Introduction
Prenatal cocaine exposure (PCE) is related to subtle cognitive, behavioral and physiological differences in infancy, childhood and adolescence. Many well-controlled prospective studies report that infants and toddlers exposed to cocaine in utero demonstrate impaired affect (Tronick et al. 2005), arousal (Bendersky and Lewis 1998), joint attention, visual recognition (Singer et al. 2005), auditory comprehension (Singer et al. 2001) and are at risk for delayed mental development (Noland et al. 2005). At school age, PCE is linked to higher risk for learning disabilities (Morrow et al. 2006) and to subtle deficits in attention, response inhibition (Bandstra et al. 2001; Accornero et al. 2007), impulsivity (Savage et al. 2005), language development (Lewis et al. 2011), working memory (Schroder et al. 2004; Mayes et al. 2007), planning and set shifting (Warner et al. 2006). Adolescents with PCE demonstrate deficits in working memory for words and faces, inhibitory control, and early sensory and higher order processing of language stimuli (Betancourt et al. 2011; Bridgett and Mayes 2011; Landi et al. 2012). Difficulty with emotional/behavioral and physiological self-regulation is demonstrated in all age groups (Minnes et al. 2005; Tronick et al. 2005; Eiden et al. 2009; Chaplin et al. 2010), and adolescents are more likely to use cocaine and other drugs compared with their non-exposed peers (Delaney-Black et al. 2011). Little is known about the effects of in utero exposure to cocaine on human early brain development that may mediate or contribute to such deficits.
Cocaine acts as a powerful central nervous system stimulant by blocking reuptake of the monamines, dopamine, serotonin and norepinephrine, resulting in prolonged, supraphysiologic synaptic and extracellular levels (Meyer and Quenzer 2005). During critical periods of fetal brain development these neurotransmitters play important roles in growth and organization (Whitaker-Azmitia et al. 1996)(25), exerting widespread effects on neuronal cell proliferation and differentiation (Lauder 1993; Popolo et al. 2004), migration (Vitalis and Parnavelas 2003; Riccio et al. 2012), and dendritic growth (Song et al. 2002; Song et al. 2004). When taken by mothers during pregnancy, cocaine and its active metabolites easily diffuse through the placenta into fetal circulation, where they cross the immature blood-brain barrier (Schenker et al. 1993). Animal research shows that cocaine exerts direct pharmacological effects on fetal brain, altering metabolism (Benveniste et al. 2010), cerebral blood supply, cortical neuron volume and functional characteristics (Ren et al. 2004; Stanwood and Levitt 2007; Frankfurt et al. 2011).
Results from neuroimaging studies conducted in late childhood and adolescence suggest that PCE may be associated with alterations in brain structure and function that persist into adolescence and that may contribute to poorer performance on executive function tasks measured at school age and in later life. Findings in preadolescent children include significant reductions in corpus callosal area, lesser occipital and parietal gray matter (GM) volume, smaller right cerebellar volume (Dow-Edwards et al. 2006) and increased levels of creatine in frontal lobe white matter, indicative of abnormal energy metabolism (Smith et al. 2001). At adolescence, PCE is linked to smaller head circumference, total brain and cortical GM volume (Rivkin et al. 2008), reduced frontal and orbital frontal cortical volume (Roussotte et al. 2010), reduced global cerebral blood flow, greater GM volume in amygdala and reduced GM volume in bilateral caudate (Avants et al. 2007; Rao et al. 2007). Functional MRI studies reveal greater resting state connectivity of the default mode network (DMN), with impaired ability for prefrontal inhibition of limbic circuitry and less deactivation of the DMN during working memory challenge (Li et al. 2009; Li et al. 2011). A very few imaging studies of infants with PCE have been reported. Delayed brain maturation is suggested by EEG findings of slower auditory brainstem response at birth, and reduced inter-hemispheric connectivity at birth and 1 year (Scher et al. 2000; Lester et al. 2003). Neonatal cranial ultrasounds reveal greater incidence of intracranial hemorrhage in infants with ‘heavy’ prenatal cocaine exposure, however infants with less exposure do not differ from drug-free infants (Frank et al. 1999). Structural MRI studies of brain development in infants with PCE have been limited to a single case study at 11 months (Gomez-Anson and Ramsey 1994), and to a group of 8 exposed infants (scanned at .6–12 months) who were then compared with published norms (Link et al. 1991). To date MRI study of neonatal brain structure in infants with PCE compared with drug-free controls and/or non-cocaine drug-exposed infants has not been reported.
