induced Tyrosine Phosphorylation of Paxillin, Cell Migration, and Proliferation—
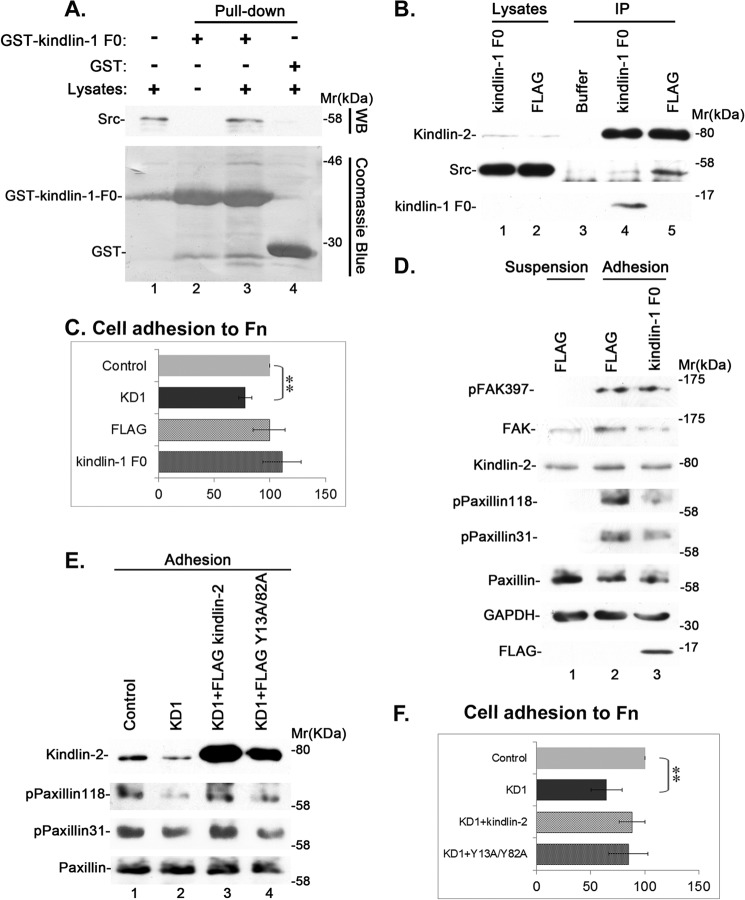
We next tested the function of the complex formation between kindlin-2 and Src. To do this, we first sought to generate a dominant negative inhibitor of the kindlin-2·Src complex. Because the kindlin-1 F0 domain is highly homologous to the kindlin-2 F0 domain, we reasoned that the kindlin-1 F0 domain may bind Src, which would compete with the kindlin-2 binding and therefore inhibit the kindlin-2·Src complex formation. To test this, we first expressed a GST-tagged kindlin-1 F0 fusion protein (containing kindlin-1 residues 1–97) and analyzed its Src binding activity in a GST pull-down experiment. The result showed that GST-kindlin-1 F0, but not GST, pulled down Src (Fig. 7A, compare lanes 3 and 4). Next, we overexpressed FLAG-kindlin-1 F0 (containing kindlin-1 residues 1–97) in cells and found that it indeed inhibited the formation of the kindlin-2·Src complex (Fig. 7B, compare lane 4 with lane 5). Unlike depletion of kindlin-2, which significantly reduced cell adhesion to fibronectin, disruption of the kindlin-2·Src complex did not reduce cell adhesion to fibronectin (Fig. 7C). Furthermore, disruption of the kindlin-2·Src complex did not impair cell-ECM adhesion-induced Tyr-397 phosphorylation of FAK (Fig. 7D). Importantly, however, disruption of the kindlin-2·Src complex, like depletion of kindlin-2, effectively inhibited cell-ECM adhesion-induced Tyr-118 and -31 phosphorylation of paxillin (Fig. 7D). Thus, although the formation of the kindlin-2·Src complex is not required for cell-ECM adhesion and the subsequent Tyr-397 phosphorylation of FAK, it is critical for cell-ECM adhesion-induced tyrosine phosphorylation of paxillin. Consistent with this, re-expression of FLAG-tagged wild type but not the Src binding-defective Y13A/Y82A mutant of kindlin-2 reversed the inhibition of Tyr-118 and -31 phosphorylation of paxillin induced by the knockdown of kindlin-2 (Fig. 7E). Furthermore, although the adhesion of kindlin-2 knockdown cells to fibronectin was significantly reduced compared with that of the control cells, the adhesion of the knockdown cells re-expressing FLAG-kindlin-2 or FLAG-Y13A/Y82A was not significantly reduced compared with that of the control cells (Fig. 7F).
