Abstract
Drug resistance poses a major challenge to ovarian cancer treatment. Understanding mechanisms of drug resistance is important for finding new therapeutic targets. In the present work, a cisplatin-resistant ovarian cancer cell line A2780-DR was established with a resistance index of 6.64. The cellular accumulation of cisplatin was significantly reduced in A2780-DR cells as compared with A2780 cells consistent with the general character of drug resistance. Quantitative proteomic analysis identified 340 differentially expressed proteins between A2780 and A2780-DR cells, which involve in diverse cellular processes, including metabolic process, cellular component biogenesis, cellular processes, and stress responses. Expression levels of Ras-related proteins Rab 5C and Rab 11B in A2780-DR cells were lower than those in A2780 cells as confirmed by real-time quantitative PCR and Western blotting. The short hairpin (sh)RNA-mediated knockdown of Rab 5C in A2780 cells resulted in markedly increased resistance to cisplatin whereas overexpression of Rab 5C in A2780-DR cells increases sensitivity to cisplatin, demonstrating that Rab 5C-dependent endocytosis plays an important role in cisplatin resistance. Our results also showed that expressions of glycolytic enzymes pyruvate kinase, glucose-6-phosphate isomerase, fructose-bisphosphate aldolase, lactate dehydrogenase, and phosphoglycerate kinase 1 were down-regulated in drug resistant cells, indicating drug resistance in ovarian cancer is directly associated with a decrease in glycolysis. Furthermore, it was found that glutathione reductase were up-regulated in A2780-DR, whereas vimentin, HSP90, and Annexin A1 and A2 were down-regulated. Taken together, our results suggest that drug resistance in ovarian cancer cell line A2780 is caused by multifactorial traits, including the down-regulation of Rab 5C-dependent endocytosis of cisplatin, glycolytic enzymes, and vimentin, and up-regulation of antioxidant proteins, suggesting Rab 5C is a potential target for treatment of drug-resistant ovarian cancer. This constitutes a further step toward a comprehensive understanding of drug resistance in ovarian cancer.
Ovarian cancer is the major cause of death in women with gynecological cancer. Early diagnosis of ovarian cancer is difficult, while its progression is fast. The standard treatment is surgical removal followed by platinum-taxane chemotherapy. However, the efficacy of the traditional surgery and chemotherapy is rather compromised and platinum resistant cancer recurs in ∼25% of patients within six months, and the overall five-year survival rate is about 31% (1–3). Virtually no efficient second line treatment is available. In order to increase survival rates from ovarian cancer and enhance patients' quality of life, new therapeutic targets are urgently required, necessitating a deeper understanding of molecular mechanisms of drug resistance.
Mechanisms of drug-resistance in ovarian cancer have been extensively studied over the last 30 years. Earlier studies have found that multiple factors are linked to drug resistance in human ovarian cancer including reduced intracellular drug accumulation, intracellular cisplatin inactivation, and increased DNA repair (4). Reduced cellular drug accumulation is mediated by the copper transporter-1 responsible for the influx of cisplatin (5–9) and MDR1, which encodes an integral membrane protein named P-glycoprotein for the active efflux of platinum drugs. Up-regulation of MDR1 has been observed in cisplatin-treated ovarian cancer cells although cisplatin is not a substrate of P-glycoprotein (10–13). A fraction of intracellular cisplatin can be converted into cisplatin-thiol conjugates by glutathione-S-transferase (GST) π, leading to inactivation of cisplatin. Up-regulation of both GSTπ and γ-glutamylcysteine synthetase has been associated with cisplatin resistance in ovarian, cervical and lung cancer cell lines (14–18). Binding of cisplatin to DNA leads to intrastrand or interstrand cross-links that alter the structure of the DNA molecule causing DNA damage. It has been amply documented that pathways for recognition and repair of damaged DNA are up-regulated in drug-resistant cancer cells (19–26). Furthermore, the secondary mutations have been identified, which restore the wild-type BRCA2 reading frame enhancing the acquired resistance to platinum-based chemotherapy (24). Alternations in other signaling pathways have also been found in drug resistant ovarian cancer (27–29). For example, DNA-PK phosphorylates RAC-alpha serine/threonine-protein kinase (AKT) and inhibits cisplatin-mediated apoptosis (28); and silencing of HDAC4 increases acetyl-STAT1 levels to prevent platinum-induced STAT1 activation and restore cisplatin sensitivity (29).
Proteomics is playing an increasingly important role in identifying differentially expressed proteins between drug-resistant and drug sensitive ovarian cancer cells (30–35). An earlier study has identified 57 differentially expressed proteins in human ovarian cancer cells and their platinum-resistant sublines, including annexin A3, destrin, cofilin 1, Glutathione-S-transferase omega 1, and cytosolic NADP+-dependent isocitrate dehydrogenase using 2D gel electrophoresis (30). Employing a similar 2D gel electrophoresis approach, changes in protein expressions of capsid glycoprotein, fructose-bisphosphate aldolase C, heterogeneous nuclear ribonucleoproteins A2/B1, putative RNA-binding protein 3, Ran-specific GTPase-activating protein, ubiquitin carboxyl-terminal hydrolase isozyme L1, stathmin, ATPSH protein, chromobox protein homolog3, and phosphoglycerate kinase 1 (PGK)1 were found in A2780 and drug-resistant A2780 cells (32). It is worth mentioning that ALDO and PGK are glycolytic enzymes, indicating that glycolysis plays a role in drug resistance. Studies have demonstrated that resistance to platinum drugs in ovarian cancer cells is linked to mitochondrial dysfunctions in oxidative phosphorylation and energy production (36–40). Mitochondrial proteomic analysis of drug-resistant cells has shown that five mitochondrial proteins (ATP-a, PRDX3, PHB, ETF, and ALDH) that participate in the electron transport respiratory chain are down-regulated in drug-resistant cell lines (41). PRDX3 is involved in redox regulation of the cell to protect radical-sensitive enzymes from oxidative damage. However, it is not clear how down-regulation of PRDX3 is associated with drug-resistance. A more recent study showed that activated leukocyte cell adhesion molecule (ALCA) and A kinase anchoring protein 12 (AKAP12) are elevated in drug-resistant A2780-CP20 cells by quantifying the mitochondrial proteins (42). Despite these efforts, the drug-resistance mechanisms are not yet well understood.
In this work, we established and characterized a drug-resistant cell line A2780-DR from A2780 cells. We employed a quantitative proteomic method to identify the differentially expressed proteins between A2780 and A2780-DR cells. Expression changes of selected proteins were confirmed by qPCR and Western blotting. We also used shRNA silencing to explore functions of Rab 5C and Rab 11B proteins in drug resistance. Our data indicate that the differentially expressed proteins participate in a variety of cellular processes and enhance our understanding of the mechanisms of drug resistance in ovarian cancer cells.
MATERIALS AND METHODS
Chemicals and Reagents
Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum, and penicillin-streptomycin were purchased from Wistent (Saint-Jean-Baptiste, CA). Dithiothreitol (DTT) was purchased from Calbiochem (San Diego, CA). The A2780 cell line was obtained from the Tumor Cell Bank of the Chinese Academy of Medical Sciences (Beijing, China). Sequencing grade modified trypsin was purchased from Promega (Fitchburg, WI). The propidium iodide staining kit was purchased from Solarbio (Beijing, China). The TMT labeling kit was purchased from Thermo-Pierce Biotechnology (Rockford, IL).
Cell Culture and Establishment of Cisplatin Resistant Subline
The human epithelial ovarian cancer cell line A2780 cells were maintained in DMEM media supplemented with 10% fetal bovine serum and penicillin (100 U/ml)–streptomycin (100 mg/ml) at 37 °C with 5% CO2. Cells were grown as monolayer cultures in 10 cm tissue culture plate and passaged when they had reached about 90% confluence. A monoclonal strain was separated by flow cytometry and further cultured to obtain A2780 cisplatin resistant strain (A2780-DR) by incubation with stepwise increasing cisplatin concentrations. Backups of all cells were stored with 10% DMSO. Every 20 passages, a new backup of cells was thawed to ascertain that resistance mechanisms were unchanged during long term cultivation. The relative cisplatin resistance was determined by cell viability assay.
Cell Cytotoxicity Assay
Effects of cisplatin on cell proliferation in A2780 and A2780-DR were analyzed with the Cell Counting Kit-8 (CCK-8) from Dojindo (Japan). A2780 and A2780-DR cells (8 × 103 each) were seeded into wells in 96-well cell culture microplates and incubated for 16 h prior to cisplatin treatment. Cells were then treated with cisplatin at different concentrations (0, 20, 40, 80, 160, and 320 μm) in triplicates for 24 h. The CCK8 reagent was added to treated cells and incubated at 37 °C for 2 h. Optical density (OD) was measured at 450 nm with a microplate reader (Bio-Rad, Hercules). Cell viability was calculated as the percentage of variable cells compared with untreated cells. The experiment was repeated three times and the IC50 was calculated by SPSS13.0 (SPSS Inc., Chicago, IL,). The lower the IC50 value, the higher the potency against cell proliferation.
Sample Preparation and Quantitative Proteomic Analysis
About 6 × 105 cells were lysed using RIPA lysis buffer (Solabio, Beijing, China), and protein concentrations were measured using the BCA method. Equal amount of proteins from untreated- and treated-samples (about 30 μg) were separated by 1D SDS-PAGE, respectively. The gel bands of interest were excised from the gel, reduced with 25 mm of dithiotreitol, and alkylated with 55 mm iodoacetamide. In gel digestion was then carried out with sequencing grade modified trypsin in 50 mm ammonium bicarbonate at 37 °C overnight. The peptides were extracted twice with 0.1% trifluoroacetic acid in 50% acetonitrile aqueous solution for 30 min. Extracts were then centrifuged in a speedvac to reduce the volume. Tryptic peptides were redissolved in 50 μl 200 mm Tetraethylammonium Bromide (TEAB), and 2 μl TMTsixplex labeling reagent was added to each sample according to the manufacture's instruction. The reaction was incubated for 1 h at room temperature. Then, 0.5 μl of 5% hydroxylamine (pH 9–10) was added to the reaction mixture and incubated for 15 min to quench the reaction. Equal amount of proteins from A2780 and A2780-DR cells were combined and analyzed by LC-MS/MS.
For LC-MS/MS analysis, the TMT-labeled peptides were separated by a 65 min gradient elution at a flow rate 0.250 μl/min with a Thermo-Dionex Ultimate 3000 HPLC system, which was directly interfaced with a Thermo Scientific Q Exactive mass spectrometer. The analytical column was a home-made fused silica capillary column (75 μm ID, 150 mm length; Upchurch, Oak Harbor, WA) packed with C-18 resin (300 Å, 5 μm, Varian, Lexington, MA). Mobile phase A consisted of 0.1% formic acid, and mobile phase B consisted of 100% acetonitrile and 0.1% formic acid. The Q Exactive mass spectrometer was operated in the data-dependent acquisition mode using Xcalibur 2.1.2 software and there was a single full-scan mass spectrum in the orbitrap (400–1800 m/z, 60,000 resolution) followed by 10 data-dependent MS/MS scans at 27% normalized collision energy.