Notably, the aforementioned cognitive, neurobehavioral and neuroimaging findings are by no means unequivocal across a large, decades-long body of research (Roussotte et al. 2010; Dow-Edwards 2011; Coyle 2013). Study is complicated by the fact that prenatal cocaine exposure is often comorbid with maternal use of other licit and illicit drugs. To address this, most investigations have compared children with in utero exposure to cocaine plus tobacco, alcohol and/or marijuana, to children with prenatal exposure to these same drugs but without cocaine. Nevertheless some studies report no observable PCE effects (Frank et al. 2001). Others reveal independent or interactive effects with nicotine, alcohol, or contextual factors (prematurity, gender, maternal care, environmental characteristics (Bandstra et al. 2010; Eiden et al. 2011; Irner 2012; Liu et al. 2013). The purpose of the current study was to examine the effects of prenatal cocaine exposure on infant brain structure during early infancy, at a time more proximal to in utero exposure, and less influenced by the postnatal environment. We used structural MRI to examine total and regional gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) brain volumes in 2–6 old week cocaine-exposed infants with or without other drug exposures, comparing them to drug-naïve infants, and also to infants with similar patterns of polydrug exposure without cocaine.
Materials and Methods
Participants
Infants were part of an ongoing study of the effects of prenatal cocaine exposure on biological mechanisms underlying mother-infant interaction and attachment characteristics. Here we report results from the first 119 infants to complete the infant MRI protocol. Mothers were recruited during pregnancy from local obstetric clinics, health departments, and programs for substance abuse treatment of pregnant and postpartum mothers. Infants were medically healthy singletons, born at ≥ 36 weeks gestation. The study sample (N=119) consisted of 33 cocaine-exposed infants with or without in utero exposure to marijuana, alcohol, nicotine, opiates and/or SSRIs (PCE), 40 infants with in utero exposure to these same drugs but without cocaine (NCOC), and 46 drug-free controls (CTL). The sample included 61 males and 58 females, and maternal report of ethnic composition was 59 White, 33 African American, 25 Multi-Racial, 2 Other. Drug use status was based on self-report on Time Line Follow Back Interview (Sobell and Sobell 1995) conducted at 1 month postpartum, response to a questionnaire about maternal substance use done at 3 months, and medical record queries of prenatal urine toxicology. If maternal self-report or urine toxicology was positive for cocaine use at any time during pregnancy, that mother-infant dyad was classified as PCE. This study was approved by the Biomedical Institutional Review Board of the University of North Carolina.
MR Image Acquisition
Infants were scanned during sleep without sedation. Infants were first fed and then swaddled. Sleeping infants were each was fitted with ear protection. A vacuum-fixation device was used to secure head position in the scanner. A nurse monitored each infant by sight, touch and by pulse oximetry for heart rate and % oxygen saturation throughout the scan. All images were acquired using a 3T MRI scanner. An unavoidable scanner replacement occurred during the study, resulting in 94 infants scanned on a 3T head only Siemens Allegra and 25 infants scanned on a 3T Siemens Tim Trio (Siemens Medical Solutions, Erlangen, Germany). Both scanners are Food and Drug Administration approved for use in all age groups. Adjustment for scanner type was added to all statistical models.
T1-weighted structural pulse sequences were obtained with a 3D magnetization prepared rapid gradient echo (MP-RAGE) sequence (repetition Time (TR)=1820 ms, echo time (TE)=3.75 ms, inversion time (TI)=1100 ms, flip angle=7°, 144 slices, voxel size: 1 × 1 × 1 mm). Proton density and T2-weighted images were acquired with turbo spin echo sequence (TR=6200ms, TE1=17ms, TE2=116ms, flip angle=150°, 58 slices, voxel size=1.3 × 1.3 × 1.5 mm).
