Summary
Generation of functional spermatids from azoospermia patients is of unusual significance in the treatment of male infertility. Here, we report an efficient approach to obtain human functional spermatids from cryptorchid patients. Spermatogonia remained whereas meiotic germ cells were rare in cryptorchid patients. Expression of numerous markers for meiotic and postmeiotic male germ cells was enhanced in human spermatogonial stem cells (SSCs) of cryptorchidism patients by retinoic acid (RA) and stem cell factor (SCF) treatment. Meiotic spreads and DNA content assays revealed that RA and SCF induced a remarkable increase of SCP3-, MLH1-, and CREST-positive cells and haploid cells. Single-cell RNA sequencing analysis reflected distinct global gene profiles in embryos derived from round spermatids and nuclei of somatic cells. Significantly, haploid spermatids generated from human SSCs of cryptorchid patients possessed fertilization and development capacity. This study thus provides an invaluable source of autologous male gametes for treating male infertility in azoospermia patients.
Graphical Abstract
Highlights
-
•
Spermatogonia remain whereas meiotic male germ cells are rare in cryptorchid patients
-
•
Human SSCs of cryptorchid patients differentiate into phenotypic haploid spermatids
-
•
Round spermatids derived from human SSCs have fertilization and development capacity
-
•
Distinct gene profiles exist in embryos from round spermatid and somatic cell nuclei
He, Li, and colleagues showed that spermatogonia remained whereas meiotic germ cells were rare or lost in cryptorchid patients. SSCs of cryptorchid patients were induced to differentiate into cells with phenotypic, DNA content, and fertilization and development potentials of haploid spermatids. These data demonstrate the generation of functional and autologous male gametes for treating male infertility in azoospermia patients.
Introduction
Male gametogenesis is a process by which spermatogonial stem cells (SSCs) divide and differentiate into haploid spermatids. Any error during male gametogenesis can result in male infertility, which is a major health problem around the world (De Kretser and Baker, 1999). Infertility affects around 15% of couples, and male factors account for 50% (Schlegel, 2009). Azoospermia has been observed in 1% of the general populations and accounts for 10%–15% of male infertility (Jarow et al., 1989; Willott, 1982). Nonobstructive azoospermia (NOA) affects 10% of infertile men, and notably it has been diagnosed in 60% of azoospermic men (Jarow et al., 1989; Matsumiya et al., 1994). Cryptorchidism is one of the most common causes that result in NOA (Sinnar et al., 2011). Severe cryptorchidism could lead to male infertility, since male germ cells (especially haploid spermatids) are significantly reduced or completely lost in cryptorchid testes (Zivkovic et al., 2009). It has been reported that the transition of gonocytes into Adark spermatogonia in cryptorchid testes is impaired (Kamisawa et al., 2012). Therefore, it is of great significance to establish an effective method to induce differentiation of human spermatogonia from cryptorchid testes into haploid spermatids for the treatment of male infertility. Previous studies have been focused on the in vitro models of male germ cell maturation (Tesarik, 2004). However, there is currently no efficient approach for generating haploid spermatids in vitro from spermatogonia of human testes.
Complete spermatogenesis in vitro to obtain male gametes has not yet been achieved in humans, although certain progress has been made in the derivation of male germ cells from mouse or human embryonic stem cells (ESCs) (Aflatoonian et al., 2009; Chen et al., 2007; Clark et al., 2004; Hübner et al., 2003; Kee et al., 2006; Mikkola et al., 2006; Nayernia et al., 2006; Tilgner et al., 2008; West et al., 2008). There are ethical issues obtaining human ESCs, which is a major obstacle for their potential use in the clinic. It has recently been demonstrated that the induced pluripotent stem cells (iPSCs) could generate primordial germ cells and finally haploid spermatids (Easley et al., 2012; Hayashi et al., 2011; Imamura et al., 2010; Park et al., 2009). Of great concern, male germ cells derived from human iPSCs may not be used for treating male infertility due to tumor-forming risks, which result from the reprogramming of somatic cells by gene transfer using viral vectors and their genetic instability. Therefore, more attention has been paid to generating male gametes from human spermatogonia of patients.
It has been suggested that several growth factors, such as bone morphogenetic proteins (BMPs), glia cell line-derived neurotrophic factor (GDNF), stem cell factor (SCF), and retinoic acid (RA), were crucial for the maintenance of normal spermatogenesis in rodents. The SCF/KIT system plays an essential role in spermatogonial proliferation, differentiation, survival, and subsequent entry into meiosis (Mithraprabhu and Loveland, 2009), and SCF has been shown to induce mouse spermatogonia to differentiate into round spermatids in vitro (Feng et al., 2000). Furthermore, SCF is required for the proliferation of mouse differentiating spermatogonia, specifically type A1 to A4 spermatogonia (Hasthorpe, 2003; Tajima et al., 1994). RA, the active derivative of vitamin A, controls the entry of germ cells into meiosis in both mice and humans (Childs et al., 2011; Ohta et al., 2010). Interestingly, RA could induce the transition of undifferentiated spermatogonia to differentiating spermatogonia and mediates the timing of meiosis by the activation of the SCF/KIT pathway (Pellegrini et al., 2008; Zhou et al., 2008). Therefore, RA and SCF were chosen in this study to induce the differentiation of human spermatogonia from cryptorchid testes. It has been recently reported by our peers and us that human SSCs can be clearly identified and cultured for a short- and long-term period (He et al., 2010; Sadri-Ardekani et al., 2011; Sadri-Ardekani et al., 2009). Round spermatids with unknown function can be derived from mouse spermatogonia (Feng et al., 2002). Nevertheless, the generation of functional haploid spermatids from SSCs in vitro has not yet been achieved in humans. Here, we present molecular and cellular evidence demonstrating the differentiation of human SSCs from cryptorchid patient into cells with phenotypic characteristics, DNA content, and fertilization and development capacity of haploid spermatids. Of unusual significance, our ability to generate human functional haploid spermatids from cryptorchid testes could offer an important source of functional and autologous male gametes for treating male infertility in azoospermia patients.
Results
Cryptorchid Patients Had a Normal Karyotype and Excluded Y Chromosome Microdeletion or Gene Mutation
We first checked the chromosome karyotype and the expression of numerous Y chromosome genes of cryptorchid patients. Karyotype analysis revealed that cryptorchid patients possessed a normal chromosome karyotype (Figure 1A). Multiplex PCR was used to check whether cryptorchid patients had Y chromosome microdeletion. As shown in Figure 1C, numerous Y chromosome genes, including SRY, sY254, sY127, sY86, sY134, sY84, and sY255, were detected in cryptorchid patients, which was comparable to the expression of these genes in normal men (Figure 1B), suggesting that cryptorchid patients did not have Y chromosome microdeletion. Mutation analyses using gene sequencing were performed to screen the mutation of INSL3 (insulin-like 3), RXFP2 (relaxin/insulin-like family peptide receptor 2), and AR (androgen receptor) genes in cryptorchid patients and normal men, and no mutation of those genes was observed (data not shown). Therefore, testicular tissues of these cryptorchid patients were used to induce differentiation.
Figure 1.
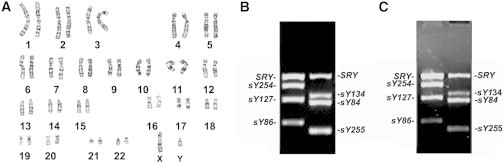
Karyotype and the Completeness of Genomic DNA Sequence of Numerous Y Chromosome Genes in Cryptorchid Patients
(A) Karyotype analysis displaying chromosome karyotype in cryptorchid patients.