The MS/MS spectra from each LC-MS/MS run were searched against the human.fasta from UniProt (release date of March 19, 2014; 68406 entries) using an in-house Proteome Discoverer (Version PD1.4, Thermo-Fisher Scientific). The search criteria were as follows: full tryptic specificity was required; one missed cleavage was allowed; carbamidomethylation (C) and TMT sixplex (K and N-terminal) were set as the fixed modifications; the oxidation (M) was set as the variable modification; precursor ion mass tolerances were set at 10 ppm for all MS acquired in an orbitrap mass analyzer; and the fragment ion mass tolerance was set at 20 mmu for all MS2 spectra acquired. The peptide false discovery rate was calculated using Percolator provided by PD. When the q value was smaller than 1%, the peptide spectrum match was considered to be correct. False discovery was determined based on peptide spectrum match when searched against the reverse, decoy database. Peptides only assigned to a given protein group were considered as unique. The false discovery rate was also set to 0.01 for protein identifications. Relative protein quantification was performed using Proteome Discoverer software (Version 1.4) according to manufacturer's instructions on the six reporter ion intensities per peptide. Quantitation was carried out only for proteins with two or more unique peptide matches. Protein ratios were calculated as the median of all peptide hits belonging to a protein. Quantitative precision was expressed as protein ratio variability. Differentially expressed proteins were further confirmed by qPCR or Western blotting. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD001176.
Real-time Quantitative PCR (qPCR)
Cells were harvested 48 h after transfection. Total RNA was extracted by the SV Total RNA Isolation System. cDNA was synthesized from 0.8 μg total RNA using the GoScriptTM Reverse Transcription System. All qPCR was performed using the Roche LightCycler® 480II Detection System with SYBR green incorporation according to the manufacturer's instructions. The primers were either designed by using the Primer Premier 5 software or from Primer Bank (http://pga.mgh.harvard.eduprimerbank/). To prevent amplification of genomic DNA, all target primers span exon-exon junctions. The specific PCR products were confirmed by melting curve analysis. Relative expression was analyzed using the 2−ΔΔCt method. Primer sequences for qPCR are listed in supplemental Table S1.
Western Blotting
Cells were harvested and lysed in RIPA lysis buffer. For shRNA transfected cells, cells were lysed at 72 h after transfection. The supernatants were collected after centrifugation at 14,000 × g for 10 min at 4 °C. Protein concentrations were determined using the BCA protein assay kit. Proteins were separated on a 12% SDS-PAGE gel and transferred onto a polyvinyl diflouride transfer membrane by electroblotting. After blocking with 5% nonfat milk for 2 h at room temperature, the membrane was incubated overnight at 4 °C with 1000× diluted primary antibody, washed with Phosphate Buffered Saline with Tween 20 (PBST) buffer for three times, then incubated with 1000× diluted anti-mouse or anti-rabbit secondary antibody labeled with horseradish peroxidase at room temperature for 2 h. The membrane was further washed with PBST buffer three times and developed using ECL reagents (Engreen, China). β-actin was detected with anti-β-actin antibody as an internal control. BioRad Image Lab software was used to analyze the images.
Determination of Cellular Platinum Accumulation
The cellular platinum accumulation was determined by the method described by Kayoko Minakata (43). Briefly, equal amount of A2780 and A2780-DR cells (about 4 × 106) were collected after 10 μm cisplatin treatment for 24 h. Cell pellets were washed three times with ice-cold PBS. Cell pellet was wet-ashed in 30 μl concentrated HNO3 at 85 °C for 8 h. The pH of wet-ashed solution was adjusted to 3–7 with either 10 m NaOH or 7 m HNO3. 30 μl of 1 m Diethyldithiocarbamate (DDC) was then added to the solution, in which DDC forms a complex with Pt by replacing other bonded ligands. After 3 min, 30 μl of isoamylalcohol was added and mixed for 30 s, and separated by centrifugation. The isoamylalcohol layer was mixed with 30 μl of 1 m oxalic acid for 10 s and centrifuged. A 1 μl aliquot of the isoamylalcohol layer was subjected to electrospray ionization mass spectrometry. Measurements were done in triplicate to determine standard errors of the mean (shown as error bars).
Short Hairpin RNA (ShRNA)-mediated Gene Silencing
The shRNAs against Rab 5C and Rab 11B were designed by the Invitrogen RNAi design tool (http://www.invitrogen.com) and synthesized by Invitrogen, LTD. Nontargeting negative control of shRNA (NCi) was also synthesized. The shRNA sequences are displayed in supplemental Table S2. The oligonucleotides were annealed and inserted into the pll3.7 siRNA expression vector to generate shRNA. The A2780 cells were plated the day before transfection and allowed to grow to 70–80% confluence. The cells were transiently transfected with Rab 5C-shRNA-pll3.7 or Rab 11B-shRNA-pll3.7 respectively with polyethylenimine (PEI) in DMEM. The effectiveness of shRNA in inhibiting Rab 5C and Rab 11B expression was evaluated by real time RT-PCR (48 h after the transfection) and Western blotting analysis (72 h after the transfection). Cells transfected with the plasmid NCi-pll3.7 served as the control.
Overexpression of Rab 5C in A2780-DR Cells
The gene of Rab 5C was cloned from the mRNA by RT-PCR from the Raji cell line, which was then subcloned into eukaryotic plasmid pcDNA3.1B (Invitrogen). The primer sequences are displayed in supplemental Table S2. Briefly, A2780-DR cells were plated the day before transfection and allowed to grow to 70–80% confluence. The cells were transiently transfected with pcDNA3.1 and Rab 5C-pcDNA3.1, respectively. The expression of Rab 5C was examined by Western blotting after 72 h transfection.
Detection of Reactive Oxygen Species (ROS) in A2780 and A2780-DR Cells
The ROS in untreated and azacytidine-treated cells was detected using the Image-iT™ LIVE Green Reactive Oxygen Species Detection Kit (Molecular Probes, Inc. Eugene, OR) following manufacturer's instructions. Briefly, A2780 and A2780-DR cells (2.5 × 105 each) were plated in triplicates in six-well plate the day before the test. After 48 h growth, the cells were collected by centrifugation and washed once with warm HBSS/Ca/Mg. Cells were resuspended with 500 μl of the 25 μm carboxy-H2DCFDA working solution for 25 min at 37 °C, followed by addition of the Hoechst 33342 reagent to the reaction mixture at the final concentration of 1.0 μm and incubation for 5 min. The final products were gently washed with 1 ml HBSS/Ca/Mg immediately followed by imaging with Zeiss 710 Confocal Microscopy.
RESULTS
Characterization of the Drug-resistant A2780 Cell Line
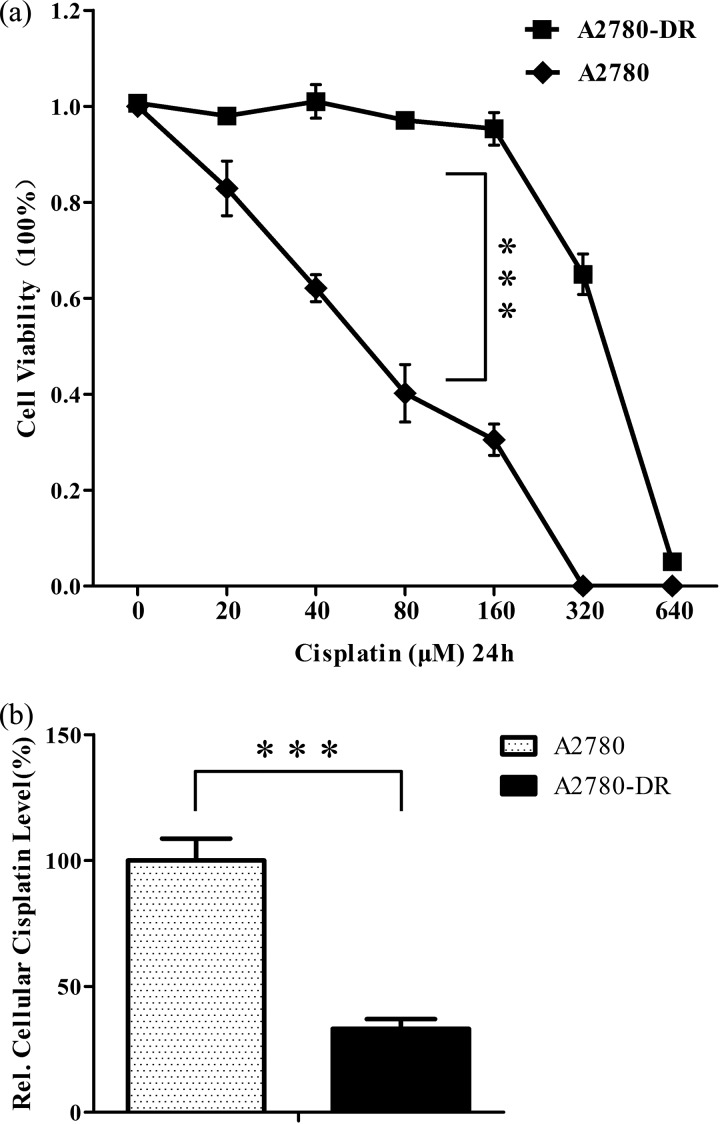
The drug-resistant cell line A2780-DR was established by the stepwise selection of A2780 cells cultured in growth media with increasing drug concentrations over a period of 6 months. To determine the sensitivity of A2780 and A2780-DR cells to cisplatin, cells were treated with different concentrations of cisplatin for 24 h and cell viability was measured by the Cell Counting Kit-8 (CCK-8) assay that allows sensitive colorimetric determination of cell viability and drug-sensitivity. The dose-dependent effects of cisplatin were represented as the percentage of viable cells as compared with untreated cells (Fig. 1A). When cells were treated with 80 μm cisplatin for 24h, percentages of viable cells were 40 and 100% for A2780 and A2780-DR cells, respectively. The inhibitory concentration 50% (IC50) and resistance index (RI) values of the two cell lines are displayed in Table I, indicating that A2780-DR is cisplatin resistant. The resistant phenotype is stable as the values of IC50 and RI have no significant changes over a period of 4 months in drug-free medium.
Fig. 1.
A, Cell cytotoxicity assays. Percentage of viable A2780-DR and A2780 cells treated with cisplatin at different concentrations for 24 h determined by using CCK-8 assay. Results are expressed as the mean of three experiments with p value <0.001; B, accumulation of cisplatin in 10 μm cisplatin treated A2780 and A2780-DR cells for 24 h as determined by electrospray ionization mass spectrometry. *** p < 0.001.
Table I. IC50 and Resistance Index of A2780-related cells to cisplatin treatment.
| IC50 (μm) | RI | |
|---|---|---|
| A2780 | 61.0 ± 2.3 | 1 |
| A2780-DR | 404.9 ± 15.1 | 6.6 |
| EV-A2780a | 54.6 ± 5.0 | 1 |
| ShRNA(Rab5C)-A2780 | 103.3 ± 6.2 | 1.9 |
| EV-A2780 | 45.1 ± 2.7 | 1 |
| ShRNA(Rab11B)-A2780 | 62.7 ± 3.2 | 1.4 |
a Empty vector transfected cells.
We also analyzed the total cellular Pt accumulation in sensitive and resistant cells following exposure to 10 μm cisplatin treatment for 24 h. The relative cisplatin concentrations were displayed in Fig. 1B as determined by mass spectrometry. After treatment with cisplatin, total intracellular Pt in A2780-DR is about one third of that in the parent cell line A2780, indicating significant reduction of cisplatin accumulation in drug-resistant cells.