Image Analysis
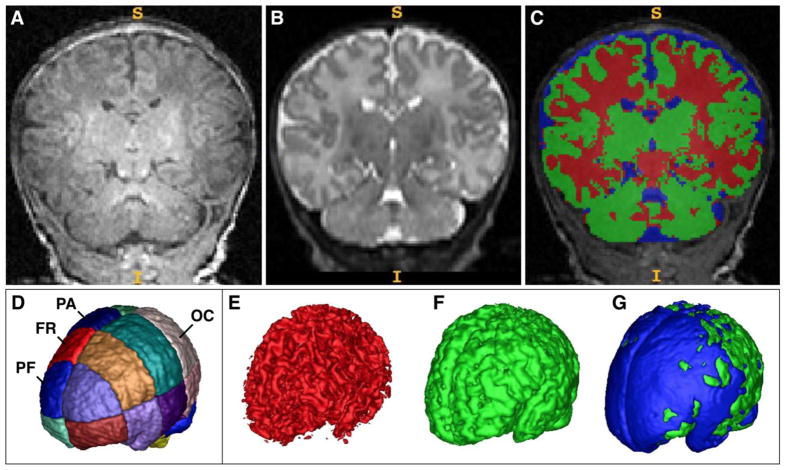
Brain tissue and cerebral spinal fluid (CSF) were segmented with an automatic segmentation tool specifically designed for the neonatal brain, described previously (Prastawa et al. 2005). The segmentation method makes use of dual contrast MRI (T1w, T2w) for optimal separation of GM, WM and CSF space and uses a co-registered probabilistic neonatal atlas, developed by our group, as a spatial prior (Figure 1A–C). The neonatal population atlas includes a lobar parcellation into 16 boxes aligned along the brain hemisphere symmetry plane and the AC-PC line (Figure 1D). The choice for a box parcellation versus more detailed anatomical lobe parcellation as often applied in adult neuroimaging was motivated by the fact that the very dense and compact cortical geometry of the neonatal brain presents a challenge for a reproducible and reliable subdivision based on sulcal features. The neonatal segmentation tool integrates multimodal T1w/T2w MRI registration, intensity bias inhomogeneity correction, tissue segmentation, brain stripping and lobe parcellation into one integrated platform, and represents a key instrument that has led to a series of publications on neonatal brain growth (Gilmore et al. 2007; Knickmeyer et al. 2008; Gilmore et al. 2010; Knickmeyer et al. 2011). Figure 1 displays representative 3-D visualizations of segmented WM (1E, red) and GM (1F, green), and of CSF shown on the brain cavity surface (1G, blue).
Figure 1.
A–C, Representative subject’s coronal views of T1-weighted (A) and T2-weighted (B) neonatal images which have been automatically segmented (C) into CSF (blue), gray matter (red) and white matter (green). Right brain is on readers’ left. D, Template of the neonatal brain for automatic parcellation into cortical regions (left and right, dorsal and ventral, prefrontal (PF), frontal (FR), parietal (PA) and occipital (OC) regions. E–G, 3-D visualizations of segmented white matter (E, red) and gray matter (F, green), and of CSF shown on the brain cavity surface (G, blue) from the same subject shown in Figure 1A–B.
Statistical Analysis
Descriptive statistics for each of the three drug exposure groups (PCE, NCOC, CTL) were computed for gestational ages at birth and MRI, birth weight, head circumference, gender, race, non-cocaine drug exposures, maternal characteristics, scanner, and brain tissue volumes. Groups were compared to determine the extent to which they differed on these variables, and how PCE and NCOC groups differed on prenatal non-cocaine drug use. Group means were compared with analyses of variance; group proportions were tested with the chi-square statistic.
Inferential analyses compared brain volumes for drug group differences using analyses of covariance (ANCOVA), including gender, scanner, maternal age, gestational age at MRI and birth weight as covariates. All reported p values are from 2-tailed tests. Gender x group interactions did not attain statistical significance and were not included in subsequent models. The first ANCOVA compared total intracranial volume (ICV). The next set of multivariate analyses of covariance (MANCOVA) added ICV as those listed above, and tested whether the three groups differed in total segmented tissue volumes (GM, CSF, WM). A top-down testing strategy was employed to control type-1 errors: multivariate comparisons of group means were examined first, and if statistically significant, then univariate test statistics were examined. If those were significant, then pairwise comparisons of the three groups were examined. The next set of analyses tested for group differences in within-region comparisons (prefrontal, frontal, parietal, occipital) when group differences were obtained in the univariate ANCOVA for a specific segmented tissue type. Repeated measures analyses of covariance were used to examine GM and CSF volume in dorsal and ventral sub-regions of regions with significant group differences. An adaptive false discovery rate (FDR) that computes adjusted p-values (Benjamini, 2000) using an adaptive linear step-up method was employed to adjust for multiple comparisons, and was applied to each MANCOVA separately.
A set of follow-up sensitivity analyses was conducted to address our concerns that PCE mothers have been reported to engage in more prenatal drug use in general than NCOC mothers, with the goal of accounting for a possible multiple drug bias in the cocaine-exposed group. The same set of analyses described above was conducted using only the data from infants in the PCE and NCOC groups with in utero exposure to 2 or more drugs, with number of different drugs used in pregnancy included as an additional covariate.