(B and C) Multiplex PCR showing the expression of numerous Y chromosome genes, including SRY, sY254, sY127, sY86, sY134, sY84, and sY255, in a normal man (B) and in a cryptorchid patient (C).
See also Table S1.
The clinic data of cryptorchid patients are shown in Table S1 (available online). The levels of testosterone (T) and estradiol (E2) of cryptorchid patients were within the normal ranges. However, both left and right testicular volumes of cryptorchid patients were significantly smaller than those of normal men. The levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin (PRL) in cryptorchid patients were statistically higher than those of normal men.
Human SSCs Remained whereas Meiotic Male Germ Cells Were Very Rare or Lost in the Testes of Cryptorchid Patients
Histological and immunohistochemical analyses of cryptorchid patients were performed to evaluate the spermatogenesis status of testicular tissues. The testes from obstructive azoospermia (OA) patients due to inflammation but with normal spermatogenesis in vivo served as the controls. Histological examination showed that seminiferous tubules of cryptorchid testes had a reduced tubular diameter and a thickened basement membrane (Figure 2B) compared to the control testes (Figure 2A). There were human spermatogonia along the basement membrane in cryptorchid testes (Figure 2B); however, differentiated male germ cells, including spermatocytes and haploid spermatids, were very rare or completely lost in the seminiferous tubules of cryptorchid testes (Figure 2B) compared with the control testes (Figure 2A).
Figure 2.
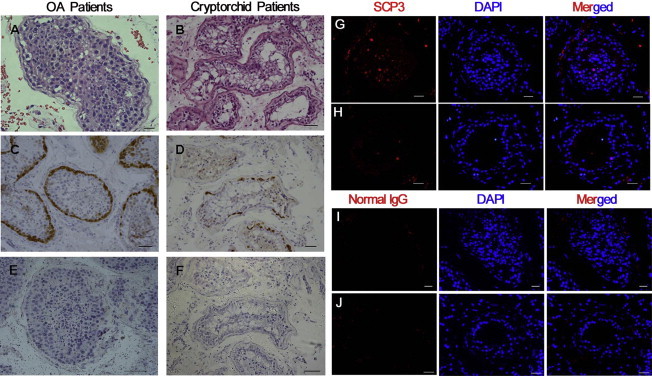
Morphology and MAGEA4 and SCP3 Expression in the Testes of Cryptorchid Patients and OA Patients
(A and B) H&E staining showing the morphology of testicular tissues from OA patients (A) and cryptorchid patients (B). Scale bar in (A), 20 μm, and scale bar in (B), 50 μm.
(C and D) Immunohistochemistry revealing MAGEA4 expression in testis sections from OA patients (C) and cryptorchid patients (D). Scale bars in (C) and (D), 50 μm.
(E and F) Replacement of anti-MAGEA4 by normal mouse IgG in testis sections of OA patients (E) and cryptorchid patients (F) served as negative controls. Scale bars in (E) and (F), 70 μm.
(G and H) Immunohistochemistry revealing SCP3 expression in testis sections from OA patients (G) and cryptorchid patients (H). Scale bars in (G) and (H), 30 μm.
(I and J) Replacement of anti-SCP3 by normal rabbit IgG in testis sections from OA patients (I) and cryptorchid patients (J) was used as negative controls. Scale bars in (I) and (J), 30 μm.
Immunohistochemistry of MAGEA4 (melanoma antigen family A, 4), a marker for human spermatogonia (He et al., 2010), revealed that the number of spermatogonia was diminished in cryptorchid testes (Figure 2D) compared to the control testes (Figure 2C). To verify specific staining of MAGEA4, replacement of primary antibody with normal mouse immunoglobulin G (IgG) was used as a negative control, and no positive reaction was seen in normal testes (Figure 2E) or the testes of cryptorchid patients (Figure 2F). The seminiferous tubules of cryptorchid testis were also characterized by immunostaining with SCP3 (synaptonemal complex protein 3), a specific marker for meiotic germ cells (West et al., 2006). Immunohistochemistry showed that very few cells were positive for SCP3 in the seminiferous tubules of cryptorchid testis (Figure 2H) compared to the control testes (Figure 2G). Replacement of primary antibody with normal rabbit IgG and PBS served as a negative control, and no staining was observed in normal testes (Figure 2I and data not shown) or the testes of cryptorchid patients (Figure 2J and data not shown), which confirmed the specific staining of SCP3. Taken together, these results reflect that spermatogonia remained whereas meiotic male germ cells were rare or lost in the testes of cryptorchid patients.
Generation of Spermatocytes and Haploid Spermatids In Vitro from Human SSCs of Cryptorchid Patients
Human male germ cells were isolated from testis tissues of 16 cryptorchid patients and 9 OA patients using two-step enzymatic digestion followed by differential plating (Figure 3A). Notably, immunocytochemistry showed that almost all of the freshly isolated male germ cells from cryptorchid patients were strongly positive for UCHL1 (Figure 3E) and GFRA1 (Figure 3F). Replacement of primary antibodies with PBS or normal IgG served as a negative control, and no staining was observed (data not shown). UCHL1 has been considered a marker for human spermatogonia, while GFRA1 is regarded as a hallmark for human SSCs (He et al., 2010). Collectively, these data suggest that the freshly isolated male germ cells were phenotypically human SSCs.
Figure 3.
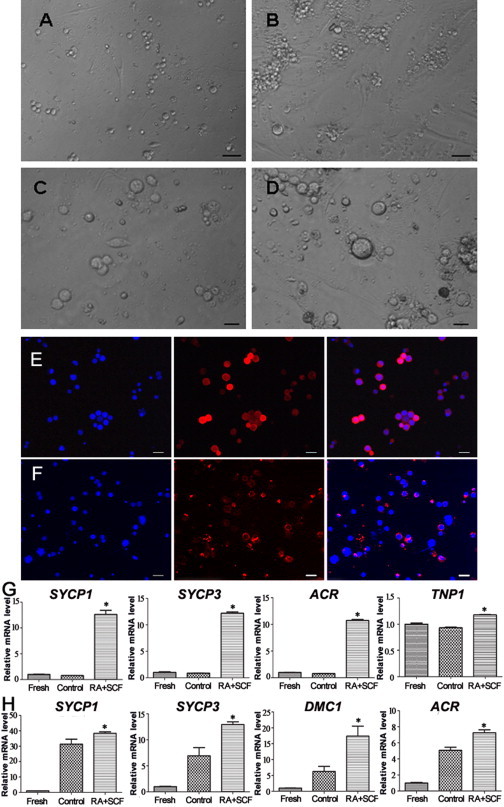
Morphological and Phenotypic Characteristics of Human SSCs without or with SCF and RA Treatment as well as UCHL1 and GFRA1 Expression in the Freshly Isolated Germ Cells
(A–D) Phase-contrast microscopy showing the morphology of the freshly isolated germ cells (A) and human SSCs with SCF and RA treatment (B and D) or without RA and SCF treatment (C). Scale bars in (A) and (B), 50 μm, and scale bars in (C) and (D), 25 μm.
(E and F) Immunocytochemistry revealing the expression of UCHL1 (E) and GFRA1 (F) in the freshly isolated male germ cells from cryptorchid patients. Scale bars in (E) and (F), 10 μm.