Proteomic Analysis of A2780 and A2780-DR Cells
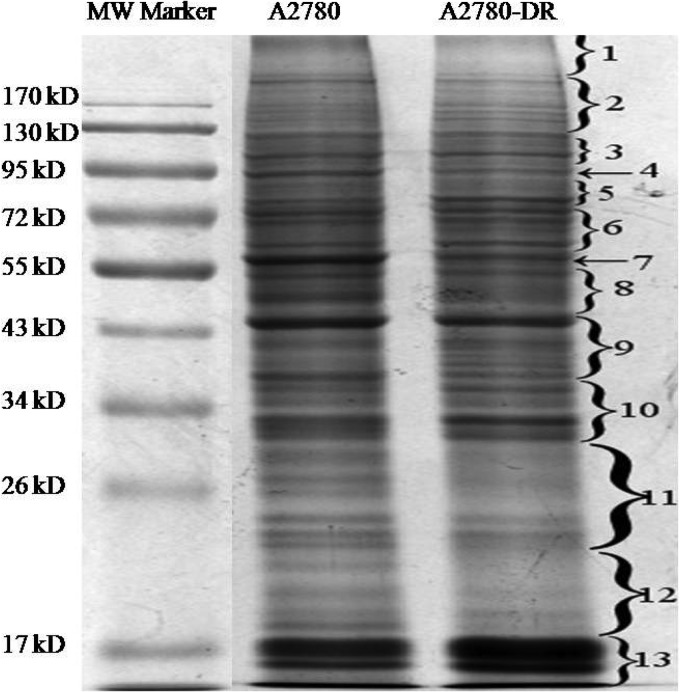
Next, proteomic analysis was carried out on A2780 and A2780-DR cells. Equal amounts of proteins from A2780 and A2780-DR cells were loaded and separated by 1D SDS-PAGE (Fig. 2). Differentially expressed proteins were identified and quantified using TMT-labeling. The experiments were repeated three times and ∼1900 proteins were identified for each cell line. The false-positive rate was set to be less than 1%. Based on TMT ratios (>2.0 or <0.6) in proteins that have two or more unique peptides, 340 proteins were found to be differentially expressed between A2780 and A2780-DR cells, of which 268 proteins are down-regulated and 72 up-regulated (Table II and III). The major protein in band 7 that was down-regulated in A2780-DR was identified as vimentin. HSP90α and HSP90β in band 4 were also down-regulated in the drug-resistant cells (Fig. 2). In order to understand the biological relevance of the identified proteins, Gene Ontology (GO) was used to categorize the differentially expressed proteins according to their molecular functions and biological processes. The annotations of gene lists are summarized via a pie plot using the PANTHER bioinformatics platform (http://www.pantherdb.org/) as shown in Fig. 3. Three hundred and thirty nine proteins were classified into several significant groups of biological processes including metabolic processes, cellular processes, cellular compartment organization, and apoptosis.
Fig. 2.
1D SDS-PAGE gel image of A2780 and A2780-DR cells. Lane 1, molecular weight markers; Lane 2, proteins from A2780 cells; Lane 3, proteins from A2780-DR cells; and bands excised from the gel with differentially expressed proteins are labeled with numeric numbers.
Table II. Up-regulated proteins in cisplatin resistant cells.
| Accession | Description | Score | Coverage (%) | Unique peptides | A2780-DR/A2780 |
|---|---|---|---|---|---|
| E9PM69 | 26S protease regulatory subunit 6A | 17 | 15 | 5 | 3.5 |
| O00231 | 26S proteasome non-ATPase regulatory subunit 11 | 18 | 16 | 6 | 5.1 |
| P36578 | 60S ribosomal protein L4 | 77 | 30 | 12 | 5.1 |
| B4DQJ8 | 6-phosphogluconate dehydrogenase, decarboxylating | 23 | 24 | 8 | 3.7 |
| G3V4F2 | Acyl-coenzyme A thioesterase 1 | 14 | 14 | 4 | 3.6 |
| P30520 | Adenylosuccinatesynthetaseisozyme 2 | 16 | 13 | 4 | 4.4 |
| P30837 | Aldehyde dehydrogenase X, mitochondrial | 22 | 18 | 6 | 6.3 |
| F8VS02 | Alpha-aminoadipicsemialdehyde dehydrogenase | 17 | 17 | 7 | 6.2 |
| P06733 | Alpha-enolase | 217 | 53 | 16 | 4.8 |
| B4DT77 | Annexin | 23 | 12 | 4 | 2.4 |
| P25705 | ATP synthase subunit alpha, mitochondrial | 136 | 45 | 19 | 6.8 |
| P06576 | ATP synthase subunit beta, mitochondrial | 199 | 52 | 20 | 5.4 |
| R4GMX5 | Basigin (Fragment) | 11 | 36 | 3 | 4.7 |
| H3BS10 | Beta-hexosaminidase | 17 | 8 | 3 | 2.3 |
| Q13895 | Bystin | 10 | 6 | 3 | 4.2 |
| P10644 | cAMP-dependent protein kinase type I-alpha regulatory subunit | 13 | 18 | 6 | 3.4 |
| C9JP16 | Cartilage-associated protein | 12 | 12 | 5 | 5.3 |
| P31930 | Cytochrome b-c1 complex subunit 1, mitochondrial | 27 | 17 | 6 | 5.7 |
| B7Z5W8 | Dihydrolipoyllysine-residue succinyltransferase component | 11 | 11 | 4 | 6.4 |
| P39656 | Dolichyl-diphosphooligosaccharide–protein glycosyltransferase 48 kDa subunit | 46 | 21 | 9 | 5.5 |
| Q05639 | Elongation factor 1-alpha 2 | 256 | 20 | 2 | 3.1 |
| P26641 | Elongation factor 1-gamma | 62 | 32 | 13 | 4.7 |
| P49411 | Elongation factor Tu, mitochondrial | 88 | 27 | 11 | 4.5 |
| F5H0C8 | Enolase | 52 | 11 | 2 | 4.1 |
| P07099 | Epoxide hydrolase 1 | 42 | 24 | 11 | 5.3 |
| Q96CG1 | ETF1 protein | 13 | 6 | 2 | 2.7 |
| P38919 | Eukaryotic initiation factor 4A-III | 31 | 21 | 6 | 4.7 |
| H7BZU1 | Eukaryotic translation initiation factor 2 subunit 3 (Fragment) | 10 | 11 | 2 | 3.9 |
| O00303 | Eukaryotic translation initiation factor 3 subunit F | 17 | 17 | 5 | 3.6 |
| J3KNT0 | Fascin | 18 | 23 | 9 | 4.1 |
| J3QRD1 | Fatty aldehyde dehydrogenase | 27 | 14 | 5 | 8.1 |
| P39748 | Flap endonuclease 1 | 12 | 8 | 3 | 4.6 |
| P00367 | Glutamate dehydrogenase 1, mitochondrial | 22 | 18 | 8 | 5.9 |
| P52597 | Heterogeneous nuclear ribonucleoprotein F | 47 | 14 | 2 | 3.6 |
| P31943 | Heterogeneous nuclear ribonucleoprotein H | 94 | 33 | 5 | 4.5 |
| P55795 | Heterogeneous nuclear ribonucleoprotein H2 | 51 | 18 | 2 | 4.3 |
| B3KWE1 | Histidine–tRNA ligase, cytoplasmic | 15 | 6 | 3 | 3.9 |
| P0C0S5 | Histone H2A.Z | 283 | 31 | 2 | 2.1 |
| H7C3I1 | Hsc70-interacting protein (Fragment) | 17 | 23 | 3 | 6.6 |
| Q6YN16 | Hydroxysteroid dehydrogenase-like protein 2 | 12 | 8 | 3 | 8.6 |
| P43686-2 | Isoform 2 of 26S protease regulatory subunit 6B | 15 | 19 | 6 | 6.0 |
| P62195-2 | Isoform 2 of 26S protease regulatory subunit 8 | 15 | 12 | 3 | 3.5 |
| Q16401-2 | Isoform 2 of 26S proteasome non-ATPase regulatory subunit 5 | 35 | 21 | 8 | 4.1 |
| P28838-2 | Isoform 2 of Cytosol aminopeptidase | 26 | 19 | 8 | 6.9 |
| Q9H0S4-2 | Isoform 2 of Probable ATP-dependent RNA helicase DDX47 | 12 | 10 | 3 | 7.3 |
| P35659-2 | Isoform 2 of Protein DEK | 17 | 11 | 4 | 4.3 |
| P50395-2 | Isoform 2 of Rab GDP dissociation inhibitor beta | 33 | 26 | 5 | 4.9 |
| Q8NBS9-2 | Isoform 2 of Thioredoxin domain-containing protein 5 | 47 | 39 | 11 | 4.3 |
| O14773-2 | Isoform 2 of Tripeptidyl-peptidase 1 | 10 | 9 | 2 | 4.9 |
| P34897-3 | Isoform 3 of Serine hydroxymethyltransferase, mitochondrial | 37 | 20 | 9 | 6.5 |
| P00390-5 | Isoform 4 of Glutathione reductase, mitochondrial | 25 | 11 | 4 | 6.1 |
| O60664-4 | Isoform 4 of Perilipin-3 | 24 | 21 | 6 | 5.6 |
| P07954-2 | Isoform Cytoplasmic of Fumaratehydratase, mitochondrial | 28 | 13 | 6 | 5.7 |
| P05455 | Lupus La protein | 19 | 16 | 6 | 6.3 |
| Q10713 | Mitochondrial-processing peptidase subunit alpha | 13 | 10 | 5 | 6.3 |
| E7ERZ4 | Mitochondrial-processing peptidase subunit beta | 11 | 12 | 3 | 5.8 |
| B4DEH8 | Polyadenylate-binding protein 2 | 19 | 17 | 3 | 5.1 |
| Q9UQ80 | Proliferation-associated protein 2G4 | 23 | 18 | 7 | 3.3 |
| B7Z254 | Protein disulfide-isomerase A6 | 95 | 35 | 12 | 7.3 |
| P49257 | Protein ERGIC-53 | 17 | 7 | 3 | 4.8 |
| P18754 | Regulator of chromosome condensation | 22 | 19 | 6 | 6.2 |
| F8W914 | Reticulon | 12 | 12 | 3 | 3.6 |
| P00352 | Retinal dehydrogenase 1 | 48 | 26 | 11 | 2.7 |
| P13489 | Ribonuclease inhibitor | 15 | 17 | 5 | 4.2 |
| Q9Y265 | RuvB-like 1 | 67 | 31 | 10 | 4.6 |
| Q9Y230 | RuvB-like 2 | 36 | 21 | 8 | 3.7 |
| O15269 | Serine palmitoyltransferase 1 | 10 | 11 | 4 | 4.5 |
| P50454 | Serpin H1 | 171 | 52 | 19 | 4.0 |
| Q13838 | Spliceosome RNA helicase DDX39B | 96 | 29 | 3 | 4.2 |
| P55084 | Trifunctional enzyme subunit beta, mitochondrial | 21 | 24 | 10 | 7.0 |
| P50552 | Vasodilator-stimulated phosphoprotein | 10 | 12 | 4 | 4.6 |
| P04004 | Vitronectin | 11 | 5 | 2 | 2.7 |
Fig. 3.
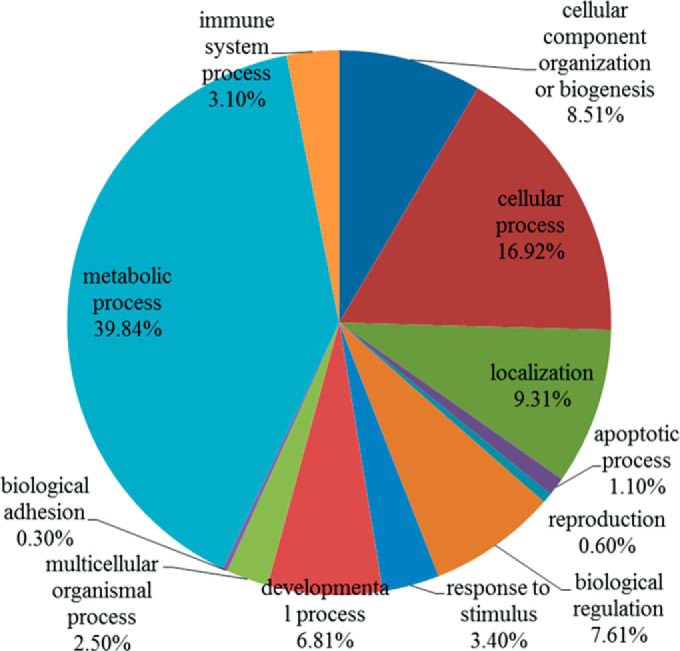
Functional classification of differentially expressed proteins between A2780 and A2780-DR cells with PANTHER (http://www.pantherdb.org).