Results
Participant characteristics
Drug group differences in participant characteristics are described in Table 1. Head circumference and gestational age at MRI visit were similar across groups, however cocaine-exposed (PCE) infants had significantly lower birth weights and lesser gestational duration compared with drug-free (CTL) and non-cocaine drug exposed (NCOC) infants. Maternal education, family income, parity and marital status differed by group. Education and income were higher in the CTL group, and they were more likely to be married and primiparous compared with PCE and NCOC groups, (p < .01 to .0001), which did not differ from one another (all p > .05). Maternal age was significantly lower in the NCOC group than PCE and CTL groups. There were no group differences in infant gender or race, and no differences between PCE and NCOC groups in the proportion of mothers who reported using nicotine, marijuana, alcohol, opiates or SSRIs during pregnancy. Notably, a majority of both PCE and NCOC mothers reported prenatal cigarette-smoking (87.88% and 87.50%, respectively), with lesser use of other drugs in similar proportions across PCE and NCOC groups.
Table 1.
Participant characteristics by prenatal drug exposure group
| PCE n = 33 | CTL n = 46 | NCOC n = 40 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SE | Mean | SE | Mean | SE | F | |
| Gestational age at MRI (days) | 307.5 | 2.9 | 303.1 | 2.5 | 304.8 | 2.7 | ns |
| Gestational age at birth (days) | 274.5a | 1.5 | 279.6b | 1.3 | 280.0b | 1.4 | 4.53* |
| Birth Weight (pounds) | 6.80a | .17 | 7.63b | .14 | 7.55b | .15 | 8.33** |
| Head Circumference (inches) | 36.26c | .36 | 36.84d | .32 | 36.78d | .32 | |
| Maternal Age (years) | 28.61b | 5.6 | 28.07b | 5.7 | 25.68a | 5.3 | 3.05* |
| Other children | 2.03 | 1.8 | 0.65 | 0.9 | 1.08 | 1.1 | 11.69**** |
|
| |||||||
| % | % | % | Chi-square | ||||
|
| |||||||
| Infant Gender | |||||||
| Male | .52 | .41 | .55 | ns | |||
| Female | .48 | .59 | .45 | ||||
| Infant Race | |||||||
| Black | .24 | .22 | .38 | ns | |||
| Multi-race | .24 | .17 | .22 | ||||
| White | .49 | .61 | .38 | ||||
| Other | .03 | .02 | |||||
| Parity | |||||||
| Primipara | .21 | .61 | .35 | 13.43** | |||
| Multipara | .79 | .39 | .65 | ||||
| Marital | |||||||
| Married | .07 | .62 | .19 | 29.23**** | |||
| Separated/Divorced | .31 | .03 | .22 | ||||
| Never Married | .62 | .35 | .59 | ||||
| Education | |||||||
| ≤ High School Grad | .48 | .23 | .46 | 42.30**** | |||
| Some college/Trade school | .52 | .07 | .33 | ||||
| ≥ College Graduate | 0 | .70 | .21 | ||||
| Income ($/year) | |||||||
| ≤ 15,000 | .84 | .15 | .53 | 44.69**** | |||
| 15,000 – 39,999 | .12 | .21 | .37 | ||||
| 40,000–80,000 | .04 | .49 | .10 | ||||
| > 80,000 | 0 | .15 | 0 | ||||
| Scanner | |||||||
| Allegra | .52 | .83 | .87 | 14.69*** | |||
| Tim Trio | .48 | .17 | .13 | ||||
| Drug Exposure | |||||||
| Cocaine | 1.0 | ||||||
| Nicotine | .88 | .88 | ns | ||||
| Marijuana | .40 | .43 | ns | ||||
| Alcohol | .30 | .43 | ns | ||||
| Opiates | .24 | .10 | ns | ||||
| SSRI | .24 | .13 | ns | ||||
PCE: Prenatal Cocaine Exposure; CTL: Drug-free Control; NCOC: Non-Cocaine Drug Exposure
p ≤ .05,
p < .01,
p < .001,
p < .0001;
a <bon pair-wise comparisons at p<.05;
n=30,
n=38; Maternal Education 17 missing; (total family) Income missing 25
However, groups did differ when more detailed drug use data, as reported on the Time Line Follow Back interview (TLFB), were examined. Table 2 provides description of drug use for each trimester in PCE and NCOC groups for subjects with complete TLFB data. Mothers in the PCE group reported smoking more cigarettes per day, on average, in first and third trimesters compared with mothers in the NCOC group.
Table 2.