(G) Real-time RT-PCR displaying the transcripts of SYCP1 (12.63 ± 0.65, n = 3), SYCP3 (12.20 ± 0.21, n = 3), ACR (10.72 ± 0.16, n = 3), and TNP1 (1.18 ± 0.005, n = 3) in cryptorchid patient SSCs with SCF and RA treatment, as well as the expression of SYCP1 (0.78 ± 0.011, n = 3), SYCP3 (0.88 ± 0.01, n = 3), ACR (0.78 ± 0.02, n = 3), and TNP1 (0.91 ± 0.001, n = 3) in cryptorchid patient SSCs without SCF and RA treatment (control). The expression of SYCP1 (1.00 ± 0.04, n = 3), SYCP3 (1.00 ± 0.11, n = 3), ACR (1.00 ± 0.01, n = 3), and TNP1 (1.00 ± 0.01, n = 3) in freshly isolated male germ cells from cryptorchid patients is also shown.
(H) Real-time RT-PCR showing the transcripts of SYCP1 (38.40 ± 0.77, n = 3), SYCP3 (12.94 ± 0.44, n = 3), ACR (17.37 ± 2.57, n = 3), and TNP1 (7.26 ± 0.21, n = 3) in OA patient SSCs with SCF and RA treatment, as well as the expression of SYCP1 (31.36 ± 2.61, n = 3), SYCP3 (6.92 ± 1.30, n = 3), ACR (6.23 ± 1.31, n = 3), and TNP1 (5.07 ± 0.31, n = 3) in OA patient SSCs without SCF and RA treatment (control). The expression of SYCP1 (1.00 ± 0.01, n = 3), SYCP3 (1.00 ± 0.07, n = 3), ACR (1.00 ± 0.02, n = 3), and TNP1 (1.00 ± 0.06, n = 3) in freshly isolated male germ cells from patients is displayed.
∗p < 0.05 in RA+SCF-treated cells compared with the control. See also Figures S1 and S2 and Table S3.
RA and SCF have been reported to play important roles in promoting spermatogenesis in rodents, and thus they were utilized to induce the differentiation of human SSCs from cryptorchid patients. After 7 days of culture, the isolated human male germ cells were able to proliferate and form colonies of ∼10–50 cells (Figure 3B). The cells in these colonies were human SSCs with proliferation potential (He et al., 2010;Sadri-Ardekani et al., 2011; Sadri-Ardekani et al., 2009), and they were treated without or with RA and SCF for differentiation. Various concentrations of SCF, ranging from 20 ng/ml to 150 ng/ml, were used to optimize the condition for inducing differentiation of human SSCs. RT-PCR assays showed that the expression of SYCP3 (Figure S1A) and ACR (acrosin) (Figure S1B) was the highest in human SSCs treated with 100 ng/ml SCF compared to other concentrations of SCF, and thus 100 ng/ml SCF was used to coax the differentiation of human SSCs. Morphological analysis revealed a different pattern of human SSCs without or with RA and SCF treatment. Interestingly, more male germ cells became enlarged in human SSCs treated with RA and SCF (Figure 3D) compared to the control group without RA or SCF (Figure 3C). Considered together, these data suggest RA and SCF induce both proliferation and differentiation of human SSCs.
We next evaluated the differentiation potential of human SSCs from cryptorchid patients. Real-time RT-PCR revealed that the transcripts of SYCP1, SYCP3, ACR, and TNP1 (transition protein 1), hallmarks of meiotic germ cells and haploid germ cells, respectively (West et al., 2006), were significantly upregulated in cryptorchid patient SSCs with RA and SCF treatment compared to the freshly isolated cells or the control without SCF and RA (Figure 3G). Likewise, the expression of SYCP1, SYCP3, ACR, and DMC1 (DNA meiotic recombinase 1), was remarkably enhanced in human SSCs of OA patients with RA and SCF treatment compared to the control without SCF and RA or the freshly isolated cells (Figure 3H). In addition, RT-PCR and real-time PCR reflected that transcripts of SYCP1, SYCP2, SYCP3, BOULE, PRM1 (protamine 1), PRM2 (protamine 2), TNP1, TNP2 (transition protein 2), and ACR, markers of meiotic cells and haploid spermatids, respectively (Lee et al., 2006; West et al., 2006), were significantly increased in human SSCs of cryptorchid patients with RA and SCF treatment compared to the control without SCF or RA treatment (Figures S2A–S2D), while mRNA levels of SYCP2, TNP1, TNP2, and PRM2 were also enhanced in human SSCs of OA patients with RA and SCF treatment compared to the control without SCF or RA (Figure S2E). These results suggest that RA and SCF induce the differentiation of human SSCs from cryptorchid patients and OA patients into meiotic male germ cells and haploid cells at transcriptional levels.
To determine whether RA and SCF could initiate the meiosis stage of male germ cells, meiotic progression was performed by examining the expression of SCP3, CREST, and MLH1, specific markers for meiosis (Kee et al., 2009; Panula et al., 2011). Meiotic spreads of human SSCs without or with SCF and RA treatment from cryptorchid patients and OA patients were analyzed by immunostaining with antibodies against SCP3 for detecting axial/lateral elements of the synaptonemal complex, MLH1 for measuring the meiotic recombination frequency, and CREST for determining centromeric regions (Holloway et al., 2008). Notably, RA and SCF induced a significantly higher percentage of SCP3-, CREST-, and MLH1-positive cells compared to human SSCs without RA or SCF treatment in cryptorchid patients (Figure 4A and 4D) and OA patients (Figure 4B and 4E). Meiotic spreads of SCP3, CREST, and MLH1 in pachytene spermatocytes from OA and NOA patients served as positive controls (Figure 4C). Together, these data indicate that RA and SCF promote the differentiation of human SSCs of from cryptorchid patients and OA patients into various meiotic stages of male germ cells.
Figure 4.
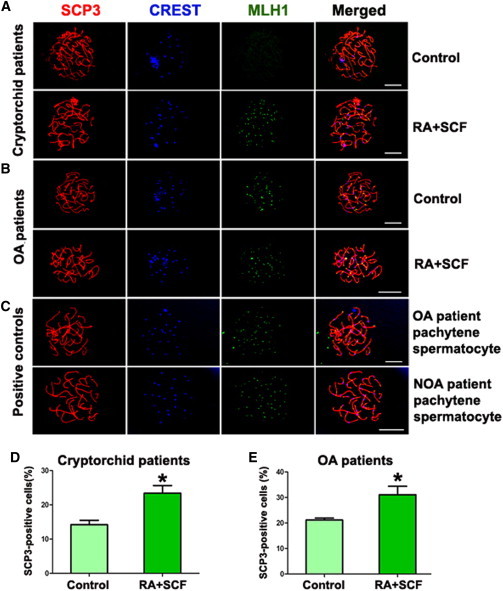
Meiotic Progression of Human SSCs without or with SCF and RA Treatment from Cryptorchid Patients and OA Patients
(A and B) Meiotic spread assays revealing the expression of SCP3 (red fluorescence), CREST (blue fluorescence), and MLH1 (green fluorescence) in human SSCs without or with SCF and RA treatment from cryptorchid patients (A) and OA patients (B). Scale bars in (A) and (B), 10 μm.
(C) The expression of SCP3 (red fluorescence), CREST (blue fluorescence), and MLH1 (green fluorescence) in pachytene spermatocytes from OA and NOA patients served as positive controls. Scale bar in (C), 10 μm.
(D) Meiotic spread assays showing the percentage of SCP3-positive cells in human SSCs with SCF and RA treatment (23.41% ± 2.22%, n = 3) and without SCF and RA treatment (control) (14.20% ± 1.29%, n = 3) from cryptorchid patients.