Verification of Differentially Expressed Proteins by Western blotting and qPCR
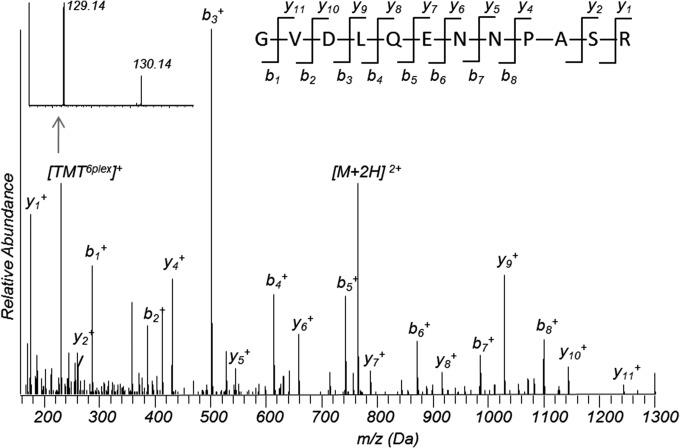
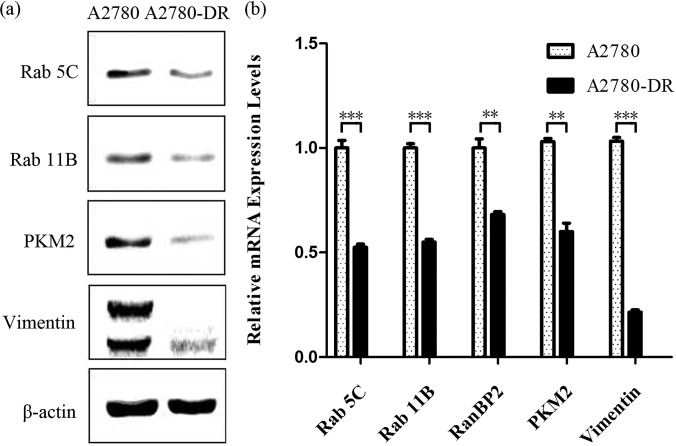
Among differentially expressed proteins (Table III), Ras-related protein Rab 5C and Rab 11B are down-regulated in the drug-resistant cells. Fig. 4 shows a ms/ms spectrum of a peptide ions that match to fragments of a tryptic peptide GVDLQENNPASR from Rab 5C and the insert shows fragments at the low mass range for the TMT-reporter ions, whose ratio indicates that the expression level of Rab 5C is three times higher in A2780 as compared with A2780-DR cells. Down-regulation of Rab 5C and Rab 11B was confirmed by Western blotting (Fig. 5A) and by qPCR analysis (Fig. 5B). Results from analysis of band intensities of Western blot are displayed in supplemental Table S3. Vimentin was the most abundant with the highest spectra count among the differentially expressed proteins and the down-regulation of vimentin was confirmed by Western blotting (Fig. 5A), showing that two bands at 54 and 56 kDa were barely visible for vimentin in A2780-DR cells. Western blotting also confirms that the expression level of PKM2 is down-regulated in A2780-DR cells (Fig. 5A). Proteomic analysis shows that a redox proteins glutathione reductase (GSR) is up-regulated in A2780-DR cells.
Table III. Down-regulated Proteins in cisplatin-resistant cells.
| Accession | Description | Score | Coverage (%) | Unique Peptides | A2780-DR/A2780 |
|---|---|---|---|---|---|
| Q04917 | 14-3-3 protein eta | 42 | 26 | 3 | 0.3 |
| P61981 | 14-3-3 protein gamma | 57 | 38 | 5 | 0.3 |
| P27348 | 14-3-3 protein theta | 55 | 27 | 2 | 0.3 |
| P63104 | 14-3-3 protein zeta/delta | 75 | 43 | 6 | 0.3 |
| P62191 | 26S protease regulatory subunit 4 | 36 | 22 | 7 | 0.6 |
| Q15008 | 26S proteasome non-ATPase regulatory subunit 6 | 31 | 11 | 4 | 0.3 |
| R4GMR5 | 26S proteasome non-ATPase regulatory subunit 8 | 19 | 14 | 5 | 0.3 |
| P82930 | 28S ribosomal protein S34, mitochondrial | 12 | 22 | 5 | 0.5 |
| P46783 | 40S ribosomal protein S10 | 24 | 23 | 3 | 0.3 |
| P62277 | 40S ribosomal protein S13 | 22 | 38 | 5 | 0.5 |
| P62269 | 40S ribosomal protein S18 | 21 | 24 | 4 | 0.6 |
| P39019 | 40S ribosomal protein S19 | 34 | 48 | 9 | 0.6 |
| P62851 | 40S ribosomal protein S25 | 26 | 21 | 3 | 0.4 |
| F2Z2S8 | 40S ribosomal protein S3 | 15 | 33 | 4 | 0.6 |
| P62701 | 40S ribosomal protein S4, X isoform | 57 | 32 | 9 | 0.3 |
| M0R0F0 | 40S ribosomal protein S5 (Fragment) | 19 | 27 | 5 | 0.5 |
| Q5JR95 | 40S ribosomal protein S8 | 21 | 38 | 6 | 0.2 |
| P46781 | 40S ribosomal protein S9 | 19 | 18 | 4 | 0.5 |
| C9J9K3 | 40S ribosomal protein SA (Fragment) | 86 | 36 | 6 | 0.2 |
| F8VU65 | 60S acidic ribosomal protein P0 (Fragment) | 26 | 25 | 6 | 0.2 |
| P62906 | 60S ribosomal protein L10a | 20 | 24 | 6 | 0.3 |
| P30050 | 60S ribosomal protein L12 | 13 | 23 | 3 | 0.5 |
| P26373 | 60S ribosomal protein L13 | 28 | 27 | 6 | 0.3 |
| E7EPB3 | 60S ribosomal protein L14 | 29 | 35 | 4 | 0.2 |
| P61313 | 60S ribosomal protein L15 | 25 | 18 | 5 | 0.4 |
| G3V203 | 60S ribosomal protein L18 | 17 | 25 | 4 | 0.5 |
| P61353 | 60S ribosomal protein L27 | 15 | 21 | 3 | 0.6 |
| E9PJD9 | 60S ribosomal protein L27a | 15 | 34 | 3 | 0.5 |
| P49207 | 60S ribosomal protein L34 | 18 | 21 | 3 | 0.5 |
| Q02878 | 60S ribosomal protein L6 | 23 | 25 | 7 | 0.2 |
| P18124 | 60S ribosomal protein L7 | 26 | 27 | 6 | 0.3 |
| O95336 | 6-phosphogluconolactonase | 11 | 14 | 3 | 0.4 |
| P24752 | Acetyl-CoA acetyltransferase, mitochondrial | 30 | 22 | 8 | 0.4 |
| H0YN26 | Acidic leucine-rich nuclear phosphoprotein 32 family member A | 15 | 18 | 2 | 0.3 |
| O95433 | Activator of 90 kDa heat shock protein ATPase homolog 1 | 19 | 18 | 5 | 0.3 |
| Q01518 | Adenylyl cyclase-associated protein 1 | 73 | 31 | 14 | 0.5 |
| P12235 | ADP/ATP translocase 1 | 128 | 32 | 2 | 0.3 |
| P05141 | ADP/ATP translocase 2 | 171 | 40 | 4 | 0.4 |
| P61204 | ADP-ribosylation factor 3 | 12 | 20 | 2 | 0.4 |
| H0YN42 | Annexin (Fragment) | 28 | 33 | 8 | 0.4 |
| P04083 | Annexin A1 | 457 | 66 | 21 | 0.2 |
| P02647 | Apolipoprotein A-I | 52 | 53 | 15 | 0.2 |
| B7Z7E9 | Aspartate aminotransferase | 17 | 16 | 5 | 0.3 |
| P00505 | Aspartate aminotransferase, mitochondrial | 48 | 18 | 8 | 0.5 |
| P48047 | ATP synthase subunit | 11 | 27 | 4 | 0.5 |
| O43681 | ATPase ASNA1 | 11 | 7 | 3 | 0.4 |
| Q08211 | ATP-dependent RNA helicase A | 346 | 34 | 36 | 0.5 |
| Q92499 | ATP-dependent RNA helicase DDX1 | 31 | 14 | 10 | 0.5 |
| O95816 | BAG family molecular chaperone regulator 2 | 21 | 27 | 6 | 0.4 |
| P51572 | B-cell receptor-associated protein 31 | 17 | 13 | 4 | 0.4 |
| E9PK09 | Bcl-2-associated transcription factor 1 (Fragment) | 13 | 6 | 5 | 0.3 |
| P07686 | Beta-hexosaminidase subunit beta | 30 | 13 | 6 | 0.4 |
| P07814 | Bifunctional glutamate/proline–tRNA ligase | 39 | 12 | 17 | 0.4 |
| P31327 | Carbamoyl-phosphate synthase [ammonia], mitochondrial | 22 | 8 | 9 | 0.4 |
| E9PFZ2 | Ceruloplasmin | 10 | 4 | 3 | 0.3 |
| F5GWX5 | Chromodomain-helicase-DNA-binding protein 4 | 23 | 3 | 6 | 0.4 |
| B4DJV2 | Citrate synthase | 56 | 18 | 3 | 0.6 |
| F5H669 | Cleavage and polyadenylation-specificity factor subunit 7 | 13 | 17 | 6 | 0.6 |
| Q9NX63 | Coiled-coil-helix-coiled-coil-helix domain-containing protein 3 | 15 | 15 | 4 | 0.4 |
| Q9P0M6 | Core histone macro-H2A.2 | 21 | 12 | 2 | 0.4 |
| H3BSJ9 | Cytochrome b-c1 complex subunit 2, mitochondrial | 31 | 33 | 8 | 0.4 |
| P47985 | Cytochrome b-c1 complex subunit Rieske, mitochondrial | 12 | 13 | 3 | 0.4 |
| Q14204 | Cytoplasmic dynein 1 heavy chain 1 | 51 | 6 | 23 | 0.4 |
| Q9Y295 | Developmentally-regulated GTP-binding protein 1 | 13 | 14 | 4 | 0.2 |
| H0Y8E6 | DNA replication licensing factor MCM2 (Fragment) | 36 | 11 | 8 | 0.3 |
| P33992 | DNA replication licensing factor MCM5 | 28 | 12 | 8 | 0.4 |
| E9PCY5 | DNA topoisomerase 2 (Fragment) | 61 | 16 | 9 | 0.5 |
| P11388 | DNA topoisomerase 2-alpha | 89 | 16 | 14 | 0.4 |
| E7EUY0 | DNA-dependent protein kinase catalytic subunit | 160 | 11 | 42 | 0.4 |
| C9J4M6 | DNA-directed RNA polymerase | 22 | 5 | 6 | 0.5 |
| B4DX52 | DnaJ homolog subfamily B member 1 | 11 | 12 | 3 | 0.3 |
| P49792 | E3 SUM | 11 | 3 | 8 | 0.5 |
| Q9HC35 | Echinoderm microtubule-associated protein-like 4 | 13 | 6 | 5 | 0.4 |
| P68104 | Elongation factor 1-alpha 1 | 345 | 34 | 6 | 0.5 |
| E9PK01 | Elongation factor 1-delta (Fragment) | 15 | 27 | 5 | 0.2 |
| Q9Y371 | Endophilin-B1 | 10 | 14 | 5 | 0.3 |
| P30040 | Endoplasmic reticulum resident protein 29 | 32 | 38 | 8 | 0.5 |
| P30084 | Enoyl-CoA hydratase, mitochondrial | 12 | 18 | 4 | 0.6 |
| P05198 | Eukaryotic translation initiation factor 2 subunit 1 | 16 | 15 | 5 | 0.2 |
| F5H335 | Eukaryotic translation initiation factor 3 subunit A | 78 | 15 | 18 | 0.3 |
| Q13347 | Eukaryotic translation initiation factor 3 subunit I | 11 | 13 | 4 | 0.2 |
| P56537 | Eukaryotic translation initiation factor 6 | 11 | 30 | 5 | 0.3 |
| Q9NPD3 | Exosome complex component RRP41 | 16 | 15 | 3 | 0.4 |
| Q9Y5B9 | FACT complex subunit SPT16 | 21 | 7 | 7 | 0.4 |
| Q08945 | FACT complex subunit SSRP1 | 45 | 16 | 12 | 0.6 |
| P49327 | Fatty acid synthase | 98 | 14 | 28 | 0.3 |