Drug exposure in PCE and NCOC groups by trimester as measured by the Time Line Follow Back Interview
| Trimester | Prenatal Cocaine Exposure (PCE) | Non-Cocaine Other Drugs (NCOC) | F | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | Range | n | Mean | SE | Range | ||||
| NICOTINE | |||||||||||
|
| |||||||||||
| Cigarettes/day | 1 | 28 | 13.72 | 1.74 | 0–40 | 26 | 8.79 | 1.60 | 0–40 | 4.31 | .0429 |
| 2 | 28 | 9.68 | 1.90 | 0–40 | 26 | 5.21 | 1.61 | 0–40 | |||
| 3 | 27 | 6.59 | 1.68 | 0–40 | 25 | 2.66 | 0.67 | 0–10 | 4.43 | .0403 | |
|
| |||||||||||
| ALCOHOL | |||||||||||
|
| |||||||||||
| Drinks/week | 1 | 10 | 5.72 | 6.02 | 0–42 | 17 | 10.77 | 4.62 | 0–84 | ||
| 2 | 10 | 4.30 | 2.66 | 0–42 | 17 | 4.30 | 2.04 | 0–14 | |||
| 3 | 10 | 0 | 0 | 0 | 17 | 1.53 | 0.72 | 0–14 | |||
| Binges/week | 1 | 9 | 1.1 | 0.78 | 0–7 | 11 | 1.66 | 0.57 | 0–7 | ||
| 2 | 9 | 0.8 | 0.78 | 0–7 | 11 | 0.64 | 0.64 | 0–7 | |||
| 3 | 9 | 0 | 0 | 0 | 11 | 0.64 | 0.64 | 0–7 | |||
|
| |||||||||||
| OPIATESa | |||||||||||
|
| |||||||||||
| Times/week | 1 | 4 | 3.25 | 1.65 | 1–7 | 3 | 7.0 | 0 | 7–7 | ||
| 2 | 4 | 2.50 | 1.55 | 1–7 | 3 | 7.0 | 0 | 7–7 | |||
| 3 | 4 | 2.13 | 1.66 | 1.5–7 | 3 | 4.67 | 2.33 | 0–7 | |||
|
| |||||||||||
| MARIJUANAb | |||||||||||
|
| |||||||||||
| Joints/week | 1 | 10 | 2.75 | 1.43 | 0–14 | 12 | 4.97 | 1.80 | 0–14 | ||
| 2 | 10 | 2.28 | 1.37 | 0–14 | 12 | 2.81 | 1.65 | 0–14 | |||
| 3 | 10 | 1.90 | 1.39 | 0–14 | 12 | 1.87 | 1.52 | 0–14 | |||
|
| |||||||||||
| COCAINE | |||||||||||
|
| |||||||||||
| Times/trimester | 1 | 33 | 39.97 | 6.36 | 0–99 | 0 | |||||
| 2 | 32 | 20.56 | 5.13 | 0–93 | 0 | ||||||
| 3 | 32 | 9.70 | 4.09 | 0–91 | 0 | ||||||
SE: standard error of the mean;
4 PCE, 1 NCOC missing opiate by trimester data;
3PCE, 5 NCOC missing marijuana by trimester data
Total brain volume differences
Table 3 displays adjusted group means and test statistics from two sets of group comparisons of intracranial volume (ICV) and total brain GM, WM and CSF volumes. The first ANCOVA analyzed group differences in ICV, which did not differ between drug exposure groups (ANCOVA (F(2,113)=2.05, p<.13) after adjusting for scanner, infant gender, birth weight, gestational age at MRI, and maternal age. The second set analyzed total segmented tissue volumes (GM, WM, CSF) using MANCOVA. This analysis indicated reliable group differences, adjusting for ICV, scanner, maternal age, infant gender, birth weight and gestational age at MRI (F(4,218)=3.62, p=.0071). Individual ANCOVA’s then revealed group differences in GM (F(2,117)=7.46, p<.001) and CSF (F(2,117)=4.74, p<.011). Pair-wise comparisons, show that the PCE group had smaller mean GM volumes than CTL and NCOC groups (−2.6% and −2.0%, respectively), and larger CSF volumes than the CTL and NCOC groups (+9.0.% and +6.2%, respectively). All group differences remained significant at p < .05 or better after adjusting for multiple comparisons.