(E) Meiotic spread assays revealing the percentage of SCP3-positive cells in human SSCs with SCF and RA treatment (27.10% ± 2.69%, n = 3) and without SCF and RA treatment (control) (21.20 ± 0.60%, n = 3) from OA patients.
∗p < 0.05 in RA+SCF-treated cells compared with the control.
Protamine 2 and acrosin are generally regarded as markers for haploid cells. Immunocytochemistry further revealed that protamine 2-positive cells were enhanced in human SSCs with RA and SCF treatment (Figure S3B) compared to the control (Figure S3A). Moreover, acrosin-positive cells were remarkably increased in human SSCs with RA and SCF treatment (Figures S3D and S3E) compared to the control (Figures S3C and S3E). The developmental stages of differentiated cells from human SSCs treated with SCF and RA were shown by expression of acrosin (Figure 5A) and protamine 2 (Figure 5B). Considered together, these results implicate that RA and SCF stimulate the differentiation of human SSCs into haploid spermatids.
Figure 5.
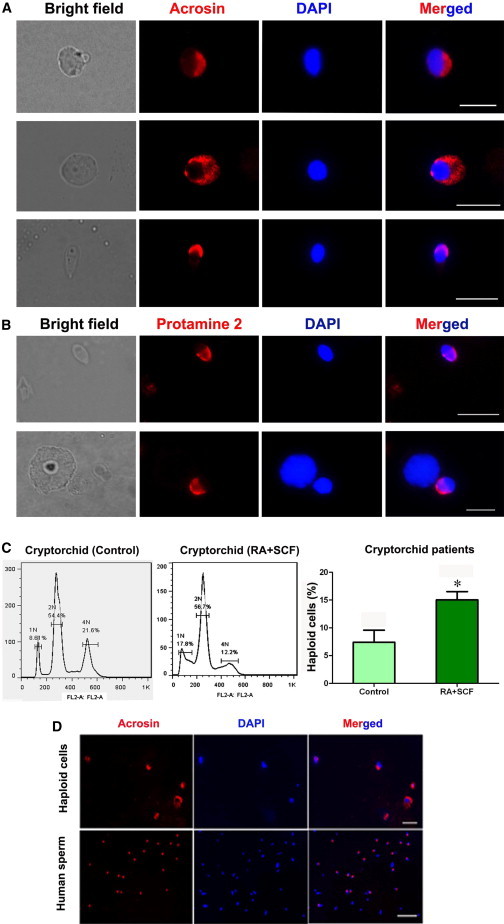
The Expression of Haploid Markers and DNA Content in Human SSCs without or with SCF and RA Treatment from Cryptorchid Patients
(A and B) Immunocytochemistry displaying the expression of acrosin (A) and protamine 2 (B) in differentiated cells from human SSCs with SCF and RA treatment. Scale bars in (A) and (B), 10 μm.
(C) Flow cytometry showing DNA content in the differentiated cells from human SSCs without SCF and RA treatment (7.40% ± 1.77%, n = 3) or with SCF and RA treatment (15.03% ± 1.22%, n = 3) from cryptorchid patients. ∗p < 0.05 in RA+SCF-treated cells compared with the control.
(D) Immunocytochemistry showing acrosin expression in the sorted cells of the differentiated cells from cryptorchid patients (upper panel). The expression of acrosin in donated normal human sperm served as positive controls (lower panel). Scale bar in upper panel, 20 μm, and scale bar in lower panel, 50 μm.
See also Figure S3.
RA and SCF Induced a Notable Increase of Haploid Cell Population of Cryptorchid Patients
To compare the ploidy levels of human SSCs with or without RA and SCF treatment, DNA contents were analyzed by flow cytometry. Notably, haploid cells were increased from 7.4% to 15.03% in human SSCs from cryptorchid patients (Figure 5C) with RA and SCF treatment compared to the control. Cell sorting of haploid cells derived from cryptorchid patients’ SSCs was performed using Hoechst 33342, and immunocytochemistry revealed that over 99% of these cells were positive for acrosin (Figure 5D). These data further suggest that RA and SCF promote the differentiation of human SSCs into haploid spermatids.
Human Round Spermatids Generated from Human SSCs of Cryptorchid Patients and OA Patients Could Fertilize Mouse Oocytes to Form Embryos with Developmental Potentials
Round spermatid microinjection (ROSI) was performed to determine the fertilization capacity of human round spermatids generated from human SSCs of cryptorchid patients and OA patients. The ROSI procedure is shown in Figure S4. Among the microinjection of human 60 round spermatids derived from cryptorchid patients, 60% of them could fertilize mouse oocytes and survived to form embryos with two pronuclei (Figure 6A; Table S2). Notably, 61% of the embryos had the potential to develop to the two-cell stage (Figure 6B; Table S2), and 16.7% and 5.6% of the embryos could develop to four-cell (Figure 6C; Table S2) and eight-cell stages (Figure 6D; Table S2), respectively.
Figure 6.
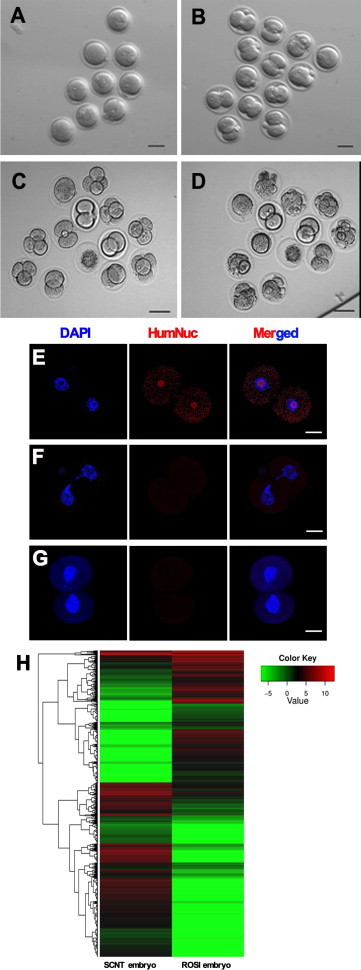
Developmental Potentials, HumNuc Expression, and Global Gene Expression Patterns of the Embryos from Mouse Oocytes Fertilized with Round Spermatids Derived from Human SSCs of Cryptorchid Patients
(A–D) Phase-contrast microscope showing the morphology of embryos with two pronuclei (PN) (A), embryos developing into the two-cell (B), four-cell (C), and eight-cell (D) stages. Scale bars in (A)–(D), 50 μm.
(E) Immunocytochemistry revealing the expression of HumNuc in the embryos from mouse oocytes fertilized with human round spermatids from human SSCs.
(F and G) Replacement of HumNuc antibody with normal rabbit IgG (F) or PBS (G) in the embryos from mouse oocytes fertilized with human round spermatids served as negative controls. Scale bars in (E)–(G), 25 μm.
(H) Clustering of the transcriptome of single embryo derived from round spermatids of cryptorchid patients and from the nucleus of Sertoli cells by single-cell RNA sequencing analysis.
See also Figures S4–S6 and Table S2.
To verify the fertilization of human round spermatids generated from human SSCs of cryptorchid patients with mouse oocytes and exclude parthenogenetic activation of mouse oocytes themselves, immunocytochemistry was carried out using human antibody against HumNuc. The embryos derived from mouse oocytes fertilized with human round spermatids of cryptorchid patients were positive for human anti-HumNuc (Figure 6E). Replacement of human anti-HumNuc with normal rabbit IgG (Figure 6F) or PBS (Figure 6G) in the embryos developed from mouse oocytes fertilized with human round spermatids served as a negative control, and no staining was seen.
