Abstract
von Willebrand factor (vWF) is essential for the induction of occlusive thrombosis in stenosed and injured pig arteries and for normal hemostasis. To separate the relative contribution of plasma and platelet vWF to arterial thrombosis, we produced chimeric normal and von Willebrand disease pigs by crossed bone marrow transplantation; von Willebrand disease (vWD) pigs were engrafted with normal pig bone marrow and normal pigs were engrafted with vWD bone marrow. Thrombosis developed in the chimeric normal pigs that showed normal levels of plasma vWF and an absence of platelet vWF; but no thrombosis occurred in the chimeric vWD pigs that demonstrated normal platelet vWF and an absence of plasma vWF. The ear bleeding times of the chimeric pigs were partially corrected by endogenous plasma vWF but not by platelet vWF. Our animal model demonstrated that vWF in the plasma compartment is essential for the development of arterial thrombosis and that it also contributes to the maintenance of bleeding time and hemostasis.
Full text
PDF

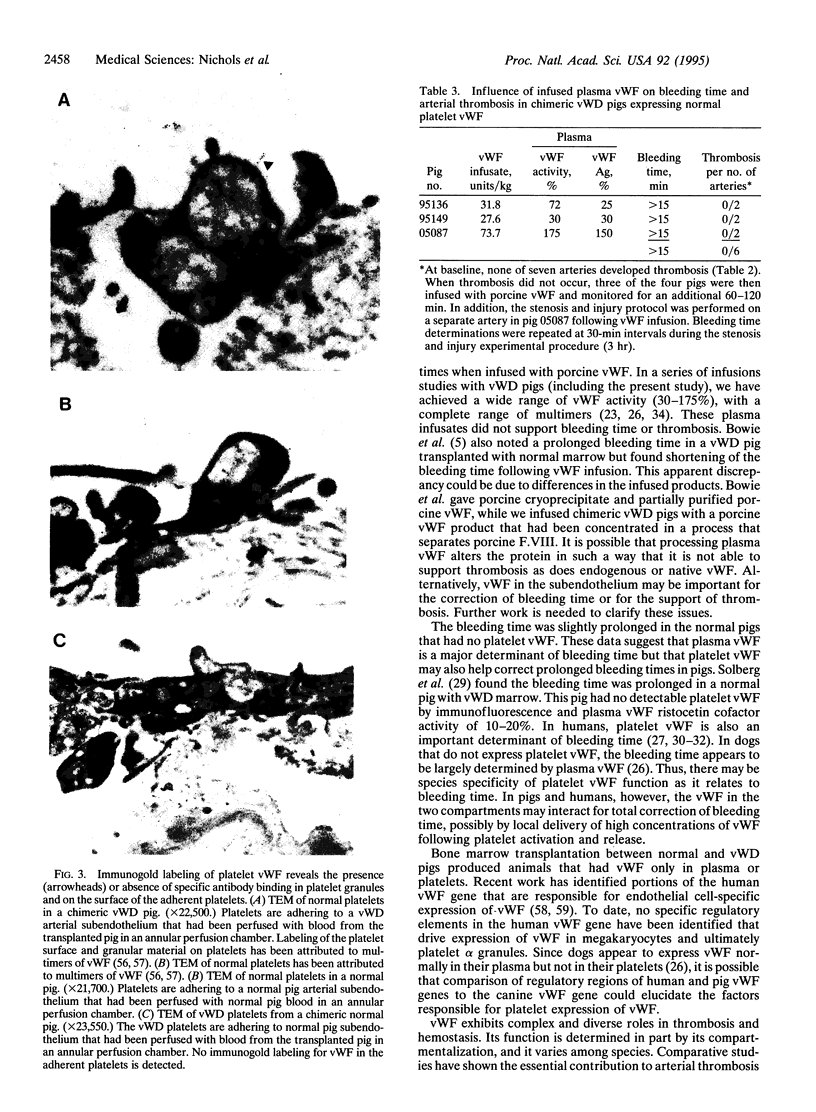

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahnak B. R., Wu Q. Y., Coulombel L., Assouline Z., Kerbiriou-Nabias D., Piétu G., Drouet L., Caen J. P., Meyer D. Expression of von Willebrand factor in porcine vessels: heterogeneity at the level of von Willebrand factor mRNA. J Cell Physiol. 1989 Feb;138(2):305–310. doi: 10.1002/jcp.1041380212. [DOI] [PubMed] [Google Scholar]
- Bellinger D. A., Nichols T. C., Read M. S., Reddick R. L., Lamb M. A., Brinkhous K. M., Evatt B. L., Griggs T. R. Prevention of occlusive coronary artery thrombosis by a murine monoclonal antibody to porcine von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8100–8104. doi: 10.1073/pnas.84.22.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie E. J., Solberg L. A., Jr, Fass D. N., Johnson C. M., Knutson G. J., Stewart M. L., Zoecklein L. J. Transplantation of normal bone marrow into a pig with severe von Willebrand's disease. J Clin Invest. 1986 Jul;78(1):26–30. doi: 10.1172/JCI112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous K. M., Reddick R. L., Read M. S., Nichols T. C., Bellinger D. A., Griggs T. R. von Willebrand factor and animal models: contributions to gene therapy, thrombotic thrombocytopenic purpura, and coronary artery thrombosis. Mayo Clin Proc. 1991 Jul;66(7):733–742. doi: 10.1016/s0025-6196(12)62087-4. [DOI] [PubMed] [Google Scholar]
- Brinkhous K. M., Sandberg H., Garris J. B., Mattsson C., Palm M., Griggs T., Read M. S. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo R., Monteagudo J., Escolar G., Ordinas A., Magallón M., Martín Villar J. Hemostatic effect of normal platelet transfusion in severe von Willebrand disease patients. Blood. 1991 May 1;77(9):1901–1905. [PubMed] [Google Scholar]
- Cramer E. M., Caen J. P., Drouet L., Breton-Gorius J. Absence of tubular structures and immunolabeling for von Willebrand factor in the platelet alpha-granules from porcine von Willebrand disease. Blood. 1986 Sep;68(3):774–778. [PubMed] [Google Scholar]
- Escolar G., White J. G. Organization of von Willebrand factor on surface-activated platelets. Arterioscler Thromb. 1993 Dec;13(12):1852–1858. doi: 10.1161/01.atv.13.12.1852. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Coumans J. V., Walker F. J. von Willebrand factor mediates protection of factor VIII from activated protein C-catalyzed inactivation. J Biol Chem. 1991 Feb 5;266(4):2172–2177. [PubMed] [Google Scholar]