Table 3.
| Group F(4,118) | PCE Mean (SE) |
CTL Mean (SE) |
NCOC Mean (SE) |
PCE vs CTL % difference | PCE vs NCOC % difference | |
|---|---|---|---|---|---|---|
| ICVa | ns | 482,825 (6558) | 481,227 (5909) | 467,072 (6545) | ||
| GMb | 7.46, 0.0009 | 230, 978 (1,166) | 237,079 (1,050) | 235,651 (1,184) | −2.6 %*** | −2.0%** |
| WMb | ns | 182,399 (859) | 182,713 (716) | 181,950 (704) | ||
| CSFb | 4.74, .0106 | 66,004 (1,411) | 60,665 (1,176) | 61,665(1,157) | +9%** | +6.2* |
PCE: Prenatal Cocaine Exposure; CTL: Drug-free Control; NCOC: Non-Cocaine Drug Exposure; ICV: Intracranial volume, GM: Total gray matter, WM: Total white matter, CSF:Total cerebral spinal fluid;
Adjusted for scanner, gender, birth weight, age at MRI, maternal age;
Adjusted for ICV, scanner, gender, birth weight, age at MRI, maternal age.
p <.05,
p <.01,
p < .005,
Regional brain volume differences
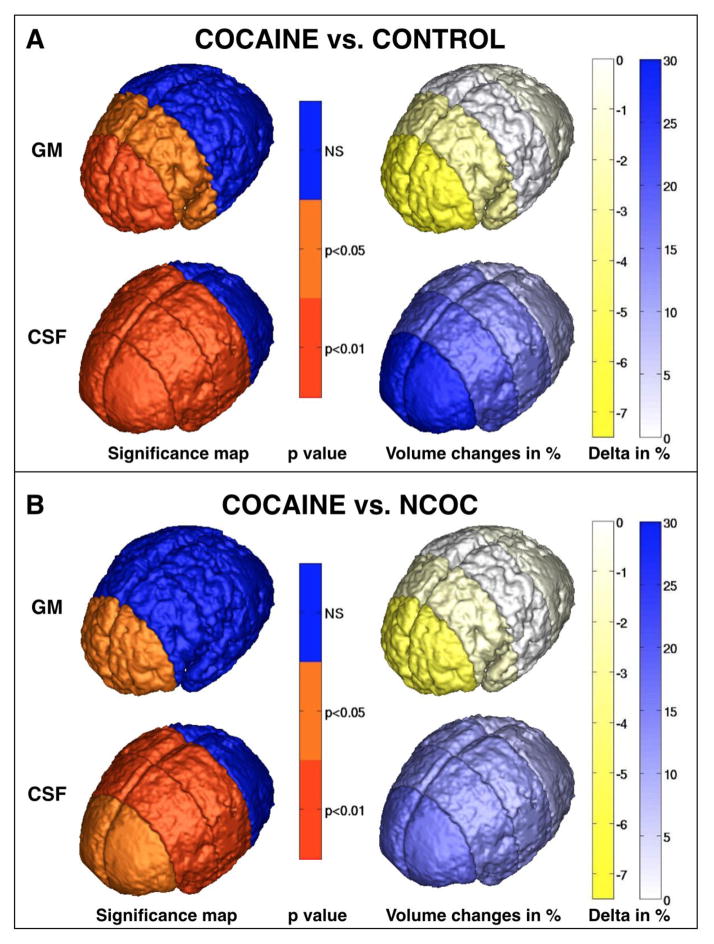
The third set of inferential analyses tested for group differences in GM and CSF in four different brain regions to determine whether the observed group differences in these two tissue types reflected the posterior-to-anterior gradient for cortical development that has been reported previously (Gilmore et al. 2007). MANCOVA’s examined group differences in GM and CSF across prefrontal, frontal, parietal and occipital brain regions (depicted in Figure 1D). Group means, unadjusted for covariates, as well as standard deviations, skewness and kurtosis values, are displayed in Supplemental Table 1 to show that brain volumes did not deviate significantly from the normal distribution. Figure 2 displays regional group differences, adjusted for covariates. Multivariate tests indicated that the magnitude of group differences in GM volumes varied across the four regions (MANCOVA group*region F(8,214)=2.18, p<.03). Univariate ANCOVA’s yielded significant group differences in prefrontal (F(2,110)=4.02, p=.021) and frontal GM (F(2,110)=3.39, p=.037). Infants with PCE had smaller mean prefrontal GM volume than CTL (−7.5%) and NCOC (−6.1%) groups, and smaller mean frontal GM volume compared with the CTL (−3.1%) and NCOC (−1.9%) groups. A multivariate test of Group*Region was significant for regional CSF volumes (F(2,110)=2.26, p=.025). PCE infants had significantly larger CSF volumes in prefrontal (F=7.98, p=.0006), frontal (F(2,110)=5.18, p=.0071) and parietal regions (F(2,110)=4.12, p=.0188) compared with CTL (+29.7%, +19.3%, +14.5%, respectively) and NCOC groups (+17.3%, +13.4%, +11.4%, respectively). Covariate-adjusted mean differences are displayed in Supplemental Table 2. Group differences between PCE and NCOC in prefrontal GM and in parietal CSF became marginally significant at p < .10 after adjustment for multiple comparisons.