Moreover, single-cell RNA sequencing analysis revealed that there were 26,186,786 total reads and 18,722,732 total mapped reads in the embryos derived from round spermatids, while 24,676,032 total reads and 13,036,264 total mapped reads were detected in the embryos generated from the nucleus of Sertoli cells. We identified 9,385 genes in the embryos derived from round spermatids and the nucleus of Sertoli cells (Figure S5A), and there were 5,699 differentially expressed genes (DEGs) (Figure 6H; Figure S5B) and distinct distribution of gene coverage (Figure S5C) between the embryos from round spermatids and the nucleus of Sertoli cells. Functional analysis of these DEGs reflected three major types and numerous subtypes of roles (Figure S5D).
To further observe the dynamic changes during embryo development, H3K9 trimethylation (H3K9-TriM) was applied to label the maternal genomes. As shown in Figure S6, maternal pronuclei were positive for H3K9-TriM, while its staining in male pronucleus was hardly detectable in each group. Sperm chromatin began to decondense immediately after fertilization (Figures S6A and S6B), and male and female pronuclei formed at 4 hr and 6 hr after fertilization (Figures S6C and S6D). At the two-cell stage, both nuclei of embryos stained positively for H3K9-TriM (Figure S6E). Interestingly, the embryos derived from round spermatids showed a different distribution pattern of H3K9-TriM compared to embryos derived from PBS (Figure S6F) or from the nucleus of Sertoli cells with both pseudopronuclei and female pronuclei (Figure S6G). Collectively, these results clearly implicate that human round spermatids derived from human SSCs of cryptorchid patients have both fertilization and development potential.
Discussion
We highlight the generation of human functional haploid spermatids from human SSCs of cryptorchid patients. Cryptorchidism is the most common etiologic factor for azoospermia in adults. Among men with untreated bilateral cryptorchidism, 89% eventually develop azoospermia (Chung and Brock, 2011). However, the exact causes of most cases of cryptorchidism remain unknown (Philibert et al., 2013). Our data indicate that cryptorchid patients have a normal chromosome karyotype with no mutations of INSL3, RXFP2, and AR genes. It has been suggested that a reduced total number of male germ cells in cryptorchid testes is the cause of male infertility (Hadziselimovic and Herzog, 2001). Therefore, in vitro differentiation of human SSCs into haploid spermatids from cryptorchid testes could be an ideal method for treating infertility in cryptorchid patients. Here, we have shown obvious evidence for rescuing germ cell development in cryptorchid patients using in vitro techniques, as evidenced by our finding that human SSCs from cryptorchid patients can progressively differentiate into meiotic and haploid spermatids by treatment with RA and SCF.
We first evaluated the spermatogenesis status of cryptorchid patients using immunohistochemistry with MAGEA4 and SCP3. Notably, human spermatogonia exist whereas meiotic male germ cells are rather rare or completely lost in the testis of cryptorchid patients, since there were numerous cells positive for MAGEA4, a marker for human spermatogonia (He et al., 2010), whereas fewer cells stained positively for SCP3, a hallmark for spermatocytes (West et al., 2006). This conclusion can also be verified by our observations that almost all freshly isolated male germ cells from cryptorchid patients were positive for UCHL1, a marker for human spermatogonia (He et al., 2010), and GFRA1, a surface hallmark for human SSCs (He et al., 2010). Our results are consistent with previous findings showing defective maturation of germ cells in cryptorchid patients (Agoulnik et al., 2012; Huff et al., 2001).
Using multiplex PCR, we found that a number of Y chromosome genes, including SRY, sY254, sY127, sY86, sY134, sY84, and sY255, were present in cryptorchid patients, thus excluding Y chromosome microdeletion in cryptorchid patients. Therefore, testis tissues of these cryptorchid patients were chosen for differentiating into spermatocytes and haploid spermatids.
The starting cells we used for differentiation were colony cells. We found that isolated cells from cryptorchid testes were able to proliferate and form colonies composed of numerous cells. We and others have revealed that the cells in these colonies were SSCs with proliferation potential (He et al., 2010; Sadri-Ardekani et al., 2009). It has been demonstrated by xenotransplantation that the cells in the colonies were actually human SSCs with self-renewal capacity (Sadri-Ardekani et al., 2009). The differentiation potential of human SSCs from cryptorchid testes was assessed by various types of approaches, including quantitative PCR, RT-PCR, immunocytochemistry, and meiotic spread assays. After treatment with RA and SCF, the expression of numerous genes for meiotic and haploid cells, including SYCP1, SYCP2, SYCP3, BOULE, PRM1, PRM2, TNP1, TNP2, and ACR (Holloway et al., 2008; Tedesco et al., 2011; West et al., 2006), in human SSCs was obviously enhanced. SCP3 can be used to measure the synaptonemal complex, while CREST is a hallmark for detecting centromeric regions and MLH1 has been utilized for measuring meiotic recombination frequency (Holloway et al., 2008). Our results, using these markers for meiosis and postmeiosis, clearly indicate that RA and SCF could induce human spermatogonia to enter the postmeiotic stage and eventually differentiate into haploid spermatids. RA has been shown to play an important role in triggering germ cells to enter meiosis (Niederreither and Dollé, 2008). We have previously reported that RA can act as a meiosis-inducing factor in the differentiation of iPSCs into male germ cells (Yang et al., 2012). SCF has been shown to be essential in spermatogonial differentiation as well as meiotic initiation (Feng et al., 2000). The SCF/KIT interaction plays a critical role in meiotic entry of differentiating spermatogonia (Rossi et al., 2008). Furthermore, the crosstalk between RA and the SCF pathway could stimulate differentiation of male germ cells toward the meiotic stages (Pellegrini et al., 2008). Consistent with these findings, we found that RA and SCF could efficiently induce the differentiation of human SSCs from cryptorchid testes into postmeiotic male germ cells.
We further explored the ploidy levels of human SSCs with RA and SCF treatment by detecting DNA content. Notably, the percentage of haploid cells was significantly increased in human SSCs by RA and SCF treatment, although spontaneous differentiation of SSCs into haploid cells was observed in control samples without RA or SCF induction. As such, RA and SCF promote the differentiation of human SSCs into haploid spermatids. The SCF/KIT signaling pathway has been proven to be essential for human ESCs to differentiate into human germ cells (West et al., 2010). Additionally, RA has a crucial role in pushing complete meiosis and generating haploid cells from mouse ESCs and human iPSCs (Eguizabal et al., 2011; Nayernia et al., 2006; Riboldi et al., 2012). Taken together, these studies from our peers and us suggest that RA and SCF are effective to coax the second meiosis of germ cells into haploid spermatids. It remains unknown whether round spermatids derived from mouse spermatogonia have fertilization ability (Feng et al., 2002). Of unusual significance, haploid spermatids generated from human SSCs of cryptorchid patients had the potential to fertilize oocytes to form embryos that were capable of developing into eight-cell stages.
In summary, we have demonstrated that RA and SCF are effective in promoting the differentiation of human SSCs from cryptorchid patients into cells with phenotypic features, DNA content, and fertilization and development capacity of haploid spermatids. This study thus offers an approach to generate human functional haploid spermatids from cryptorchid testes, which could provide autologous male gametes for clinical treatment of infertile cryptorchid patients using assisted reproductive technology.