- Ferreira V., Assouline Z., Schwachtgen J. L., Bahnak B. R., Meyer D., Kerbiriou-Nabias D. The role of the 5'-flanking region in the cell-specific transcription of the human von Willebrand factor gene. Biochem J. 1993 Aug 1;293(Pt 3):641–648. doi: 10.1042/bj2930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folts J. D., Crowell E. B., Jr, Rowe G. G. Platelet aggregation in partially obstructed vessels and its elimination with aspirin. Circulation. 1976 Sep;54(3):365–370. doi: 10.1161/01.cir.54.3.365. [DOI] [PubMed] [Google Scholar]
- Gebrane-Younès J., Drouet L., Caen J. P., Orcel L. Heterogeneous distribution of Weibel-Palade bodies and von Willebrand factor along the porcine vascular tree. Am J Pathol. 1991 Dec;139(6):1471–1484. [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Sadler J. E. von Willebrand disease: a database of point mutations, insertions, and deletions. For the Consortium on von Willebrand Factor Mutations and Polymorphisms, and the Subcommittee on von Willebrand Factor of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1993 Feb 1;69(2):177–184. [PubMed] [Google Scholar]
- Gralnick H. R., Rick M. E., McKeown L. P., Williams S. B., Parker R. I., Maisonneuve P., Jenneau C., Sultan Y. Platelet von Willebrand factor: an important determinant of the bleeding time in type I von Willebrand's disease. Blood. 1986 Jul;68(1):58–61. [PubMed] [Google Scholar]
- Griggs T. R., Cooper H. A., Webster W. P., Wagner R. H., Brinkhous K. M. Plasma aggregating factor (bovine) for human platelets: a marker for study of antihemophilic and von Willebrand Factors. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2814–2818. doi: 10.1073/pnas.70.10.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs T. R., Webster W. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Von Willebrand factor: gene dosage relationships and transfusion response in bleeder swine--a new bioassay. Proc Natl Acad Sci U S A. 1974 May;71(5):2087–2090. doi: 10.1073/pnas.71.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Eubanks D. C., Lollar P. von Willebrand factor is a cofactor for thrombin-catalyzed cleavage of the factor VIII light chain. J Biol Chem. 1990 Oct 15;265(29):17854–17858. [PubMed] [Google Scholar]
- Hourdillé P., Gralnick H. R., Heilmann E., Derlon A., Ferrer A. M., Vezon G., Nurden A. T. von Willebrand factor bound to glycoprotein Ib is cleared from the platelet surface after platelet activation by thrombin. Blood. 1992 Apr 15;79(8):2011–2021. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahroudi N., Lynch D. C. Endothelial-cell-specific regulation of von Willebrand factor gene expression. Mol Cell Biol. 1994 Feb;14(2):999–1008. doi: 10.1128/mcb.14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedam J. A., Hamer R. J., Beeser-Visser N. H., Bouma B. N., Sixma J. J. The effect of von Willebrand factor on activation of factor VIII by factor Xa. Eur J Biochem. 1990 Apr 30;189(2):229–234. doi: 10.1111/j.1432-1033.1990.tb15481.x. [DOI] [PubMed] [Google Scholar]
- Koedam J. A., Meijers J. C., Sixma J. J., Bouma B. N. Inactivation of human factor VIII by activated protein C. Cofactor activity of protein S and protective effect of von Willebrand factor. J Clin Invest. 1988 Oct;82(4):1236–1243. doi: 10.1172/JCI113721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelman S. J., Koedam J. A., van Wijnen M., Stern D. M., Nawroth P. P., Sixma J. J., Bouma B. N. von Willebrand factor as a regulator of intrinsic factor X activation. J Lab Clin Med. 1994 Apr;123(4):585–593. [PubMed] [Google Scholar]