Figure 2.
Regional gray matter (GM) and cerebral spinal fluid (CSF) volume (mm3) differences between cocaine-exposed infants (PCE) compared with (A) drug-free Controls and (B) infants with non-cocaine other drug exposures (NCOC). The left column illustrates significance maps based on p values of ANCOVA comparisons of GM and CSF parcellation volumes depicted in Figure 1F and G. Color scale columns at right depict % difference between group means. Greater blue intensity signifies greater increases in tissue volume in the Cocaine-exposed group; greater yellow intensity denotes greater deficits in the Cocaine-exposed group.
Follow-up analyses of brain volumes for infants with more than one drug exposure
Concerns that PCE mothers are often reported to engage in more prenatal drug use than NCOC mothers led to a follow-up sensitivity analysis to determine whether differences between PCE and NCOC infants were present when we included only mothers who reported similar levels of drug use during pregnancy. A total of 31 mothers in the PCE group reported using at least one other drug in addition to cocaine during pregnancy, and 29 NCOC mothers endorsed using at least two drugs during pregnancy. These mothers ranged from reporting using 2 drugs (32% PCE; 72% NCOC), 3 drugs (26% PCE; 24% NCOC) to 4–5 drugs (42% PCE; 4% NCOC). Drug use included nicotine (94% PCE; 93% NCOC); alcohol (32% PCE; 55% NCOC), marijuana (42% PCE, 55% NCOC); opiates (26% PCE; 14% NCOC), and SSRIs (26% PCE; 14% NCOC). On average, the NCOC group reported using fewer different drug types during gestation compared with the PCE group based on t-tests that accounted for possible differences in variability (number of drugs: PCE M=3.12, SD 1.01; NCOC M=2.31, SD=0.54; t(58) =4.25, p<.0001). Therefore the number of drugs used was included in statistical models.
Comparisons of brain volumes in these two groups are shown in Table 4. ICV volume did not differ between PCE and NCOC groups after adjusting for scanner, infant sex, gestational age at MRI, birth weight, maternal age and number of drug types used during pregnancy. Differences in total tissue volumes were similar to those observed in the total sample after adjusting for the same covariates with the addition of ICV to the model, (multivariate F(2,50) = 2.78, p = .072). PCE infants had significantly smaller mean total GM (−2.5%; Group F(1,50) = 5.53, p=.023) and non-significantly larger total CSF volume (+7.0%; F(1,50)=2.47, p<.13) compared with infants with NCOC exposure. Regional differences in GM were similar to results seen in the larger sample. The multivariate test of regional GM volume group differences across prefrontal, frontal, parietal and occipital regions was suggestive but not significant (GM: F(4,48) = 2.24, p=.079). Compared with the NCOC group, the PCE group had significantly smaller GM volume in prefrontal cortex (−8.8%; F(1,48)=4.48, p=0.039), while mean GM volumes in frontal, parietal and occipital regions did not differ. These analyses provided less support for the widespread group differences in regional CSF volume that had been observed in the larger sample. The multivariate test of CSF regional volume differences was not significant, (F(4,48)=2.79, p=.10), with larger CSF volumes present only in prefrontal cortex in infants with PCE compared with the NCOC group (+24%; univariate F(1,48)=4.30, p=.043). Group differences in prefrontal GM and CSF were no longer significant after adjustment for multiple comparisons.
Table 4.
Group comparisons of brain volumes (mm3) for infants with in utero exposure to > 2 drugs.