Experimental Procedures
Procurement of Testicular Biopsy Specimens from Cryptorchid Patients and Obstructive Azoospermia Patients
Testicular biopsy specimens were obtained from 16 cryptorchid patients and 9 obstructive azoospermia (OA) patients 13 to 47 years of age (28.93 ± 2.25 years) from January 2012 to June 2014. Cryptorchid patients were selected by excluding abnormal karyotype; Y chromosome microdeletion; gene mutation of INSL3, RXFP2, and AR; and Sertoli cell-only syndrome. Clinical data of cryptorchid patients are shown in Table S1. OA patients with normal spermatogenesis in vivo were used as controls. All OA was caused by inflammation and vasoligation, but not by congenital absence of the vas deferens or other diseases including cancer. This study was approved by the institutional ethical review committee of Ren Ji Hospital (license number of ethics statement: 2012-01), Shanghai Jiao Tong University School of Medicine. The collected testis tissues were immediately placed aseptically in Dulbecco’s modified Eagle’s medium (DMEM) containing penicillin and streptomycin (Gibco).
Karyotype Analysis
Chromosomal karyotype analysis of peripheral blood lymphocytes from cryptorchid patients was performed, and the karyotypes were interpreted using the recommendation of the International System for Human Cytogenetic Nomenclature.
Multiplex PCR
Peripheral venous blood was obtained from cryptorchid patients and normal men. Multiplex PCR was performed to check the expression of numerous Y chromosome genes, including SRY, sY254, sY127, sY86, sY134, sY84, and sY255, according to a procedure we described previously (Ma et al., 2013). The primers of chosen genes were shown previously (Sun et al., 2012), and PCR without primers served as negative controls.
Histological Examination
Testicular tissues from cryptorchid patients and OA patients were fixed in 4% paraformaldehyde for 3 hr or in Bouin’s fixative overnight, embedded in paraffin, and sectioned at 5 μm thickness. The sections were stained with hematoxylin and eosin (H&E) and observed under a microscope.
Isolation and Culture of Male Germ Cells from Cryptorchid Patients
The seminiferous tubules were isolated from testicular tissues of 16 cryptorchid patients and nine OA patients using the first enzymatic digestion including collagenase IV (Sigma) and DNase I using a procedure previously described (He et al., 2010). Human testicular cells were obtained using a second enzymatic digestion with collagenase IV, hyaluronidase (Sigma), trypsin (Sigma), and DNase I and followed by differential plating to remove human Sertoli cells using a procedure previously described (He et al., 2010). For differential plating, cell suspensions were seeded into culture plates in DMEM/F-12 (Gibco) supplemented with 10% fetal bovine serum (FBS; Hyclone) and incubated at 34°C in 5% CO2 for 3 hr. After incubation, Sertoli cells attached to culture plates, while male germ cells were suspended and collected by centrifuge. Male germ cells were cultured in DMEM/F12 containing 10% FBS, 2 μM RA (Sigma), and 20–150 ng/ml SCF (Peprotech) for 7 to 10 days. The cells cultured with DMEM/F12 and 10% FBS but without RA or SCF were used as controls. All cultures were maintained at 34°C in a humidified 5% CO2 incubator.
Meiotic Spread Assays
Meiotic spread assays were performed to determine the meiotic progression in human SSCs of cryptorchid patients and OA patients without or with RA and SCF treatment as well as in pachytene spermatocytes of OA and NOA patients according to a method described previously (Panula et al., 2011). Briefly, cells were lysed by a hypotonic solution and spread evenly over slides layered with 1% paraformaldehyde (PFA) and 0.15% Triton X-100. Slides were dried for 24 hr at room temperature in a humid chamber. The cells were treated with 0.04% photoflo for 5 min and blocked with 4% goat serum. Triple staining was performed in cells incubated with primary antibodies, including rabbit polyclonal to SCP3 (Abcam), human anti-centromere (CREST) (Immunovision), and mouse MLH1 monoclonal antibody (Abcam), overnight at 37°C in a humid chamber. Goat anti-rabbit Alexa Fluor 555 (Invitrogen), AMCA-AffiniPure donkey anti-human IgG, and goat anti-mouse Alexa 488 secondary antibodies (Jackson) were applied at 1:1,000 dilution and incubated for 90 min at 37°C. Cells were washed three times with PBS, and images were captured with a fluorescence microscope (Leica).
Flow Cytometry and Isolation of 1N Haploid Cells
Flow cytometry was performed to measure DNA content of human SSCs of cryptorchid patients and OA patients without or with RA and SCF treatment. In brief, cells were washed twice in PBS and fixed in cold 70% ethanol. After being stained in a solution containing 25 μg/ml propidium iodide (PI) (Sigma), 40 μg/ml RNase (Invitrogen), and 0.3% Tween-20 in PBS at room temperature for 20 min, cells were analyzed with a FACSCalibur system (Becton Dickinson).
To isolate 1N haploid cells, cells was stained with 10 μg/ml Hoechst 33342 (Sigma) in culture medium at 34°C for 60 min. The haploid 1N peak was collected and fixed with 4% PFA before immunostaining.
ROSI Derived from Human SSCs of Cryptorchid and OA Patients into Mouse Oocytes
Microinjection of round spermatids (ROSI) was performed to detect the fertilization capacity of round spermatids derived from human SSCs of cryptorchid and OA patients using a procedure described previously (Li et al., 2010). Female B6D2F1 mice were superovulated by the injection of 5 IU equine chorionic gonadotropin followed by 5 IU human chorionic gonadotropin (hCG) 48 hr later. Cumulus-oocyte complexes were collected from oviducts at 14–16 hr after hCG injection, and they were placed in HEPES Chatot-Ziomek-Bavister (CZB) medium and treated with 0.1% hyaluronidase to disperse cumulus cells. The oocytes were washed twice and then moved to a new 20 μl CZB drop for culture. Oocytes were incubated for at least 15 min after collection in plain CZB media, and they were placed into Ca2+-free CZB containing 10 mM SrCl2 for 1 hr in order to activate the oocytes artificially. After being washed twice, oocytes were transferred to CZB medium to resume incubation. Human round spermatids were collected from fresh testicular cells of obstructive azoospermia patients or human SSCs of cryptorchid patients with RA and SCF treatment. Round spermatids can easily be recognized by their small size and the presence of a round nucleus with a centrally located nucleolus (Yazawa et al., 2007). Human Sertoli cells were isolated from OA patients using a two-step enzymatic digestion and differential plating according to a procedure described previously (He et al., 2010). ROSI was performed using a micromanipulator with piezoelectric elements, and injection of the nucleus of human Sertoli cells or PBS into oocytes served as controls. The oocytes fertilized with human round spermatids from cryptorchid patients and OA patients were incubated in CZB medium at 37°C under 5% CO2 in air to examine pronucleus formation and in vitro development.
Single-Cell RNA Sequencing Analysis of Embryos Derived from Round Spermatids of Cryptorchid Patients and the Nucleus of Sertoli Cells
To compare global gene expression, single-cell RNA sequencing analysis of embryos derived from round spermatids of cryptorchid patients and the nucleus of Sertoli cells was performed according to a procedure described previously (Yan et al., 2013). Briefly, single blastomeres were isolated from embryos derived from the microinjection of round spermatids or the nucleus of Sertoli cells to mouse oocytes after removing the zona pellucida using acidic Tyrode solution (Sigma), and total RNA was extracted from the blastomeres using lysate buffer and mRNA was enriched by Oligo(dT) and followed by mRNA fragment. The cDNA was synthesized by using random hexamer-primer and dinucleotide triphosphates and size selected, and PCR amplification was performed. Agilent 2100 Bioanaylzer and ABI StepOnePlus Real-Time PCR System were used to qualify and quantify of the sample library. The library products were used for Illumina sequencing (Beijing Genomics Institute, Shenzhen, China).