FIGURE 7.
The kindlin-2·Src interaction is critical for cell-ECM adhesion-induced tyrosine phosphorylation of paxillin but not that of FAK. A, the lysates of human podocytes (lane 1), GST pull-downs (lane 4), and GST-kindlin-1 F0 pull-downs (lane 3) were analyzed by Western blotting with anti-Src antibody or Coomassie Blue staining. The sample in lane 2 was prepared as that in lane 3 except that cell lysates were omitted. B, kindlin-2 was immunoprecipitated from human podocytes transfected with FLAG-kindlin-1 F0 or FLAG control vectors. The cell lysates (lanes 1 and 2) and anti-kindlin-2 immunoprecipitates or anti-FLAG immunoprecipitates (lanes 4 and 5) were analyzed by Western blotting with antibodies recognizing kindlin-2, Src, or FLAG, as indicated. The sample in lane 3 was prepared as those in lanes 4 and 5 except that the cell lysates were omitted. C, human podocytes were transfected with kindlin-2 siRNA KD1, an irrelevant small RNA as a control, FLAG-kindlin-1 F0 expression vector, and FLAG vector as a control. Cell adhesion to fibronectin-coated plates was analyzed as described under “Experimental Procedures.” Bars, means ± S.D. (error bars) from three independent experiments. **, p < 0.01 versus the control. D, human podocytes were transfected with FLAG-kindlin-1 F0 expression vector or FLAG vector as a control. Cells were kept in suspension for 30 min and harvested (suspension), or replated on fibronectin-coated dishes for 1 h and then harvested (adhesion). The lysates of cells were analyzed by Western blotting with antibodies as indicated in the figure. E and F, human podocytes were transfected with a control RNA (lane 1), kindlin-2 siRNA KD1 (lane 2), kindlin-2 siRNA KD1 and a DNA vector encoding FLAG-kindlin-2 (lane 3), or kindlin-2 siRNA KD1 and a DNA vector encoding FLAG-Y13A/Y82A (lane 4) as described under “Experimental Procedures.” The cells were kept in suspension for 30 min, replated on fibronectin-coated dishes for 1 h, and analyzed by Western blotting with antibodies, as indicated (E). Cell adhesion to fibronectin (F) was analyzed as described in C. Bars, means ± S.D. from three independent experiments. **, p < 0.01 versus the control.
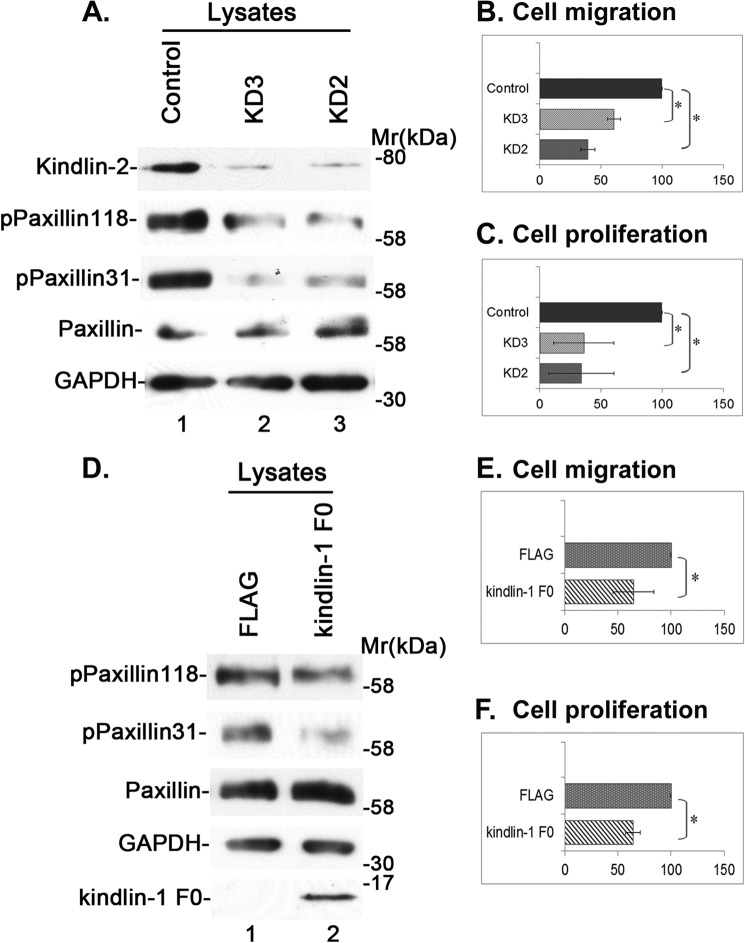
To assess the functions of the kindlin-2·Src-paxillin signaling axis, we analyzed the migration and proliferation of cells that were transfected with kindlin-2 siRNAs or overexpressing FLAG-kindlin-1 F0. The results showed that depletion of kindlin-2 markedly inhibited paxillin Tyr-118 and -31 phosphorylation (Fig. 8A, compare lanes 2 and 3 with lane 1) and reduced cell migration (Fig. 8B) and proliferation (Fig. 8C). Similarly, overexpression of FLAG-kindlin-1 F0, which inhibited paxillin Tyr-118 and -31 phosphorylation (Fig. 8D, compare lane 2 with lane 1), effectively reduced cell migration (Fig. 8E) and proliferation (Fig. 8F). Thus, the kindlin-2·Src·paxillin signaling axis, which is regulated by cell-ECM adhesion, is critical for cell migration and proliferation.
FIGURE 8.