- Koutts J., Walsh P. N., Plow E. F., Fenton J. W., 2nd, Bouma B. N., Zimmerman T. S. Active release of human platelet factor VIII-related antigen by adenosine diphosphate, collagen, and thrombin. J Clin Invest. 1978 Dec;62(6):1255–1263. doi: 10.1172/JCI109246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGDELL R. D., WAGNER R. H., BRINKHOUS K. M. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assy procedure. J Lab Clin Med. 1953 Apr;41(4):637–647. [PubMed] [Google Scholar]
- Mannucci P. M., Lombardi R., Castaman G., Dent J. A., Lattuada A., Rodeghiero F., Zimmerman T. S. von Willebrand disease "Vicenza" with larger-than-normal (supranormal) von Willebrand factor multimers. Blood. 1988 Jan;71(1):65–70. [PubMed] [Google Scholar]
- Mannucci P. M., Moia M., Rebulla P., Altieri D., Monteagudo J., Castillo R. Correction of the bleeding time in treated patients with severe von Willebrand disease is not solely dependent on the normal multimeric structure of plasma von Willebrand factor. Am J Hematol. 1987 May;25(1):55–65. doi: 10.1002/ajh.2830250106. [DOI] [PubMed] [Google Scholar]
- Nichols T. C., Bellinger D. A., Johnson T. A., Lamb M. A., Griggs T. R. von Willebrand's disease prevents occlusive thrombosis in stenosed and injured porcine coronary arteries. Circ Res. 1986 Jul;59(1):15–26. doi: 10.1161/01.res.59.1.15. [DOI] [PubMed] [Google Scholar]
- Nichols T. C., Bellinger D. A., Reddick R. L., Smith S. V., Koch G. G., Davis K., Sigman J., Brinkhous K. M., Griggs T. R., Read M. S. The roles of von Willebrand factor and factor VIII in arterial thrombosis: studies in canine von Willebrand disease and hemophilia A. Blood. 1993 May 15;81(10):2644–2651. [PubMed] [Google Scholar]
- Nichols T. C., Bellinger D. A., Tate D. A., Reddick R. L., Read M. S., Koch G. G., Brinkhous K. M., Griggs T. R. von Willebrand factor and occlusive arterial thrombosis. A study in normal and von Willebrand's disease pigs with diet-induced hypercholesterolemia and atherosclerosis. Arteriosclerosis. 1990 May-Jun;10(3):449–461. doi: 10.1161/01.atv.10.3.449. [DOI] [PubMed] [Google Scholar]
- Parker R. I., McKeown L. P., Gallin J. I., Gralnick H. R. Absence of the largest platelet-von Willebrand multimers in a patient with lactoferrin deficiency and a bleeding tendency. Thromb Haemost. 1992 Mar 2;67(3):320–324. [PubMed] [Google Scholar]
- Read M. S., Smith S. V., Lamb M. A., Brinkhous K. M. Role of botrocetin in platelet agglutination: formation of an activated complex of botrocetin and von Willebrand factor. Blood. 1989 Aug 15;74(3):1031–1035. [PubMed] [Google Scholar]
- Reddick R. L., Griggs T. R., Lamb M. A., Brinkhous K. M. Platelet adhesion to damaged coronary arteries: Comparison in normal and von Willebrand disease swine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5076–5079. doi: 10.1073/pnas.79.16.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick R. L., Read M. S., Brinkhous K. M., Bellinger D., Nichols T., Griggs T. R. Coronary atherosclerosis in the pig. Induced plaque injury and platelet response. Arteriosclerosis. 1990 Jul-Aug;10(4):541–550. doi: 10.1161/01.atv.10.4.541. [DOI] [PubMed] [Google Scholar]