| P30043 | Flavin reductase (NADPH) | 11 | 31 | 4 | 0.5 |
| P04075 | Fructose-bisphosphatealdolase A | 176 | 57 | 18 | 0.2 |
| K7EQ48 | Glucose-6-phosphate isomerase | 45 | 16 | 8 | 0.5 |
| B4DWJ2 | Glutamine–tRNA ligase | 17 | 11 | 8 | 0.5 |
| O76003 | Glutaredoxin-3 | 11 | 12 | 3 | 0.2 |
| P78417 | Glutathione S-transferase omega-1 | 28 | 14 | 3 | 0.4 |
| P09211 | Glutathione S-transferase P | 15 | 25 | 4 | 0.4 |
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | 247 | 55 | 16 | 0.2 |
| H3BM42 | Golgi apparatus protein 1 | 10 | 2 | 2 | 0.4 |
| Q08379 | Golgin subfamily A member 2 | 16 | 6 | 5 | 0.4 |
| Q9UIJ7 | GTP:AMP phosphotransferase AK3, mitochondrial | 12 | 26 | 6 | 0.5 |
| P62826 | GTP-binding nuclear protein Ran | 15 | 25 | 5 | 0.4 |
| Q5T3Q7 | HEAT repeat-containing protein 1 | 12 | 3 | 5 | 0.6 |
| P07900 | Heat shock protein HSP 90-alpha | 716 | 55 | 24 | 0.5 |
| P08238 | Heat shock protein HSP 90-beta | 925 | 56 | 24 | 0.5 |
| D6R9P3 | Heterogeneous nuclear ribonucleoprotein A/B | 24 | 18 | 5 | 0.4 |
| F8W6I7 | Heterogeneous nuclear ribonucleoprotein A1 | 102 | 44 | 10 | 0.3 |
| G3V4W0 | Heterogeneous nuclear ribonucleoproteins C1/C2 (Fragment) | 72 | 41 | 13 | 0.4 |
| Q86YZ3 | Hornerin | 11 | 4 | 2 | 0.4 |
| Q9Y4L1 | Hypoxia up-regulated protein 1 | 182 | 39 | 33 | 0.5 |
| P52292 | Importin subunit alpha-1 | 17 | 10 | 6 | 0.6 |
| Q12905 | Interleukin enhancer-binding factor 2 | 68 | 22 | 7 | 0.5 |
| P50213 | Isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial | 27 | 26 | 8 | 0.3 |
| O75874 | Isocitrate dehydrogenase [NADP] cytoplasmic | 15 | 16 | 6 | 0.2 |
| B4DFL2 | Isocitrate dehydrogenase [NADP] | 19 | 19 | 6 | 0.4 |
| Q9P2E9-2 | Isoform 1 of Ribosome-binding protein 1 | 38 | 20 | 12 | 0.4 |
| Q99714-2 | Isoform 2 of 3-hydroxyacyl-CoA dehydrogenase type-2 | 51 | 54 | 8 | 0.5 |
| Q92688-2 | Isoform 2 of Acidic leucine-rich nuclear phosphoprotein 32 | 11 | 19 | 2 | 0.3 |
| P23526-2 | Isoform 2 of Adenosylhomocysteinase | 13 | 7 | 3 | 0.3 |
| O00571-2 | Isoform 2 of ATP-dependent RNA helicase DDX3X | 54 | 24 | 14 | 0.6 |
| Q00610-2 | Isoform 2 of Clathrin heavy chain 1 | 230 | 31 | 40 | 0.4 |
| O15160-2 | Isoform 2 of DNA-directed RNA polymerases I and III subunit | 10 | 17 | 4 | 0.3 |
| P21333-2 | Isoform 2 of Filamin-A | 385 | 29 | 56 | 0.4 |
| P78347-2 | Isoform 2 of General transcription factor II-I | 57 | 13 | 12 | 0.5 |
| P51991-2 | Isoform 2 of Heterogeneous nuclear ribonucleoprotein A3 | 39 | 22 | 6 | 0.5 |
| P31942-2 | Isoform 2 of Heterogeneous nuclear ribonucleoprotein H3 | 25 | 17 | 4 | 0.3 |
| Q86UP2-2 | Isoform 2 of Kinectin | 66 | 15 | 17 | 0.4 |
| Q9NZM1-2 | Isoform 2 of Myoferlin | 35 | 7 | 12 | 0.5 |
| P12036-2 | Isoform 2 of Neurofilament heavy polypeptide | 94 | 8 | 5 | 0.4 |
| Q9Y617-2 | Isoform 2 of Phosphoserine aminotransferase | 14 | 17 | 5 | 0.2 |
| P11940-2 | Isoform 2 of Polyadenylate-binding protein 1 | 70 | 31 | 15 | 0.6 |
| O75400-2 | Isoform 2 of Pre-mRNA-processing factor 40 homolog A | 19 | 10 | 7 | 0.4 |
| P28370-2 | Isoform 2 of Probable global transcription activator SNF2L1 | 42 | 9 | 2 | 0.4 |
| P25788-2 | Isoform 2 of Proteasome subunit alpha type-3 | 24 | 24 | 6 | 0.3 |
| Q5VT52-2 | Regulation of nuclear pre-mRNA domain-containing protein 2 | 10 | 5 | 5 | 0.5 |
| Q92900-2 | Isoform 2 of Regulator of nonsense transcripts 1 | 18 | 7 | 6 | 0.4 |
| Q5JTH9-2 | Isoform 2 of RRP12-like protein | 24 | 8 | 8 | 0.5 |
| P16615-2 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 | 43 | 15 | 12 | 0.5 |
| Q9H2G2-2 | Isoform 2 of STE20-like serine/threonine-protein kinase | 11 | 4 | 5 | 0.4 |
| A6NHR9-2 | Structural maintenance of CFH domain-containing protein 1 | 15 | 2 | 4 | 0.3 |
| O14776-2 | Isoform 2 of Transcription elongation regulator 1 | 15 | 4 | 6 | 0.4 |
| P60174-1 | Isoform 2 of Triosephosphateisomerase | 112 | 62 | 14 | 0.3 |
| P07951-2 | Isoform 2 of Tropomyosin beta chain | 21 | 20 | 4 | 0.2 |
| O43399-2 | Isoform 2 of Tumor protein D54 | 19 | 31 | 4 | 0.4 |
| Q9UIG0-2 | Isoform 2 of Tyrosine-protein kinase BAZ1B | 33 | 9 | 12 | 0.4 |
| Q9NYU2-2 | Isoform 2 of UDP-glucose:glycoproteinglucosyltransferase 1 | 27 | 7 | 8 | 0.6 |
| P30622-2 | Isoform 3 of CAP-Gly domain-containing linker protein 1 | 16 | 4 | 6 | 0.4 |
| Q9Y281-3 | Isoform 3 of Cofilin-2 | 11 | 18 | 2 | 0.3 |
| P33993-3 | Isoform 3 of DNA replication licensing factor MCM7 | 18 | 17 | 7 | 0.6 |
| Q14103-3 | Isoform 3 of Heterogeneous nuclear ribonucleoprotein D0 | 23 | 27 | 7 | 0.4 |
| P06756-3 | Isoform 3 of Integrin alpha-V | 111 | 25 | 23 | 0.5 |
| Q7L2E3-3 | Isoform 3 of Putative ATP-dependent RNA helicase DHX30 | 22 | 8 | 8 | 0.4 |
| P08559-3 | Pyruvate dehydrogenase E1 component subunit alpha, | 18 | 14 | 5 | 0.4 |
| Q13813-3 | Isoform 3 of Spectrin alpha chain, non-erythrocytic 1 | 135 | 16 | 34 | 0.4 |
| Q99832-3 | Isoform 3 of T-complex protein 1 subunit eta | 75 | 36 | 14 | 0.5 |
| Q86W42-3 | Isoform 3 of TH | 11 | 24 | 5 | 0.3 |
| Q14669-4 | Isoform 4 of E3 ubiquitin-protein ligase TRIP12 | 18 | 4 | 5 | 0.4 |
| Q8N766-4 | Isoform 4 of ER membrane protein complex subunit 1 | 14 | 5 | 3 | 0.5 |
| P54819-5 | Isoform 5 of Adenylate kinase 2, mitochondrial | 16 | 30 | 5 | 0.3 |
| O75369-6 | Isoform 6 of Filamin-B | 48 | 5 | 8 | 0.5 |
| P27816-6 | Isoform 6 of Microtubule-associated protein 4 | 25 | 10 | 10 | 0.4 |
| Q04637-7 | Isoform 7 of Eukaryotic translation initiation factor 4 gamma 1 | 29 | 8 | 11 | 0.3 |
| Q15149-7 | Isoform 7 of Plectin | 99 | 9 | 34 | 0.4 |
| Q00325-2 | Isoform B of Phosphate carrier protein, mitochondrial | 40 | 15 | 6 | 0.4 |
| P02788-2 | Isoform DeltaLf of Lactotransferrin | 22 | 8 | 5 | 0.4 |
| P31946-2 | Isoform Short of 14–3-3 protein beta/alpha | 48 | 36 | 3 | 0.3 |
| P46013-2 | Isoform Short of Antigen KI-67 | 20 | 4 | 8 | 0.5 |
| O75534-2 | Isoform Short of Cold shock domain-containing protein E1 | 11 | 9 | 6 | 0.6 |
| Q15056-2 | Isoform Short of Eukaryotic translation initiation factor 4H | 10 | 29 | 5 | 0.3 |
| J3KR24 | Isoleucine–tRNA ligase, cytoplasmic | 25 | 6 | 7 | 0.3 |
| P42704 | Leucine-rich PPR motif-containing protein, mitochondrial | 334 | 32 | 43 | 0.5 |
| P00338 | l-lactate dehydrogenase A chain | 15 | 9 | 2 | 0.4 |
| P07195 | l-lactate dehydrogenase B chain | 14 | 9 | 2 | 0.5 |
| O00264 | Membrane-associated progesterone receptor component 1 | 17 | 16 | 3 | 0.6 |
| B4E1E9 | Mitochondrial dicarboxylate carrier | 12 | 19 | 5 | 0.5 |
| Q9BQG0 | Myb-binding protein 1A | 60 | 15 | 18 | 0.5 |
| P19105 | Myosin regulatory light chain 12A | 13 | 19 | 3 | 0.5 |
| P35580 | Myosin-10 | 36 | 5 | 3 | 0.4 |
| P35579 | Myosin-9 | 163 | 20 | 28 | 0.4 |
| O75489 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 3 | 26 | 33 | 7 | 0.5 |
| P48681 | Nestin | 40 | 9 | 13 | 0.4 |
| Q09666 | Neuroblast differentiation-associated protein AHNAK | 17 | 5 | 7 | 0.4 |
| P69849 | Nodal modulator 3 | 25 | 9 | 9 | 0.5 |
| Q14980 | Nuclear mitotic apparatus protein 1 | 130 | 22 | 39 | 0.6 |
| P49790 | Nuclear pore complex protein Nup153 | 16 | 6 | 7 | 0.6 |
| E9PF10 | Nuclear pore complex protein Nup155 | 14 | 5 | 6 | 0.6 |
| Q92621 | Nuclear pore complex protein Nup205 | 20 | 4 | 9 | 0.5 |
| Q8TEM1 | Nuclear pore membrane glycoprotein 210 | 21 | 6 | 8 | 0.5 |