Depletion of kindlin-2 or disruption of the kindlin-Src complex inhibits cell migration and proliferation. A–C, depletion of kindlin-2 inhibits cell migration and proliferation. Rat mesangial cells were transfected with kindlin-2 siRNA KD2 (lane 3), KD3 (lane 2), or a control RNA (lane 1) and analyzed by Western blotting as described under “Experimental Procedures” (A). The migration (B) and proliferation (C) of the kindlin-2 knockdown and control cells were analyzed as described under “Experimental Procedures.” Bars, means ± S.D. from three independent experiments. *, p < 0.05. D–F, disruption of the kindlin-2·Src complex inhibits cell migration and proliferation. Rat mesangial cells were transfected with FLAG-kindlin-1 F0 (lane 2) or FLAG vector as a control (lane 1). The transfectants were analyzed by Western blotting with antibodies as indicated (D). The migration (E) and proliferation (F) of FLAG-kindlin-1 F0-overexpressing or FLAG control cells were analyzed as described under “Experimental Procedures.” Bars, means ± S.D. from three independent experiments. *, p < 0.05.
DISCUSSION
Kindlin-2 is emerging as an important regulator of integrin inside-out signaling (35–41). Our results presented in this paper reveal a novel and equally important function of kindlin-2 in integrin outside-in signaling. We show that cell-ECM adhesion promotes tyrosine phosphorylation of kindlin-2. Several lines of evidence suggest that Src is intimately involved in the tyrosine phosphorylation of kindlin-2. First, Src forms a complex with kindlin-2. Furthermore, the complex formation of kindlin-2 with Src is closely correlated with its tyrosine phosphorylation during integrin outside-in signaling. Last, using several different experimental strategies (e.g. oxidation-induced Src activation, treatment with Src inhibitor PP2, or mutations that activate or inactivate Src), we show that activation of Src promotes, whereas inhibition of Src reduces, tyrosine phosphorylation of kindlin-2. Interestingly, our mutagenesis studies have shown that the complex formation between kindlin-2 and Src is mediated largely by Tyr-13 and Tyr-82 located in the kindlin-2 F0 domain and Src SH2 domain, albeit the Src SH3 domain may also contribute to it. Based on these findings and previous findings that Src is recruited to cell-ECM adhesions, where it binds to Tyr-397-phosphorylated FAK and is activated, we propose a model by which cell-ECM adhesion stimulates kindlin-2 tyrosine phosphorylation and complex formation with Src. In this model, upon cell-ECM adhesion, Src is recruited to the sites of contact, where it is activated via interactions with its binding partners at cell-ECM adhesions (e.g. Tyr-397-phosphorylated FAK). Src, through the SH3 domain, initially forms a weak complex with kindlin-2. The presence of active Src in close proximity to kindlin-2 facilitates tyrosine phosphorylation of kindlin-2. Increased tyrosine phosphorylation of kindlin-2, in turn, promotes Src SH2-mediated interaction and consequently enhances the formation of the kindlin-2·Src complex. This model not only provides an explanation for our findings delineated above but also explains why FAK is required for the optimal formation of the kindlin-2·Src complex (Fig. 2).
