Abstract
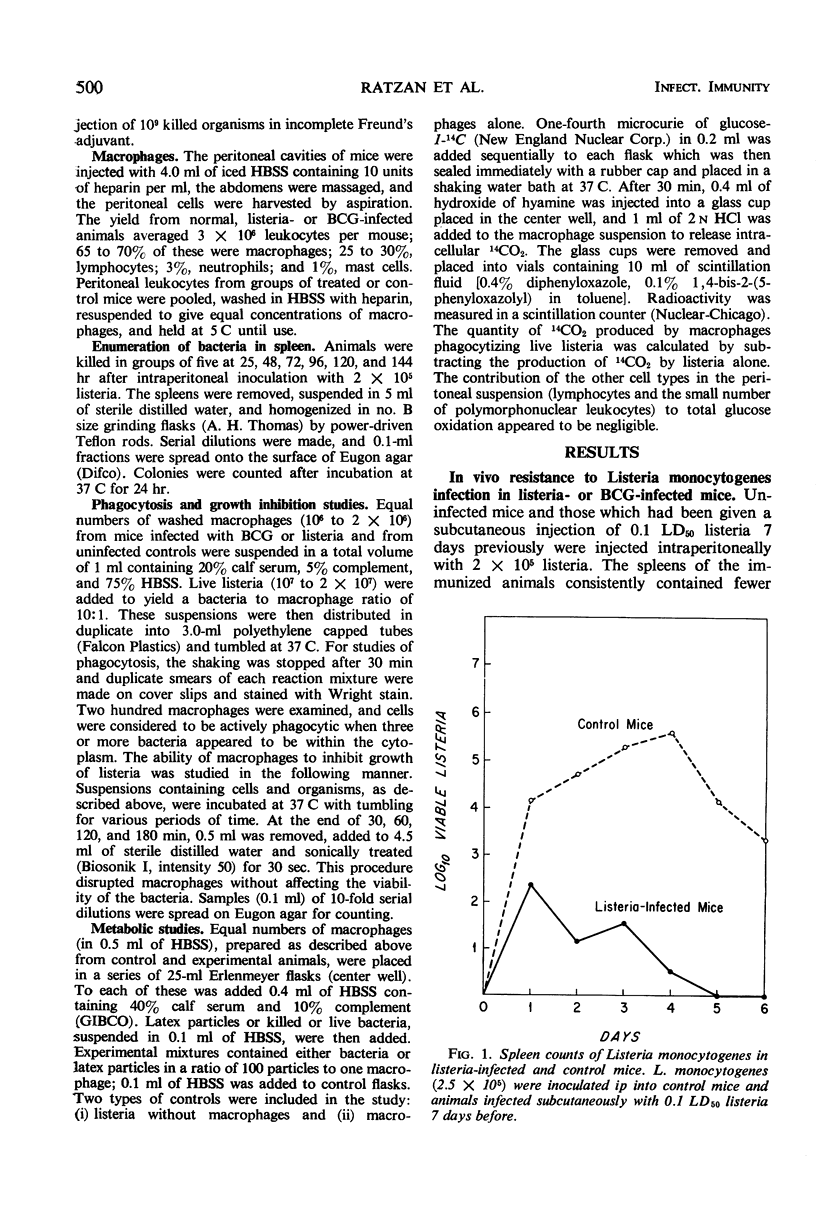
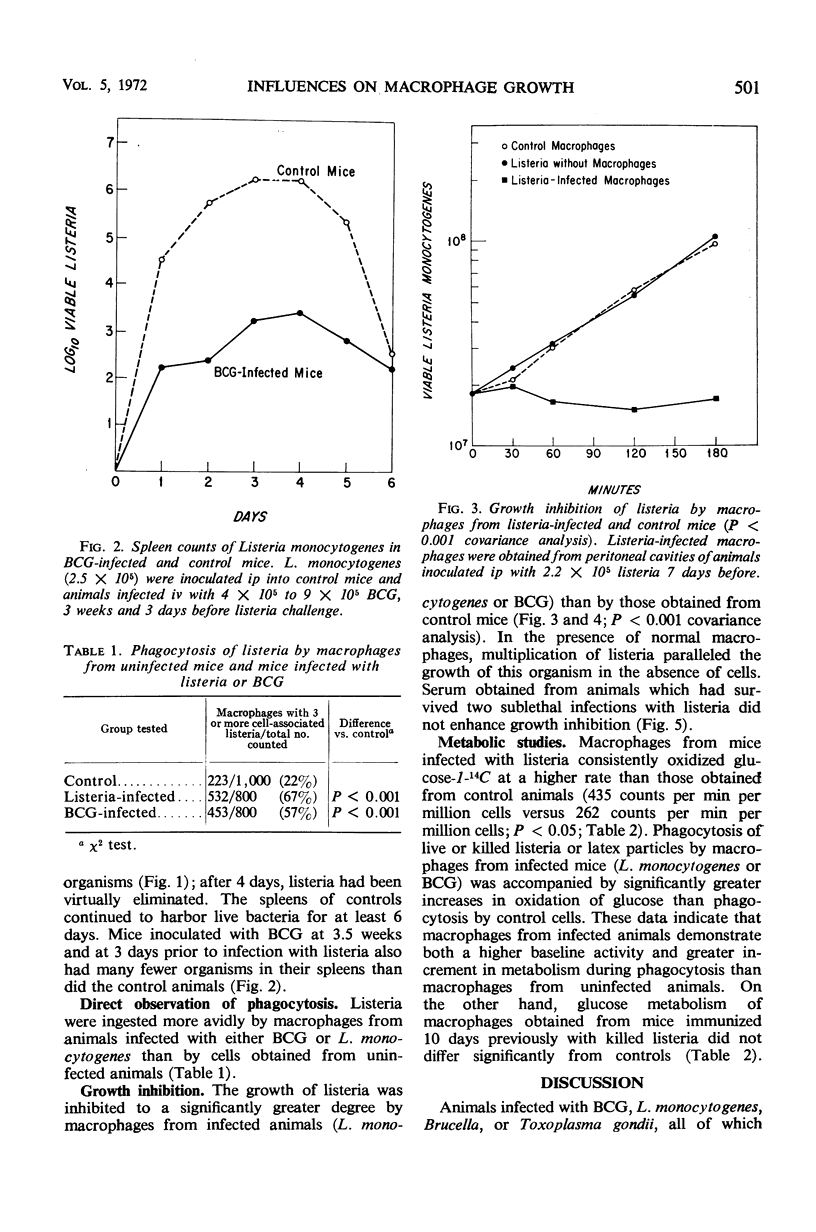
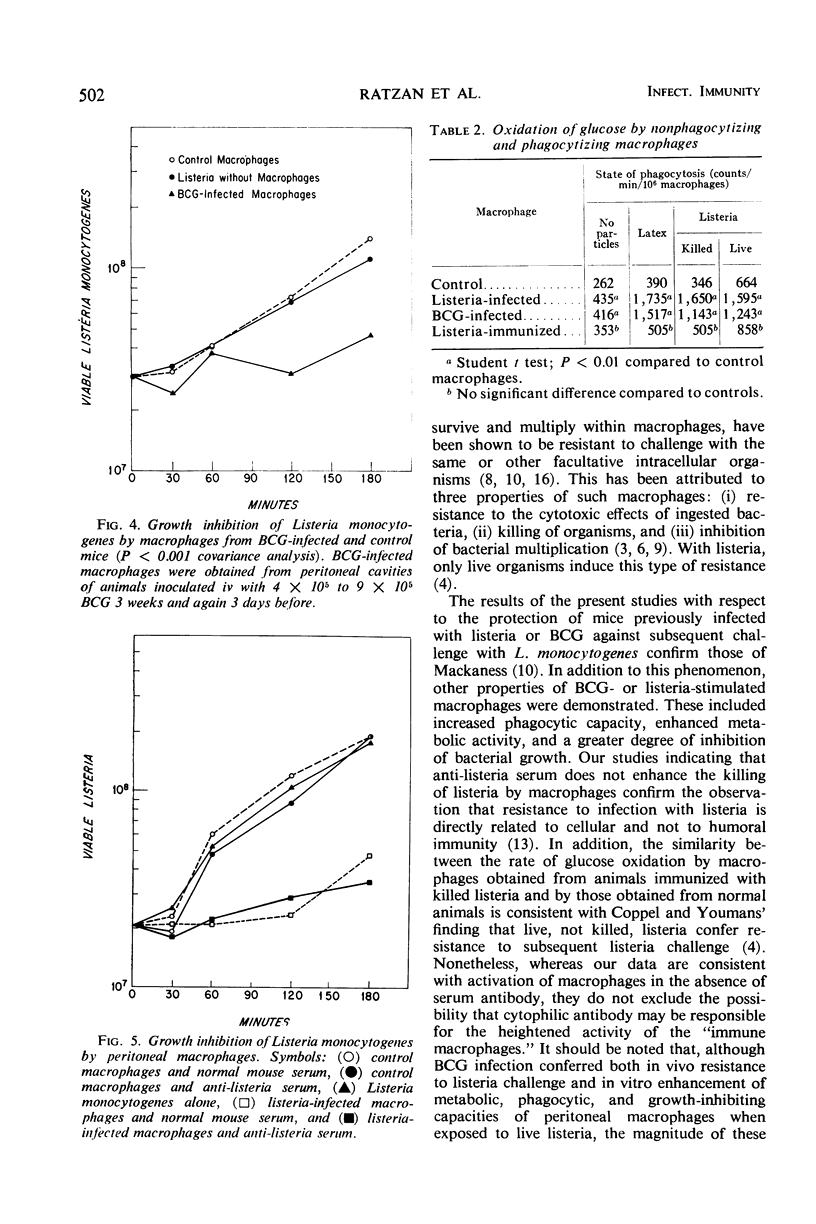
Macrophages from mice infected with facultative intracellular organisms such as Listeria monocytogenes and BCG have been shown to resist infection by antigenically unrelated intracellular bacterial parasites. This study compares phagocytosis, bacterial growth inhibition, and oxidation of glucose by macrophages from normal mice, mice infected with listeria or BCG, or mice immunized with killed listeria in incomplete Freund's adjuvant. Macrophages from listeria- and BCG-infected mice ingested more listeria; 67 and 57%, respectively, had three or more cell-associated bacteria versus 22% of controls (P < 0.001). Peritoneal macrophages from listeria- and BCG-infected animals significantly (P < 0.001 covariance analysis) inhibited growth of listeria in suspension, whereas control macrophages had no such inhibitory effect. The rate of oxidation of glucose-1-14C was higher in macrophages from listeria- and BCG-infected mice than from either uninfected animals or those immunized with killed listeria. During phagocytosis of killed or live bacteria, or latex particles, the rate of glucose oxidation was increased (P < 0.01). These