| Q14978 | Nucleolar and coiled-body phosphoprotein 1 | 28 | 17 | 10 | 0.5 |
| P06748 | Nucleophosmin | 113 | 46 | 13 | 0.3 |
| P12270 | Nucleoprotein TPR | 82 | 12 | 28 | 0.6 |
| Q02790 | Peptidyl-prolylcis-trans isomerase FKBP4 | 36 | 25 | 10 | 0.5 |
| P32119 | Peroxiredoxin-2 | 45 | 26 | 5 | 0.5 |
| H7C3T4 | Peroxiredoxin-4 (Fragment) | 36 | 39 | 4 | 0.5 |
| P30041 | Peroxiredoxin-6 | 49 | 24 | 6 | 0.3 |
| O95571 | Persulfidedioxygenase ETHE1, mitochondrial | 12 | 16 | 3 | 0.4 |
| P00558 | Phosphoglycerate kinase 1 | 37 | 30 | 10 | 0.2 |
| P18669 | Phosphoglyceratemutase 1 | 71 | 43 | 9 | 0.3 |
| E9PBS1 | Phosphoribosylaminoimidazole carboxylase (Fragment) | 10 | 9 | 3 | 0.4 |
| O15067 | Phosphoribosylformylglycinamidine synthase | 10 | 6 | 5 | 0.3 |
| Q15102 | Platelet-activating factor acetylhydrolase IB subunit gamma | 24 | 22 | 5 | 0.3 |
| Q15365 | Poly(rC)-binding protein 1 | 35 | 28 | 4 | 0.2 |
| H3BRU6 | Poly(rC)-binding protein 2 (Fragment) | 33 | 30 | 4 | 0.3 |
| O75915 | PRA1 family protein 3 | 13 | 16 | 2 | 0.4 |
| Q6P2Q9 | Pre-mRNA-processing-splicing factor 8 | 61 | 11 | 21 | 0.4 |
| Q8IY81 | pre-rRNA processing protein FTSJ3 | 19 | 8 | 7 | 0.4 |
| P07737 | Profilin-1 | 95 | 65 | 9 | 0.4 |
| P25789 | Proteasome subunit alpha type-4 | 14 | 16 | 5 | 0.3 |
| P28066 | Proteasome subunit alpha type-5 | 18 | 23 | 4 | 0.3 |
| P60900 | Proteasome subunit alpha type-6 | 42 | 35 | 9 | 0.2 |
| P20618 | Proteasome subunit beta type-1 | 17 | 17 | 4 | 0.4 |
| J3KSM3 | Proteasome subunit beta type-3 | 15 | 23 | 2 | 0.4 |
| P28070 | Proteasome subunit beta type-4 | 20 | 19 | 4 | 0.2 |
| P28074 | Proteasome subunit beta type-5 | 11 | 17 | 4 | 0.5 |
| P28072 | Proteasome subunit beta type-6 | 19 | 13 | 3 | 0.4 |
| Q99436 | Proteasome subunit beta type-7 | 11 | 18 | 4 | 0.3 |
| Q14690 | Protein RRP5 homolog | 12 | 3 | 5 | 0.5 |
| Q99584 | Protein S100-A13 | 23 | 48 | 5 | 0.6 |
| P05109 | Protein S100-A8 | 11 | 40 | 4 | 0.6 |
| P06702 | Protein S100-A9 | 22 | 38 | 4 | 0.4 |
| P14618 | Pyruvate kinase PKM | 371 | 64 | 32 | 0.7 |
| P46940 | RasGTPase-activating-like protein IQGAP1 | 65 | 13 | 18 | 0.5 |
| Q15907 | Ras-related protein Rab-11B | 48 | 39 | 9 | 0.6 |
| P61106 | Ras-related protein Rab-14 | 26 | 25 | 4 | 0.3 |
| P62820 | Ras-related protein Rab-1A | 37 | 36 | 6 | 0.5 |
| B4DJA5 | Ras-related protein Rab-5A | 13 | 21 | 2 | 0.4 |
| P51148 | Ras-related protein Rab-5C | 26 | 19 | 2 | 0.3 |
| J3QR09 | Ribosomal protein L19 | 16 | 21 | 4 | 0.1 |
| P38159 | RNA-binding motif protein, X chromosome | 61 | 44 | 17 | 0.6 |
| P49756 | RNA-binding protein 25 | 11 | 7 | 4 | 0.4 |
| Q5QPM1 | RNA-binding protein Raly (Fragment) | 16 | 27 | 5 | 0.5 |
| Q14151 | Scaffold attachment factor B2 | 18 | 4 | 2 | 0.5 |
| P35270 | Sepiapterin reductase | 14 | 33 | 6 | 0.4 |
| B5MCX3 | Septin-2 | 15 | 25 | 6 | 0.2 |
| B4E241 | Serine/arginine-rich-splicing factor 3 | 21 | 25 | 3 | 0.6 |
| P62136 | Serine/threonine-protein phosphatase PP1-alpha catalytic | 21 | 25 | 3 | 0.3 |
| P02768 | Serum albumin | 123 | 43 | 25 | 0.4 |
| Q9Y5M8 | Signal recognition particle receptor subunit beta | 11 | 14 | 3 | 0.4 |
| J3QLE5 | Small nuclear ribonucleoprotein-associated protein N | 21 | 26 | 4 | 0.3 |
| Q01082 | Spectrin beta chain, non-erythrocytic 1 | 62 | 10 | 21 | 0.5 |
| O75533 | Splicing factor 3B subunit 1 | 93 | 19 | 21 | 0.4 |
| Q13435 | Splicing factor 3B subunit 2 | 40 | 10 | 8 | 0.6 |
| Q15393 | Splicing factor 3B subunit 3 | 56 | 10 | 10 | 0.5 |
| B4E1K7 | Stomatin-like protein 2, mitochondrial | 36 | 32 | 7 | 0.5 |
| Q14683 | Structural maintenance of chromosomes protein 1A | 20 | 5 | 7 | 0.4 |
| Q9UQE7 | Structural maintenance of chromosomes protein 3 | 12 | 5 | 5 | 0.4 |
| Q6UWP8 | Suprabasin | 17 | 15 | 2 | 0.4 |
| O60264 | SWI/SNF-related matrix-associated actin-dependent regulator | 73 | 16 | 9 | 0.5 |
| Q92797 | Symplekin | 11 | 4 | 4 | 0.5 |
| Q5TCU6 | Talin-1 | 39 | 6 | 12 | 0.4 |
| P17987 | T-complex protein 1 subunit alpha | 85 | 40 | 19 | 0.6 |
| P40227 | T-complex protein 1 subunit zeta | 97 | 33 | 15 | 0.5 |
| Q9BRA2 | Thioredoxin domain-containing protein 17 | 17 | 33 | 4 | 0.5 |
| E9PH29 | Thioredoxin-dependent peroxide reductase, mitochondrial | 73 | 26 | 6 | 0.6 |
| Q8NI27 | TH | 14 | 5 | 8 | 0.4 |
| Q9Y2W1 | Thyroid hormone receptor-associated protein 3 | 35 | 13 | 10 | 0.3 |
| P37837 | Transaldolase | 19 | 13 | 5 | 0.3 |
| Q01995 | Transgelin | 13 | 35 | 6 | 0.4 |
| P37802 | Transgelin-2 | 15 | 23 | 4 | 0.5 |
| Q92616 | Translational activator GCN1 | 31 | 5 | 11 | 0.4 |
| P09661 | U2 small nuclear ribonucleoprotein A' | 26 | 31 | 7 | 0.5 |
| P08579 | U2 small nuclear ribonucleoprotein B'' | 17 | 24 | 5 | 0.4 |
| O75643 | U5 small nuclear ribonucleoprotein 200 kDa helicase | 67 | 10 | 18 | 0.5 |
| P09936 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | 22 | 26 | 6 | 0.3 |
| D6RDM7 | Ubiquitin-conjugating enzyme E2 K (Fragment) | 13 | 22 | 2 | 0.5 |
| P54727 | UV excision repair protein RAD23 homolog B | 18 | 18 | 7 | 0.5 |
| P26640 | Valine–tRNA ligase | 13 | 4 | 4 | 0.4 |
| O75396 | Vesicle-trafficking protein SEC22b | 15 | 15 | 3 | 0.4 |
| Q00341 | Vigilin | 25 | 8 | 8 | 0.4 |
| P08670 | Vimentin | 1987 | 81 | 43 | 0.4 |
| P13010 | X-ray repair cross-complementing protein 5 | 58 | 17 | 11 | 0.6 |
Fig. 4.
MS/MS spectra for identification of Rab 5C: A MS/MS spectrum of a doubly charged TMT-labeled peptide ion at m/z 764. 9025 for MH22+ corresponding to the mass of the peptide GVDLQENNPASR from Rab 5C. Fig. inserts show peaks of TMT reporter ions of two labeled peptides.
Fig. 5.
Confirmation of differentially expressed proteins by Western and qPCR. A, Western blot analysis of selected proteins from A2780 and A2780-DR cells. B, qPCR analysis of selected genes from A2780 and A2780-DR cells. ** for p value <0.001; and *** for p value <0.001.
Rab 5C Mediated Cisplatin-resistance
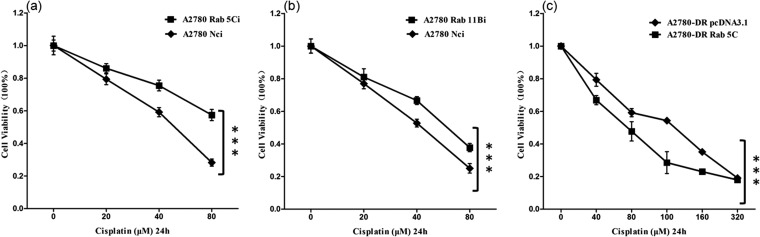
To understand the role of Rab 5C and Rab 11B in drug-resistance, shRNAs were used to silence Rab 5C and Rab 11B in A2780 cells. The plasmid NCi-pll3.7 was also transfected into A2780 as the control to exclude effects of cytotoxicity caused by transfection. The silencing of Rab 5C and Rab 11B was verified by Western blotting and qPCR assays (supplemental Fig. S1). The expression level of Rab 5C mRNA decreased to 30% of that observed for NCi-pll3.7 -transfected cells, whereas the expression level of Rab 11B mRNA decreased to 57% of the control. The sensitivities of shRNA-transfected cells to cisplatin were analyzed by CCK8 assay after cells were treated with different concentrations of cisplatin for 24 h. When cells were treated with 40 μm cisplatin for 24 h, differences in sensitivity to cisplatin were observed among NCi-pll3.7- and Rab 5C shRNA-transfected cells. The change of drug resistance in Rab 11B shRNA-transfected cells is less significant as compared with A2780 cells. Results demonstrate that transfection of A2780 cells with shRNA against Rab 5C increases cell resistance to cisplatin. The IC50 and resistance index values of shRNA-transfected cell lines are 103.3 and 1.89 for Rab 5C, and 62.7 and 1.39 for Rab 11B (Table I).