In addition to demonstrating that kindlin-2 can sense (i.e. is chemically modified) and respond to cell-ECM adhesion (i.e. forming a complex with Src), we have provided evidence showing that kindlin-2 is functionally required for integrin outside-in signaling that controls cell migration and proliferation because depletion of kindlin-2 diminished cell-ECM adhesion-induced tyrosine phosphorylation of paxillin and inhibited cell migration and proliferation. On the one hand, the requirement for kindlin-2 reflects the fact that kindlin-2 is critical for integrin activation (reviewed in Refs. 35–41), a prerequisite for optimal integrin signaling. On the other hand, this is due to a direct role of kindlin-2 in the regulation of tyrosine phosphorylation of paxillin (this work), a prominent effector of integrin outside-in signaling that controls cell migration and proliferation (13, 14, 53). Because 1) kindlin-2 forms a complex with Src and the latter can catalyze tyrosine phosphorylation of paxillin and 2) disruption of the kindlin-2·Src complex impaired cell-ECM adhesion-induced tyrosine phosphorylation of paxillin, kindlin-2 probably regulates cell-ECM adhesion-induced tyrosine phosphorylation of paxillin through its complex formation with Src. Collectively, our results presented in this paper, together with those of previous studies, suggest that kindlin-2 plays two important and mechanistically distinct roles in integrin signaling. First, kindlin-2, through its F3 domain-mediated interaction with integrin β cytoplasmic domains and pleckstrin homology domain-mediated interactions with membrane phospholipids (35–37, 39–41, 43, 44, 48, 54), promotes inside-out signaling of integrins. The requirement for kindlin-2 in this process explains why depletion of kindlin-2 reduces integrin-mediated cell-ECM adhesion as well as tyrosine phosphorylation of FAK and paxillin that are dependent on it. Second, once integrins are activated, they engage extracellularly with ECM proteins, such as fibronectin, and intracellularly with signaling proteins, such as FAK, resulting in autophosphorylation of FAK at Tyr-397 and interaction with and activation of Src. Active Src promotes tyrosine phosphorylation of kindlin-2 and consequently enhances the complex formation between kindlin-2 and Src, which in turn promotes Tyr-31 and Tyr-118 phosphorylation of paxillin and downstream signaling, resulting in increased cell migration and proliferation.
It is worth noting that although the Src-kindlin-2 interaction is clearly important for regulation of Tyr-31 and Tyr-118 phosphorylation of paxillin, our results do not rule out a role for FAK in this process. Instead, our results suggest that FAK also plays important roles in this process. Indeed, we have found that FAK can be pulled down by recombinant kindlin-2, albeit FAK was not detected in kindlin-2 immunoprecipitates, suggesting that the association of kindlin-2 with FAK is weak (or indirect). Although the precise role of this weak or indirect association between kindlin-2 and FAK in regulation of paxillin tyrosine phosphorylation requires further investigation, given the prominent role of FAK in regulation of the Src-kindlin-2 complex, FAK probably plays an important regulatory role in relaying cell-ECM adhesion signals from kindlin-2 and Src to paxillin.
This work was supported, in whole or in part, by National Institutes of Health Grant GM65188 (to C. W.).
- ECM
- extracellular matrix
- FAK
- focal adhesion kinase
- MBP
- maltose-binding protein
- ILK
- integrin-linked kinase
- N-ter
- kindlin-2 N-terminal fragment encompassing the F0 and part of the F1 domain (residues 1–256)
- SH2
- Src homology 2
- SH3
- Src homology 3
- IP
- immunoprecipitation.