- Renard C., Kristensen B., Gautschi C., Hruban V., Fredholm M., Vaiman M. Joint report of the first international comparison test on swine lymphocyte alloantigens (SLA). Anim Genet. 1988;19(1):63–72. doi: 10.1111/j.1365-2052.1988.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Rudek Z., Kwiatkowska L. The possibility of detecting fetal lymphocytes in the maternal blood of the domestic pig, Sus scrofa. Cytogenet Cell Genet. 1983;36(3):580–583. doi: 10.1159/000131976. [DOI] [PubMed] [Google Scholar]
- Samama C. M., Mazoyer E., Bruneval P., Ciostek P., Bonnin P., Bonneau M., Roussi J., Bailliart O., Pignaud G., Viars P. Aprotinin could promote arterial thrombosis in pigs: a prospective randomized, blind study. Thromb Haemost. 1994 May;71(5):663–669. [PubMed] [Google Scholar]
- Smiley R. K., Tittley P., Rock G. Studies on the prolonged bleeding time in von Willebrand's disease. Thromb Res. 1989 Mar 1;53(5):417–426. doi: 10.1016/0049-3848(89)90196-5. [DOI] [PubMed] [Google Scholar]
- Sporn L. A., Chavin S. I., Marder V. J., Wagner D. D. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest. 1985 Sep;76(3):1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham E. G., Lane R. S., Rotblat F., Johnson A. J., Snape T. J., Middleton S., Kernoff P. B. Response to infusions of polyelectrolyte fractionated human factor VIII concentrate in human haemophilia A and von Willebrand's disease. Br J Haematol. 1982 Oct;52(2):259–267. doi: 10.1111/j.1365-2141.1982.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Turitto V. T., Weiss H. J., Zimmerman T. S., Sussman I. I. Factor VIII/von Willebrand factor in subendothelium mediates platelet adhesion. Blood. 1985 Apr;65(4):823–831. [PubMed] [Google Scholar]
- Uchida Y., Yoshimoto N., Murao S. Cyclic fluctuations in coronary blood pressure and flow induced by coronary artery constriction. Jpn Heart J. 1975 Jul;16(4):454–464. doi: 10.1536/ihj.16.454. [DOI] [PubMed] [Google Scholar]
- Vaiman M., Daburon F., Remy J., Villiers P. A., de Riberolles C., Lecompte Y., Mahouy G., Fradelizi D. Allograft tolerance in pigs after fractionated lymphoid irradiation. I. Skin grafts after partial lateral irradiation and bone marrow cell grafting. Transplantation. 1981 May;31(5):358–364. doi: 10.1097/00007890-198105010-00011. [DOI] [PubMed] [Google Scholar]
- Vaiman M. Histocompatibility systems in pigs. Prog Vet Microbiol Immunol. 1988;4:108–133. [PubMed] [Google Scholar]
- Webster W. P., Mandel S. R., Strike L. E., Penick G. D., Griggs T. R., Brinkhous K. M. Factor VIII synthesis: hepatic and renal allografts in swine with von Willebrand's disease. Am J Physiol. 1976 May;230(5):1342–1348. doi: 10.1152/ajplegacy.1976.230.5.1342. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Hoffmann T., Yoshioka A., Ruggeri Z. M. Evidence that the arg1744 gly1745 asp1746 sequence in the GPIIb-IIIa-binding domain of von Willebrand factor is involved in platelet adhesion and thrombus formation on subendothelium. J Lab Clin Med. 1993 Sep;122(3):324–332. [PubMed] [Google Scholar]
- Weiss H. J., Sussman I. I., Hoyer L. W. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand's disease. J Clin Invest. 1977 Aug;60(2):390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J. von Willebrand factor and platelet function. Ann N Y Acad Sci. 1991;614:125–137. doi: 10.1111/j.1749-6632.1991.tb43698.x. [DOI] [PubMed] [Google Scholar]
- Wu Q. Y., Drouet L., Carrier J. L., Rothschild C., Berard M., Rouault C., Caen J. P., Meyer D. Differential distribution of von Willebrand factor in endothelial cells. Comparison between normal pigs and pigs with von Willebrand disease. Arteriosclerosis. 1987 Jan-Feb;7(1):47–54. doi: 10.1161/01.atv.7.1.47. [DOI] [PubMed] [Google Scholar]