Statistical Analysis
All data are presented as mean ± SEM. Experiments with samples from three patients were performed three times unless otherwise stated, and the number of independent experiments performed is given as “n” in the figure legends. Statistical analyses were performed using Student’s t test, and p < 0.05 was considered statistically significant.
Acknowledgments
We thank Professor Giulio C. Spagnoli (University Hospital of Basel, Switzerland) for providing an antibody to MAGEA4. We are grateful to Dr. Chong Li, associate professor at Tong Ji University, China, for his kind assistance in round spermatid microinjection (ROSI). This study was supported by key grants from National Natural Science Foundation of China (31230048) and Chinese Ministry of Science and Technology (2013CB947901, 2014CB943101, and 2010CB945200), grants from National Natural Science Foundation of China (31201109 and 31171422), The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, a key grant from the Science and Technology Commission of Shanghai Municipality (12JC1405900), Key Discipline and Specialty Foundation of Shanghai Municipal Commission of Health and Family Planning, and the Shanghai Pujiang Program (11PJ1406400).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Contributor Information
Zheng Li, Email: lizhengboshi@163.com.
Zuping He, Email: zupinghe@sjtu.edu.cn.
Accession Numbers
Sequence data for the sequences of embryos derived from round spermatids of human SSCs and the nucleus of human Sertoli cells reported in this paper have been deposited to the NCBI Sequence Read Archive (SRA) under accession number SRP044280.
Supplemental Information
References
- Aflatoonian B., Ruban L., Jones M., Aflatoonian R., Fazeli A., Moore H.D. In vitro post-meiotic germ cell development from human embryonic stem cells. Hum. Reprod. 2009;24:3150–3159. doi: 10.1093/humrep/dep334. [DOI] [PubMed] [Google Scholar]
- Agoulnik A.I., Huang Z., Ferguson L. Spermatogenesis in cryptorchidism. Methods Mol. Biol. 2012;825:127–147. doi: 10.1007/978-1-61779-436-0_11. [DOI] [PubMed] [Google Scholar]
- Chen H.F., Kuo H.C., Chien C.L., Shun C.T., Yao Y.L., Ip P.L., Chuang C.Y., Wang C.C., Yang Y.S., Ho H.N. Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum. Reprod. 2007;22:567–577. doi: 10.1093/humrep/del412. [DOI] [PubMed] [Google Scholar]
- Childs A.J., Cowan G., Kinnell H.L., Anderson R.A., Saunders P.T. Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE. 2011;6:e20249. doi: 10.1371/journal.pone.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Brock G.B. Cryptorchidism and its impact on male fertility: a state of art review of current literature. Can Urol Assoc J. 2011;5:210–214. doi: 10.5489/cuaj.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.T., Bodnar M.S., Fox M., Rodriquez R.T., Abeyta M.J., Firpo M.T., Pera R.A. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 2004;13:727–739. doi: 10.1093/hmg/ddh088. [DOI] [PubMed] [Google Scholar]
- De Kretser D.M., Baker H.W. Infertility in men: recent advances and continuing controversies. J. Clin. Endocrinol. Metab. 1999;84:3443–3450. doi: 10.1210/jcem.84.10.6101. [DOI] [PubMed] [Google Scholar]
- Easley C.A., 4th, Phillips B.T., McGuire M.M., Barringer J.M., Valli H., Hermann B.P., Simerly C.R., Rajkovic A., Miki T., Orwig K.E., Schatten G.P. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Reports. 2012;2:440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal C., Montserrat N., Vassena R., Barragan M., Garreta E., Garcia-Quevedo L., Vidal F., Giorgetti A., Veiga A., Izpisua Belmonte J.C. Complete meiosis from human induced pluripotent stem cells. Stem Cells. 2011;29:1186–1195. doi: 10.1002/stem.672. [DOI] [PubMed] [Google Scholar]
- Feng L.X., Ravindranath N., Dym M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J. Biol. Chem. 2000;275:25572–25576. doi: 10.1074/jbc.M002218200. [DOI] [PubMed] [Google Scholar]
- Feng L.X., Chen Y., Dettin L., Pera R.A., Herr J.C., Goldberg E., Dym M. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- Hadziselimovic F., Herzog B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet. 2001;358:1156–1157. doi: 10.1016/S0140-6736(01)06274-2. [DOI] [PubMed] [Google Scholar]
- Hasthorpe S. Clonogenic culture of normal spermatogonia: in vitro regulation of postnatal germ cell proliferation. Biol. Reprod. 2003;68:1354–1360. doi: 10.1095/biolreprod.102.008458. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Ohta H., Kurimoto K., Aramaki S., Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–532. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- He Z., Kokkinaki M., Jiang J., Dobrinski I., Dym M. Isolation, characterization, and culture of human spermatogonia. Biol. Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway J.K., Booth J., Edelmann W., McGowan C.H., Cohen P.E. MUS81 generates a subset of MLH1-MLH3-independent crossovers in mammalian meiosis. PLoS Genet. 2008;4:e1000186. doi: 10.1371/journal.pgen.1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner K., Fuhrmann G., Christenson L.K., Kehler J., Reinbold R., De La Fuente R., Wood J., Strauss J.F., 3rd, Boiani M., Schöler H.R. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- Huff D.S., Fenig D.M., Canning D.A., Carr M.G., Zderic S.A., Snyder H.M., 3rd Abnormal germ cell development in cryptorchidism. Horm. Res. 2001;55:11–17. doi: 10.1159/000049957. [DOI] [PubMed] [Google Scholar]
- Imamura M., Aoi T., Tokumasu A., Mise N., Abe K., Yamanaka S., Noce T. Induction of primordial germ cells from mouse induced pluripotent stem cells derived from adult hepatocytes. Mol. Reprod. Dev. 2010;77:802–811. doi: 10.1002/mrd.21223. [DOI] [PubMed] [Google Scholar]
- Jarow J.P., Espeland M.A., Lipshultz L.I. Evaluation of the azoospermic patient. J. Urol. 1989;142:62–65. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- Kamisawa H., Kojima Y., Mizuno K., Imura M., Hayashi Y., Kohri K. Attenuation of spermatogonial stem cell activity in cryptorchid testes. J. Urol. 2012;187:1047–1052. doi: 10.1016/j.juro.2011.10.170. [DOI] [PubMed] [Google Scholar]
- Kee K., Gonsalves J.M., Clark A.T., Pera R.A. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- Kee K., Angeles V.T., Flores M., Nguyen H.N., Reijo Pera R.A. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Kim H.J., Kim H., Lee S.J., Gye M.C. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006;27:2845–2853. doi: 10.1016/j.biomaterials.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Li C., Mizutani E., Ono T., Wakayama T. An efficient method for generating transgenic mice using NaOH-treated spermatozoa. Biol. Reprod. 2010;82:331–340. doi: 10.1095/biolreprod.109.078501. [DOI] [PubMed] [Google Scholar]
- Ma M., Yang S., Zhang Z., Li P., Gong Y., Liu L., Zhu Y., Tian R., Liu Y., Wang X. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype. Hum. Reprod. 2013;28:1863–1873. doi: 10.1093/humrep/det068. [DOI] [PubMed] [Google Scholar]