REFERENCES
- 1. Schwartz M. A., Schaller M. D., Ginsberg M. H. (1995) Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 11, 549–599 [DOI] [PubMed] [Google Scholar]
- 2. Geiger B., Bershadsky A., Pankov R., Yamada K. M. (2001) Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2, 793–805 [DOI] [PubMed] [Google Scholar]
- 3. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 4. Guan J. L., Trevithick J. E., Hynes R. O. (1991) Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 2, 951–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. (1991) Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of β1 integrins. Proc. Natl. Acad. Sci. U.S.A. 88, 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turner C. E. (1991) Paxillin is a major phosphotyrosine-containing protein during embryonic development. J. Cell Biol. 115, 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burridge K., Turner C. E., Romer L. H. (1992) Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 119, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. (1992) pp125fak a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. U.S.A. 89, 5192–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan J. L., Shalloway D. (1992) Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature 358, 690–692 [DOI] [PubMed] [Google Scholar]
- 10. Schwartz M. A. (1997) Integrins, oncogenes, and anchorage independence. J. Cell Biol. 139, 575–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LaFlamme S. E., Auer K. L. (1996) Integrin signaling. Semin. Cancer Biol. 7, 111–118 [DOI] [PubMed] [Google Scholar]
- 12. LaFlamme S. E., Homan S. M., Bodeau A. L., Mastrangelo A. M. (1997) Integrin cytoplasmic domains as connectors to the cell's signal transduction apparatus. Matrix Biol. 16, 153–163 [DOI] [PubMed] [Google Scholar]
- 13. Turner C. E. (2000) Paxillin and focal adhesion signalling. Nat. Cell Biol. 2, E231–E236 [DOI] [PubMed] [Google Scholar]
- 14. Schaller M. D. (2001) Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20, 6459–6472 [DOI] [PubMed] [Google Scholar]
- 15. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 16. Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 17. Katz E., Streuli C. H. (2007) The extracellular matrix as an adhesion checkpoint for mammary epithelial function. Int. J. Biochem. Cell Biol. 39, 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lietha D., Cai X., Ceccarelli D. F. J., Li Y., Schaller M. D., Eck M. J. (2007) Structural basis for the autoinhibition of focal adhesion kinase. Cell 129, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai X., Lietha D., Ceccarelli D. F., Karginov A. V., Rajfur Z., Jacobson K., Hahn K. M., Eck M. J., Schaller M. D. (2008) Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol. Cell. Biol. 28, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frame M. C., Patel H., Serrels B., Lietha D., Eck M. J. (2010) The FERM domain: organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 11, 802–814 [DOI] [PubMed] [Google Scholar]
- 21. Zhao X., Guan J.-L. (2011) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 63, 610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hall J. E., Fu W., Schaller M. D. (2011) Focal adhesion kinase: exploring FAK structure to gain insight into function. Int. Rev. Cell Mol. Biol. 288, 185–225 [DOI] [PubMed] [Google Scholar]
- 23. Bellis S. L., Miller J. T., Turner C. E. (1995) Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J. Biol. Chem. 270, 17437–17441 [DOI] [PubMed] [Google Scholar]
- 24. Brown M. C., Turner C. E. (2004) Paxillin: adapting to change. Physiol. Rev. 84, 1315–1339 [DOI] [PubMed] [Google Scholar]
- 25. Playford M. P., Schaller M. D. (2004) The interplay between Src and integrins in normal and tumor biology. Oncogene 23, 7928–7946 [DOI] [PubMed] [Google Scholar]
- 26. Schaller M. D., Parsons J. T. (1995) pp125fak-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol. Cell. Biol. 15, 2635–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frisch S. M., Vuori K., Ruoslahti E., Chan-Hui P. Y. (1996) Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 134, 793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaller M. D., Sasaki T. (1997) Differential signaling by the focal adhesion kinase and cell adhesion kinase β. J. Biol. Chem. 272, 25319–25325 [DOI] [PubMed] [Google Scholar]
- 29. Richardson A., Parsons T. (1996) A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature 380, 538–540 [DOI] [PubMed] [Google Scholar]