To further explore Rab 5C mediated drug resistance, Rab 5C was sub-cloned into eukaryotic plasmid pcDNA3.1 that was transfected into A2780-DR cells. The overexpression of Rab 5C in A2780 cells was examined by Western blotting (supplemental Fig. S1C), showing that the expression level of Rab 5C in A2780-DR cells is three times higher than that in empty vector-transfected cells. The sensitivities of Rab 5C overexpressing cells to cisplatin were analyzed by CCK8 assay after cells were treated with different concentrations of cisplatin for 24 h (Fig. 6C), demonstrating that overexpression of Rab 5C in A2780-DR cells increases cell susceptibility to cisplatin.
Fig. 6.
Cell cytotoxicity assays. Percentage of viable A2780, shRNA-transfected A2780, and Rab 5C-pcDNA3.1-transfected cells treated with cisplatin at different concentrations for 24 h determined by using CCK-8 assay. Results are expressed as the mean of three experiments; A, Rab 5Ci; B, Rab 11Bi; and C, Rab 5C-pcDNA3.1. ***p < 0.001.
DISCUSSION
Multidrug resistance is the main reason for the failure of ovarian cancer chemotherapy. The establishment of drug resistant cancer cell lines is an important step in providing an in vitro model for understanding the mechanism of drug resistance and for identifying new therapeutic targets. Cisplatin is a traditional anticancer drug used in clinical settings. In this study, we used human ovarian cell line A2780 as a model system to establish a cisplatin resistant cell line A2780-DR. By the stepwise increase of cisplatin concentration in the growth medium and selection of drug-resistance colonies for six months, we successfully established a cisplatin-resistant cell line with the resistance index 6.76. Using mass spectrometry analysis, it was found that Pt accumulation in A2780-DR cells was only one third of that in A2780 cells, suggesting that a reduction in cellular Pt accumulation is the major cause of drug-resistance in A2780-DR cells.
To identify factors leading to drug resistance, we used TMT labeling to quantify proteins from two types of cells. TMT-labeling uses an isobaric tag with an amine-reactive NHS-ester group, which enables quantitation of two samples with a single LC-MS/MS run that eliminates experimental variation. It also enhances the ionization efficiency of peptides to make them more amenable for MS analysis. We identified about 1900 proteins in three repeated experiments. Among them, 340 proteins were differentially expressed between A2780 and A2780-DR cells, which participate in a variety of cellular processes including cell metabolism, stress responses, cell cycle, and DNA repair. Based on GO analysis, 135 proteins are associated with the metabolic processes including ferredoxin metabolic process (GO:0006124); nitrogen compound metabolic process (GO:0006807); oxygen and reactive oxygen species metabolic process (GO:0006800); phosphate metabolic process (GO:0006796); and primary metabolic process (GO:0044238).
Five out of ten glycolytic proteins were down-regulated including Glucose-6-phosphate isomerase (GPI), fructose-bisphosphate aldolase (ALDO), lactate dehydrogenase (LDH), PGK, and pyruvate kinase (PKM), indicating that glycolysis was down-regulated in drug-resistant cells. This is consistent with an earlier report showing that ALDO and PGK are down-regulated in drug-resistant cells (32). The other proteomic study has also linked the decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer, showing tumors with the lowest PKM2 levels attain the lowest oxaliplatin response rates and the high PKM2 levels are associated with high p53 levels (44). In the present study, the expression level of PKM in A2780-DR cells was about half of that in A2780 cells (Table III) by quantitative proteomic analysis. This was confirmed by Western blotting (Fig. 5). The mRNA level of PKM2 in A2780-DR cells was also down-regulated compared with A2780 cells by qPCR analysis. PKM2 catalyzes the rate-limiting step of glycolysis, in which phosphoenolpyruvate is converted to pyruvate. As a key enzyme for cancer metabolism and tumor growth, PKM2 and other glycolytic enzymes were found to be up-regulated in most cancer cells (45–47). However, our results and previous studies have shown that down-regulation of glycolytic enzymes are a characteristic of drug-resistant ovarian cancer and colorectal cancer cells (32, 44). Although the molecular events leading to down-regulation of glycolytic enzymes are still not clear, we have found that expression levels of c-Myc and HIF1A are down-regulated in drug-resistant cells. Western blots of c-Myc and HIF1A are displayed in supplemental Fig. S2, showing that both c-Myc and HIF1a are down-regulated in A2780-DR cells. c-Myc is an oncogene that regulates transcription of many growth related genes. HIF1A is a master transcriptional regulator of the adaptive response to hypoxia that activates the transcription of glycolytic enzymes. Down-regulation of c-Myc and HIF1A results in decreases in expression levels of glycolytic enzymes that may contribute to drug resistance in ovarian cancer cells.
Cisplatin is a potent electrophile covalently modifying nucleophilic sites on proteins, lipids, DNA, and RNA to generate reactive oxygen species and to induce cell apoptosis. To explore the difference in endogenous ROS levels between A280 and A2780-DR, the intracellular ROS levels were measured with the Image-iT™ LIVE Green Reactive Oxygen Species Detection Kit in both cells. Results show that the ROS level in A2780-DR cells is lower than that in A2780 cells (supplemental Fig. S3), indicating that A2780_DR cells possess a higher capacity to accommodate cisplatin-induced ROS stress. However, quantitative proteomics showed that some redox proteins such as peroxiredoxin-6 (PRDX6) and thioredoxin reductase 1 (TR1) were down-regulated, whereas glutathione reductase (GSR) is up-regulated in A2780-DR cells (Table II). GSR maintains high levels of reduced glutathione in the cytosol, and the up-regulation of GSR may lead to a decrease of ROS in A2780-DR cells. Studies are underway to understand the complex interactions of ROS and the cellular antioxidant system.
On 1D SDS-PAGE (Fig. 2), band 7 has the largest change in intensity, in which vimentin was identified as the major protein. Quantitative proteomics showed that expression of vimentin was decreased 2-fold in A2780-DR cells as compared with A2780 cells, which was confirmed by Western blotting (Fig. 5A). Vimentin is a major intermediate filament protein and is ubiquitously expressed to maintain cellular integrity. Overexpression of vimentin has been observed in various epithelial cancers and correlated with accelerated tumor growth, invasion, and poor prognosis (50). Furthermore, etoposide resistance in neuroblastoma cell and vinca alkaloid resistance in acute lymphoblastic leukemia have been linked to overexpression of vimentin (51–52). However, down-regulation of vimentin has been found in resistant1A9 cells to the microtubule stabilizing agents, PLA and LAU (53), consistent with results from the present study. Therefore, changes in expression levels of vimentin are cancer-type dependent and vimentin is a potential marker for drug-resistance in ovarian cancer.
Decreased cellular drug accumulation is the most commonly observed phenomenon among drug resistant cells. In the present study, we found that cisplatin accumulation was lower in drug-resistant cells (Fig. 1). It is known that cisplatin was not a substrate of P-glycoprotein the key mediator for drug efflux (10–13). Therefore, reduction of cisplatin accumulation in drug-resistant cells may be related to uptake of cisplatin. Uptake of cisplatin can be governed by different mechanisms including passive diffusion, carrier-mediated transporting, and endocytosis (54). An elegant study has shown that several small GTPases (Rab 5, Rac 1, and Rho A) were down-regulated in cisplatin-resistant human hepatoma and epidermal carcinoma cells, demonstrating that GTPase-regulated endocytosis is an important factor in drug-resistance (55). Quantitative proteomic analysis in this study shows that Ras-related proteins Rab 5C and Rab 11B are down-regulated in A2780-DR cells as confirmed by Western blotting and qPCR. Rab 5C is a member of the Rab protein family and a key regulator in endocytosis and early endosome fusion, whereas Rab 11 has been associated with endosome recycling (56). Therefore, down-regulation of Rab 5C and Rab 11B may result in reduced accumulation of cisplatin. To further confirm Rab 5C and Rab 11B mediated drug-resistance, shRNAs against Rab 5C and Rab 11B were used to silence these genes in A2780 cells. As predicted, silencing Rab 5C in A2780 cells lead to increased drug resistance (Fig. 6), but effects of Rab 11B shRNA are less significant (Table I). On the other hand, overexpression of Rab5C in A2780-DR cells increases its sensitivity to cisplatin treatment. These results suggest for the first time that Rab 5C mediated endocytosis regulates drug resistance in ovarian cancer cells.
CONCLUSIONS
Taken together, our results show that multiple cellular processes contribute to drug resistance in ovarian cancer cells. Although increased glycolysis is observed in most cancer cells, glycolytic enzymes PKM2, GPI, ALDO, LDH, and PGK are down-regulated in drug-resistant ovarian cancer cells. Drug resistance is also associated with a decrease of the endogenous ROS level, the up-regulation of GSR, as well as down-regulation of vimentin. Furthermore, the down-regulation of Rab 5C-mediated endocytosis contributes to the reduction of cellular cisplatin accumulation and drug-resistance. These results further our understanding of the multifactorial mechanisms in acquisition and development of cisplatin resistance in human cancer cells.
Supplementary Material
Acknowledgments
We thank the Protein Chemistry Facility at the Center for Biomedical Analysis of Tsinghua University for sample analysis. We thank Dr Zhenyu Zhang for valuable discussions.
Footnotes
Author contributions: Q.L., R.G., and Haiteng Deng designed research; L.J., Y.H., Z.Z., X.J., and Haiyun Deng performed research; L.J., Y.H., Z.Z., Y.C., and Haiteng Deng analyzed data; Haiteng Deng wrote the paper.
* This work was supported in part by NSFC 30871434 (R.S.G.) and NSFC 31270871 (H.T.D), the Chinese Ministry of Science and Technology 2014CBA02005 (H.T.D) and the Global Science Alliance Program of Thermo-Fisher Scientific.
 This article contains supplemental Figs. S1 to S3 and Tables S1 to S3.
This article contains supplemental Figs. S1 to S3 and Tables S1 to S3.
1 The abbreviations used are:
- PGP
- phosphoglycerate kinase
- ROS
- reactive oxygen species
- GO
- Gene Ontology
- PKM
- pyruvate kinase.