- Matsumiya K., Namiki M., Takahara S., Kondoh N., Takada S., Kiyohara H., Okuyama A. Clinical study of azoospermia. Int. J. Androl. 1994;17:140–142. doi: 10.1111/j.1365-2605.1994.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Mikkola M., Olsson C., Palgi J., Ustinov J., Palomaki T., Horelli-Kuitunen N., Knuutila S., Lundin K., Otonkoski T., Tuuri T. Distinct differentiation characteristics of individual human embryonic stem cell lines. BMC Dev. Biol. 2006;6:40. doi: 10.1186/1471-213X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithraprabhu S., Loveland K.L. Control of KIT signalling in male germ cells: what can we learn from other systems? Reproduction. 2009;138:743–757. doi: 10.1530/REP-08-0537. [DOI] [PubMed] [Google Scholar]
- Nayernia K., Nolte J., Michelmann H.W., Lee J.H., Rathsack K., Drusenheimer N., Dev A., Wulf G., Ehrmann I.E., Elliott D.J. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev. Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Niederreither K., Dollé P. Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Ohta K., Lin Y., Hogg N., Yamamoto M., Yamazaki Y. Direct effects of retinoic acid on entry of fetal male germ cells into meiosis in mice. Biol. Reprod. 2010;83:1056–1063. doi: 10.1095/biolreprod.110.085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula S., Medrano J.V., Kee K., Bergström R., Nguyen H.N., Byers B., Wilson K.D., Wu J.C., Simon C., Hovatta O., Reijo Pera R.A. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum. Mol. Genet. 2011;20:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T.S., Galic Z., Conway A.E., Lindgren A., van Handel B.J., Magnusson M., Richter L., Teitell M.A., Mikkola H.K., Lowry W.E. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M., Filipponi D., Gori M., Barrios F., Lolicato F., Grimaldi P., Rossi P., Jannini E.A., Geremia R., Dolci S. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle. 2008;7:3878–3888. doi: 10.4161/cc.7.24.7262. [DOI] [PubMed] [Google Scholar]
- Philibert P., Boizet-Bonhoure B., Bashamboo A., Paris F., Aritake K., Urade Y., Leger J., Sultan C., Poulat F. Unilateral cryptorchidism in mice mutant for Ptgds. Hum. Mutat. 2013;34:278–282. doi: 10.1002/humu.22231. [DOI] [PubMed] [Google Scholar]
- Riboldi M., Rubio C., Pellicer A., Gil-Salom M., Simón C. In vitro production of haploid cells after coculture of CD49f+ with Sertoli cells from testicular sperm extraction in nonobstructive azoospermic patients. Fertil. Steril. 2012;98:580–590, e4. doi: 10.1016/j.fertnstert.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Rossi P., Lolicato F., Grimaldi P., Dolci S., Di Sauro A., Filipponi D., Geremia R. Transcriptome analysis of differentiating spermatogonia stimulated with kit ligand. Gene Expr. Patterns. 2008;8:58–70. doi: 10.1016/j.modgep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H., Mizrak S.C., van Daalen S.K., Korver C.M., Roepers-Gajadien H.L., Koruji M., Hovingh S., de Reijke T.M., de la Rosette J.J., van der Veen F. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H., Akhondi M.A., van der Veen F., Repping S., van Pelt A.M. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305:2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- Schlegel P.N. Evaluation of male infertility. Minerva Ginecol. 2009;61:261–283. [PubMed] [Google Scholar]
- Sinnar S.A., Small C.L., Evanoff R.M., Reinholdt L.G., Griswold M.D., Kopito R.R., Ryu K.Y. Altered testicular gene expression patterns in mice lacking the polyubiquitin gene Ubb. Mol. Reprod. Dev. 2011;78:415–425. doi: 10.1002/mrd.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Chen X.F., Zhu X.B., Hu H.L., Zhang W., Shao F.M., Li P., Miao Q.L., Huang Y.R., Li Z. A new molecular diagnostic approach to assess Y chromosome microdeletions in infertile men. J. Int. Med. Res. 2012;40:237–248. doi: 10.1177/147323001204000124. [DOI] [PubMed] [Google Scholar]
- Tajima Y., Sawada K., Morimoto T., Nishimune Y. Switching of mouse spermatogonial proliferation from the c-kit receptor-independent type to the receptor-dependent type during differentiation. J. Reprod. Fertil. 1994;102:117–122. doi: 10.1530/jrf.0.1020117. [DOI] [PubMed] [Google Scholar]
- Tedesco M., Farini D., De Felici M. Impaired meiotic competence in putative primordial germ cells produced from mouse embryonic stem cells. Int. J. Dev. Biol. 2011;55:215–222. doi: 10.1387/ijdb.103108mt. [DOI] [PubMed] [Google Scholar]
- Tesarik J. Overcoming maturation arrest by in vitro spermatogenesis: search for the optimal culture system. Fertil. Steril. 2004;81:1417–1419. doi: 10.1016/j.fertnstert.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Tilgner K., Atkinson S.P., Golebiewska A., Stojkovic M., Lako M., Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–3085. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- West J.A., Park I.H., Daley G.Q., Geijsen N. In vitro generation of germ cells from murine embryonic stem cells. Nat. Protoc. 2006;1:2026–2036. doi: 10.1038/nprot.2006.303. [DOI] [PubMed] [Google Scholar]
- West F.D., Machacek D.W., Boyd N.L., Pandiyan K., Robbins K.R., Stice S.L. Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling. Stem Cells. 2008;26:2768–2776. doi: 10.1634/stemcells.2008-0124. [DOI] [PubMed] [Google Scholar]
- West F.D., Roche-Rios M.I., Abraham S., Rao R.R., Natrajan M.S., Bacanamwo M., Stice S.L. KIT ligand and bone morphogenetic protein signaling enhances human embryonic stem cell to germ-like cell differentiation. Hum. Reprod. 2010;25:168–178. doi: 10.1093/humrep/dep338. [DOI] [PubMed] [Google Scholar]
- Willott G.M. Frequency of azoospermia. Forensic Sci. Int. 1982;20:9–10. doi: 10.1016/0379-0738(82)90099-8. [DOI] [PubMed] [Google Scholar]
- Yan L., Yang M., Guo H., Yang L., Wu J., Li R., Liu P., Lian Y., Zheng X., Yan J. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 2013;20:1131–1139. doi: 10.1038/nsmb.2660. [DOI] [PubMed] [Google Scholar]
- Yang S., Bo J., Hu H., Guo X., Tian R., Sun C., Zhu Y., Li P., Liu P., Zou S. Derivation of male germ cells from induced pluripotent stem cells in vitro and in reconstituted seminiferous tubules. Cell Prolif. 2012;45:91–100. doi: 10.1111/j.1365-2184.2012.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa H., Yanagida K., Sato A. Human round spermatids from azoospermic men exhibit oocyte-activation and Ca2+ oscillation-inducing activities. Zygote. 2007;15:337–346. doi: 10.1017/S0967199407004339. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Li Y., Nie R., Friel P., Mitchell D., Evanoff R.M., Pouchnik D., Banasik B., McCarrey J.R., Small C., Griswold M.D. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 2008;78:537–545. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic D., Varga J., Konstantinidis G., Vlaski J., Snyder H.M., Hadziselimovic F. Regional differences in maturation of germ cells of cryptorchid testes: role of environment. Acta Paediatr. 2009;98:1339–1343. doi: 10.1111/j.1651-2227.2009.01325.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