- 30. Schaller M. D., Hildebrand J. D., Parsons J. T. (1999) Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell 10, 3489–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glenney J. R., Jr., Zokas L. (1989) Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J. Cell Biol. 108, 2401–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas S. M., Soriano P., Imamoto A. (1995) Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature 376, 267–271 [DOI] [PubMed] [Google Scholar]
- 33. Klinghoffer R. A., Sachsenmaier C., Cooper J. A., Soriano P. (1999) Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18, 2459–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schaller M. D. (2000) UNC112. A new regulator of cell-extracellular matrix adhesions? J. Cell Biol. 150, F9–F11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larjava H., Plow E. F., Wu C. (2008) Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9, 1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plow E. F., Qin J., Byzova T. (2009) Kindling the flame of integrin activation and function with kindlins. Curr. Opin. Hematol. 16, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meves A., Stremmel C., Gottschalk K., Fässler R. (2009) The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 19, 504–513 [DOI] [PubMed] [Google Scholar]
- 38. Shattil S. J., Kim C., Ginsberg M. H. (2010) The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11, 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malinin N. L., Plow E. F., Byzova T. V. (2010) Kindlins in FERM adhesion. Blood 115, 4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai-Cheong J. E., Parsons M., McGrath J. A. (2010) The role of kindlins in cell biology and relevance to human disease. Int. J. Biochem. Cell Biol. 42, 595–603 [DOI] [PubMed] [Google Scholar]
- 41. Ye F., Snider A. K., Ginsberg M. H. (2014) Talin and kindlin: the one-two punch in integrin activation. Front. Med. 8, 6–16 [DOI] [PubMed] [Google Scholar]
- 42. Tu Y., Wu S., Shi X., Chen K., Wu C. (2003) Migfilin and mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47 [DOI] [PubMed] [Google Scholar]
- 43. Qu H., Tu Y., Shi X., Larjava H., Saleem M. A., Shattil S. J., Fukuda K., Qin J., Kretzler M., Wu C. (2011) Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J. Cell Sci. 124, 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi X., Ma Y. Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F., Wu C. (2007) The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455–20466 [DOI] [PubMed] [Google Scholar]
- 45. Kohno M., Yasunari K., Minami M., Kano H., Maeda K., Mandal A. K., Inoki K., Haneda M., Yoshikawa J. (1999) Regulation of rat mesangial cell migration by platelet-derived growth factor, angiotensin II, and adrenomedullin. J. Am. Soc. Nephrol. 10, 2495–2502 [DOI] [PubMed] [Google Scholar]
- 46. Wu C. (2005) Migfilin and its binding partners: from cell biology to human diseases. J. Cell Sci. 118, 659–664 [DOI] [PubMed] [Google Scholar]
- 47. Mackinnon A. C., Qadota H., Norman K. R., Moerman D. G., Williams B. D. (2002) C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12, 787–797 [DOI] [PubMed] [Google Scholar]
- 48. Montanez E., Ussar S., Schifferer M., Bosl M., Zent R., Moser M., Fässler R. (2008) Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosado J. A., Redondo P. C., Salido G. M., Gómez-Arteta E., Sage S. O., Pariente J. A. (2004) Hydrogen peroxide generation induces pp60src activation in human platelets: evidence for the involvement of this pathway in store-mediated calcium entry. J. Biol. Chem. 279, 1665–1675 [DOI] [PubMed] [Google Scholar]
- 50. Twamley-Stein G. M., Pepperkok R., Ansorge W., Courtneidge S. A. (1993) The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc. Natl. Acad. Sci. U.S.A. 90, 7696–7700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bibbins K. B., Boeuf H., Varmus H. E. (1993) Binding of the Src SH2 domain to phosphopeptides is determined by residues in both the SH2 domain and the phosphopeptides. Mol. Cell. Biol. 13, 7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Destaing O., Sanjay A., Itzstein C., Horne W. C., Toomre D., De Camilli P., Baron R. (2008) The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell 19, 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deakin N. O., Turner C. E. (2008) Paxillin comes of age. J. Cell Sci. 121, 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma Y. Q., Qin J., Wu C., Plow E. F. (2008) Kindlin-2 (Mig-2): a co-activator of β3 integrins. J. Cell Biol. 181, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]