data suggest that the cellular immunity after infection by an intracellular organism is associated with an increase in metabolic activity of macrophages, namely, an increase in the rate of glucose oxidation resulting in enhancement of phagocytosis and killing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., ZAPPASODI P., LURIE M. B. Metabolic studies on mononuclear cells from rabbits of varying genetic resistance to tuberculosis. I. Studies on cells of normal noninfected animals. Am Rev Respir Dis. 1961 Sep;84:364–370. doi: 10.1164/arrd.1961.84.3.364. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Coppel S., Youmans G. P. Specificity of Acquired Resistance Produced by Immunization with Listeria monocytogenes and Listeria Fractions. J Bacteriol. 1969 Jan;97(1):121–126. doi: 10.1128/jb.97.1.121-126.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Myrvik Q. N. Biology of the mycobacterioses. Studies on glucose oxidation during the interaction of alveolar macrophages and bacteria. Ann N Y Acad Sci. 1968 Sep 5;154(1):167–176. doi: 10.1111/j.1749-6632.1968.tb16707.x. [DOI] [PubMed] [Google Scholar]
- Goihman-Yahr M. Raffel S, Ferraresi RW: Delayed hypersensitivity in relation to suppression of growth of Listeria monocytogenes by guinea pig macrophages. J Bacteriol. 1969 Nov;100(2):635–640. doi: 10.1128/jb.100.2.635-640.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard G. C. Some biochemical aspects of the immune macrophage. Br J Exp Pathol. 1970 Feb;51(1):97–105. [PMC free article] [PubMed] [Google Scholar]
- Herman R., Baron S. Effects of interferon inducers on the intracellular growth of the protozoan parasite, Leishmania donovani. Nature. 1970 Apr 11;226(5241):168–170. doi: 10.1038/226168a0. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- SAITO K., SUTER E. LYSOSOMAL ACID HYDROLASES IN MICE INFECTED WITH BCG. J Exp Med. 1965 May 1;121:727–738. doi: 10.1084/jem.121.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORBECKE G. J., OLD L. J., BENACERRAF B., CLARKE D. A. A histochemical study of acid and alkaline phosphatase in mouse livers during various conditions modifying activity of the reticuloendothelial system. J Histochem Cytochem. 1961 Jul;9:392–399. doi: 10.1177/9.4.392. [DOI] [PubMed] [Google Scholar]
- West J., Morton D. J., Esmann V., Stjernholm R. L. Carbohydrate metabolism in leukocytes. 8. Metabolic activities of the macrophage. Arch Biochem Biophys. 1968 Mar 20;124(1):85–90. doi: 10.1016/0003-9861(68)90306-8. [DOI] [PubMed] [Google Scholar]