REFERENCES
- 1. Bell D., Berchuck A., Birrer M., Chien J., Cramer D., Dao F., Dhir R., Disaia P., Gabra H., Glenn P. (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller D. S., Blessing J. A., Krasner C. N., Mannel R. S., Hanjani P., Pearl M. L., Waggoner S. E., Boardman C. H. (2009) Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J. Clin. Oncol. 27, 2686–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M. J. (2009) Cancer statistics, 2009. CA Cancer J. Clin. 59, 225–249 [DOI] [PubMed] [Google Scholar]
- 4. Fojo A., Hamilton T. C., Young R. C., Ozols R. F. (1987) Multidrug resistance in ovarian cancer. Cancer 60, 2075–2080 [DOI] [PubMed] [Google Scholar]
- 5. Safaei R., Howell S. B. (2005) Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit. Rev. Oncol. Hemat. 53, 13–23 [DOI] [PubMed] [Google Scholar]
- 6. Ishida S., Lee J., Thiele D. J., Herskowitz I. (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. 99, 14298–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Safaei R., Katano K., Samimi G., Naerdemann W., Stevenson J. L., Rochdi M., Howell S. B. (2004) Cross-resistance to cisplatin in cells with acquired resistance to copper. Cancer Chemoth. Pharm. 53, 239–246 [DOI] [PubMed] [Google Scholar]
- 8. Lin X., Okuda T., Holzer A., Howell S. B. (2002) The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol. Pharmacol. 62, 1154–1159 [DOI] [PubMed] [Google Scholar]
- 9. Holzer A. K., Katano K., Klomp L. W., Howell S. B. (2004) Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin. Cancer Res. 10, 6744–6749 [DOI] [PubMed] [Google Scholar]
- 10. Breier A., Gibalova L., Seres M., Barancik M., Sulova Z. (2013) New Insight into P-glycoprotein as a drug target. Anti-Cancer Agent. Me. 13, 159–170 [PubMed] [Google Scholar]
- 11. Cocker H. A., Tiffin N., Pritchard-Jones K., Pinkerton C. R., Kelland L. R. (2001) In vitro prevention of the emergence of multidrug resistance in a pediatric rhabdomyosarcoma cell line. Clin. Cancer Res. 7, 3193–3198 [PubMed] [Google Scholar]
- 12. Yang X., Page M. (1995) P-glycoprotein expression in ovarian cancer cell line following treatment with cisplatin. Oncol. Res. 7, 619–624 [PubMed] [Google Scholar]
- 13. Stordal B., Hamon M., McEneaney V., Roche S., Gillet J.-P., O'Leary J. J., Gottesman M., Clynes M. (2012) Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PLoS One 7, e40717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelland L. (2007) The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584 [DOI] [PubMed] [Google Scholar]
- 15. Sakamoto M., Kondo A., Kawasaki K., Goto T., Sakamoto H., Miyake K., Koyamatsu Y., Akiya T., Iwabuchi H., Muroya T. (2001) Analysis of gene expression profiles associated with cisplatin resistance in human ovarian cancer cell lines and tissues using cDNA microarray. Human Cell 14, 305–315 [PubMed] [Google Scholar]
- 16. Li M., Balch C., Montgomery J. S., Jeong M., Chung J. H., Yan P., Huang T. H., Kim S., Nephew K. P. (2009) Integrated analysis of DNA methylation and gene expression reveals specific signaling pathways associated with platinum resistance in ovarian cancer. BMC Med. Genomics 2, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Godwin A. K., Meister A., O'Dwyer P. J., Huang C. S., Hamilton T. C., Anderson M. E. (1992) High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl. Acad. Sci. 89, 3070–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hector S., Nava M. E., Clark K., Murphy M., Pendyala L. (2007) Characterization of a clonal isolate of an oxaliplatin resistant ovarian carcinoma cell line A2780/C10. Cancer Lett. 245, 195–204 [DOI] [PubMed] [Google Scholar]
- 19. Martin L. P., Hamilton T. C., Schilder R. J. (2008) Platinum resistance: the role of DNA repair pathways. Clin. Cancer Res. 14, 1291–1295 [DOI] [PubMed] [Google Scholar]
- 20. Selvakumaran M., Pisarcik D. A., Bao R., Yeung A. T., Hamilton T. C. (2003) Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 63, 1311–1316 [PubMed] [Google Scholar]
- 21. Dabholkar M., Vionnet J., Bostick-Bruton F., Yu J. J., Reed E. (1994) Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J. Clin. Invest. 94, 703–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohn E. C., Sarosy G., Bicher A., Link C., Christian M., Steinberg S. M., Rothenberg M., Adamo D. O., Davis P., Ognibene F. P. (1994) Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. J. Natl. Cancer I. 86, 18–24 [DOI] [PubMed] [Google Scholar]
- 23. Murphy M. A., Wentzensen N. (2011) Frequency of mismatch repair deficiency in ovarian cancer: a systematic review. Int. J. Cancer 129, 1914–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakai W., Swisher E. M., Karlan B. Y., Agarwal M. K., Higgins J., Friedman C., Villegas E., Jacquemont C., Farrugia D. J., Couch F. J. (2008) Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edwards S. L., Brough R., Lord C. J., Natrajan R., Vatcheva R., Levine D. A., Boyd J., Reis-Filho J. S., Ashworth A. (2008) Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451, 1111–1115 [DOI] [PubMed] [Google Scholar]
- 26. Swisher E. M., Sakai W., Karlan B. Y., Wurz K., Urban N., Taniguchi T. (2008) Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 68, 2581–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ali A. Y., Farrand L., Kim J. Y., Byun S., Suh J. Y., Lee H. J., Tsang B. K. (2012) Molecular determinants of ovarian cancer chemoresistance: new insights into an old conundrum. Ann. NY Acad. Sci. 1271, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stronach E. A., Chen M., Maginn E. N., Agarwal R., Mills G. B., Wasan H., Gabra H. (2011) DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired platinum resistance. Neoplasia 13, 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stronach E. A., Alfraidi A., Rama N., Datler C., Studd J. B., Agarwal R., Guney T. G., Gourley C., Hennessy B. T., Mills G. B. (2011) HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res. 71, 4412–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan X., Pan L., Yuan Y., Lang J., Mao N. (2007) Identification of platinum-resistance associated proteins through proteomic analysis of human ovarian cancer cells and their platinum-resistant sublines. J. Proteome Res. 6, 772–780 [DOI] [PubMed] [Google Scholar]
- 31. Jinawath N., Vasoontara C., Jinawath A., Fang X., Zhao K., Yap K.-L., Guo T., Lee C. S., Wang W., Balgley B. M. (2010) Oncoproteomic analysis reveals co-upregulation of RELA and STAT5 in carboplatin resistant ovarian carcinoma. PLoS One 5, e11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong F., Peng X., Zeng Z., Yu M., Zhao Y., Tong A. (2011) Proteomic analysis of cisplatin resistance in human ovarian cancer using 2-DE method. Mol. Cell. Biochem. 348, 141–147 [DOI] [PubMed] [Google Scholar]
- 33. Di Michele M., Della Corte A., Cicchillitti L., Del Boccio P., Urbani A., Ferlini C., Scambia G., Donati M. B., Rotilio D. (2009) A proteomic approach to paclitaxel chemoresistance in ovarian cancer cell lines. Biochim. Biophys. Acta 1794, 225–236 [DOI] [PubMed] [Google Scholar]
- 34. Stewart J. J., White J. T., Yan X., Collins S., Drescher C. W., Urban N. D., Hood L., Lin B. (2006) Proteins associated with Cisplatin resistance in ovarian cancer cells identified by quantitative proteomic technology and integrated with mRNA expression levels. Mol. Cell. Proteomics 5, 433–443 [DOI] [PubMed] [Google Scholar]
- 35. Cicchillitti L., Di Michele M., Urbani A., Ferlini C., Donati M. B., Scambia G., Rotilio D. (2009) Comparative proteomic analysis of paclitaxel sensitive A2780 epithelial ovarian cancer cell line and its resistant counterpart A2780TC1 by 2D-DIGE: the role of ERp57. J. Proteome Res. 8, 1902–1912 [DOI] [PubMed] [Google Scholar]
- 36. Yang Z., Schumaker L. M., Egorin M. J., Zuhowski E. G., Guo Z., Cullen K. J. (2006) Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin. Cancer Res. 12, 5817–5825 [DOI] [PubMed] [Google Scholar]
- 37. Custódio J., Cardoso C. M., Santos M. S., Almeida L. M., Vicente J. A., Fernandes M. A. (2009) Cisplatin impairs rat liver mitochondrial functions by inducing changes on membrane ion permeability: prevention by thiol group protecting agents. Toxicology 259, 18–24 [DOI] [PubMed] [Google Scholar]
- 38. Saitou M., Isonishi S., Hamada T., Kiyokawa T., Tachibana T., Ishikawa H., Yasuda M. (2009) Mitochondrial ultrastructure-associated chemotherapy response in ovarian cancer. Oncology Reports 21, 199–204 [PubMed] [Google Scholar]
- 39. Liang X., Finkel T., Shen D., Yin J., Aszalos A., Gottesman M. M. (2008) SIRT1 contributes in part to cisplatin resistance in cancer cells by altering mitochondrial metabolism. Mol. Cancer Res, 6, 1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andrews P. A., Albright K. D. (1992) Mitochondrial defects in cis-diamminedichloroplatinum (II)-resistant human ovarian carcinoma cells. Cancer Res. 52, 1895–1901 [PubMed] [Google Scholar]
- 41. Dai Z., Yin J., He H., Li W., Hou C., Qian X., Mao N., Pan L. (2010) Mitochondrial comparative proteomics of human ovarian cancer cells and their platinum-resistant sublines. Proteomics 10, 3789–3799 [DOI] [PubMed] [Google Scholar]
- 42. Chappell N. P., Teng P.-n., Hood B. L., Wang G., Darcy K. M., Hamilton C. A., Maxwell G. L., Conrads T. P. (2012) Mitochondrial proteomic analysis of cisplatin resistance in ovarian cancer. J. Proteome Res. 11, 4605–4614 [DOI] [PubMed] [Google Scholar]
- 43. Minakata K., Nozawa H., Okamoto N., Suzuki O. (2006) Determination of platinum derived from cisplatin in human tissues using electrospray ionization mass spectrometry. J. Chromatogr. B 832, 286–291 [DOI] [PubMed] [Google Scholar]
- 44. Martinez-Balibrea E., Plasencia C., Ginés A., Martinez-Cardús A., Musulén E., Aguilera R., Manzano J. L., Neamati N., Abad A. (2009) A proteomic approach links decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer and in human cell lines. Mol. Cancer Ther. 8, 771–778 [DOI] [PubMed] [Google Scholar]
- 45. Christofk H. R., Vander Heiden M. G., Harris M. H., Ramanathan A., Gerszten R. E., Wei R., Fleming M. D., Schreiber S. L., Cantley L. C. (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 [DOI] [PubMed] [Google Scholar]
- 46. Mazurek S. (2012) Pyruvate kinase M2: a key enzyme of the tumor metabolome and its medical relevance. Biomedical Res. 23, 133–141 [Google Scholar]
- 47. Xu R., Pelicano H., Zhou Y., Carew J. S., Feng L., Bhalla K. N., Keating M. J., Huang P. (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 65, 613–621 [PubMed] [Google Scholar]
- 48. Chung Y., Yoo Y., Park J., Kim Y., Kim H. J. (2000) Increased expression of peroxiredoxin II confers resistance to cisplatin. Anticancer Res. 21, 1129–1133 [PubMed] [Google Scholar]
- 49. Pak J. H., Choi W. H., Lee H. M., Joo W.-D., Kim J.-H., Kim Y.-T., Kim Y.-M., Nam J.-H. (2011) Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Invest. 29, 21–28 [DOI] [PubMed] [Google Scholar]
- 50. Satelli A., Li S. (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 68, 3033–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verrills N. M., Walsh B. J., Cobon G. S., Hains P. G., Kavallaris M. (2003) Proteome analysis of vinca alkaloid response and resistance in acute lymphoblastic leukemia reveals novel cytoskeletal alterations. J. Biol. Chem. 278, 45082–45093 [DOI] [PubMed] [Google Scholar]
- 52. Urbani A., Poland J., Bernardini S., Bellincampi L., Biroccio A., Schnölzer M., Sinha P., Federici G. (2005) A proteomic investigation into etoposide chemo-resistance of neuroblastoma cell lines. Proteomics 5, 796–804 [DOI] [PubMed] [Google Scholar]
- 53. Kanakkanthara A., Rawson P., Northcote P. T., Miller J. H. (2012) Acquired resistance to Peloruside A and laulimalide is associated with down-regulation of vimentin in human ovarian carcinoma cells. Pharm. Res. 29, 3022–3032 [DOI] [PubMed] [Google Scholar]
- 54. Arnesano F., Natile G. (2009) Mechanistic insight into the cellular uptake and processing of cisplatin 30 years after its approval by FDA. Coordin. Chem. Rev. 253, 2070–2081 [Google Scholar]
- 55. Shen D., Su A., Liang X., Pai-Panandiker A., Gottesman M. (2004) Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br. J. Cancer 91, 270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwartz S. L., Cao C., Pylypenko O., Rak A., Wandinger-Ness A. (2007) Rab GTPases at a glance. J. Cell Sci. 120, 3905–3910 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.