| Tissue Type | PCE Mean (SE) n = 31 |
NCOC Mean (SE) n = 29 |
Univariate Group F2,527 (p) |
PCE vs NCOC % difference |
|---|---|---|---|---|
| ICVa | 486,592 (7,230) | 462,907 (8,294) | 2.24 (.141) | + 4.4 |
|
| ||||
|
Total Tissue Volumesb
| ||||
| GM | 228,932 (1,448) | 234,672 (1615) | 5.53 (.023) | − 2.5 |
| CSF | 65,562 (1608) | 61298 (1794) | 2.47 (.122) | +7.0 |
| WM | 181,825 (1046) | 180,348 (1167) | 0.70 | |
|
| ||||
|
Regional GM Volumesb
| ||||
| Prefrontal | 20,170 (547) | 22,122 (610) | 4.48 (.039)c | − 8.8* |
| Frontal | 39,613 (436) | 40,240 (487) b | 0.73 | |
| Parietal | 56,730 (446) | 56,508 (411) | 0.09 | |
| Occipital | 53,735 (476) | 54,909 (531) | 2.14 | |
|
| ||||
|
Regional CSF Volumesb
| ||||
| Prefrontal | 9,454 (527) b | 7,607 (589) | 4.30 (.043)c | +24.3* |
| Frontal | 11,596 (505) | 10,614 (563) | 1.33 | |
| Parietal | 10,066 (333) | 9,632 (372) | 0.60 | |
| Occipital | 5,727 (315) | 5,486 (352) | 0.21 | |
ICV: intracranial volume; GM: gray matter; WM: white matter; CSF: cerebral spinal fluid; PCE: Prenatal Cocaine Exposure; NCOC: Non-Cocaine Drug Exposure;
Adjusted for scanner, sex, gestational age at scan, birth weight, maternal age, number of drugs during pregnancy;
Adjusted for scanner, ICV, sex, gestational age at scan, birth weight, maternal age, number of drugs during pregnancy;
p >.10 after adjustment for multiple comparisons
Dorsal and ventral GM and CSF volume differences
In brain regions where the PCE group demonstrated smaller GM volumes, group differences were further examined separately in dorsal and ventral sub-regions. Covariate-adjusted means, per cent differences and univariate ANCOVA F values are reported in Supplemental Table 3. In models adjusted for scanner, ICV, birth weight, gestational age at MRI, gender, and maternal age, MANCOVA testing of GM volumes across subregions was marginally significant (F(16,206) = 1.57, p = .079). Univariate analyses showed that infants with PCE had smaller mean GM volumes in the dorsal prefrontal cortex (F = 5.93, p =.0036) compared with CTL (−12.1%) and NCOC (−10.4%) groups, and in frontal cortex (F = 4.22, p = .0172) compared with the CTL group (−4.8%). Larger CSF volumes (multivariate Group F(16,206)=2.29, p=.0041) were revealed in dorsal prefrontal (F=9.85, p=.0001), frontal (F=7.35, p=.001) and parietal (F=6.01, p=.033) sub-regions in infants with PCE compared with CTL (+34.6%, +32.9%, +21.2%, respectively) and NCOC groups (+26.9%,+23.7%, +11.4%, respectively). Greater CSF volumes were also present in ventral prefrontal cortex in infants with PCE compared with the CTL group (+23.6%). Group difference in frontal GM between PCE and NCOC groups was no longer significant after adjustment for multiple comparisons.
In the sub-sample of participants who used at least two drugs during pregnancy, dorsal and ventral prefrontal cortex sub-region differences in prefrontal GM and CSF were compared. Significant differences were revealed for GM (multivariate F= 3.39, p=.0415) and CSF (multivariate F=4.09, p=.0226) volumes. PCE infants had significantly smaller dorsal prefrontal GM (−14%, univariate F=6.70, p=.0125) and larger dorsal prefrontal CSF volumes (+32%, univariate F=6.32, p=.0151), while ventral prefrontal GM and CSF volumes did not differ between groups.
Discussion
To our knowledge this is the first MRI study of neonatal brain structure comparing prenatally cocaine-exposed (PCE) infants to both drug-naive infants (CTL) and also to infants exposed to licit and illicit drugs other than cocaine that are commonly used in pregnancy (NCOC). Despite comparable head circumference, intracranial volume and gestational age at testing, PCE infants had smaller total cortical GM and larger CSF volumes compared with both CTL and NCOC infants. Notably, these deficits were seen only in the PCE group, while CTL and NCOC groups did not differ from one another. Cocaine-related differences in GM were restricted to prefrontal and frontal brain regions, and post hoc analyses suggest that these may be driven by group differences in dorsal, as opposed to ventral, regions. Cocaine-related differences in CSF were larger, with greater CSF volumes evident in prefrontal, frontal and parietal regions in PCE compared with both CTL and NCOC groups, albeit with most pronounced differences in dorsal prefrontal cortex. A similar pattern of results emerged when subsets of infants with exposure to two or more drugs in PCE and NCOC groups were compared, however significant differences in GM and CSF were restricted to prefrontal cortex only. These findings suggest that observed differences in this region are not due to the higher likelihood of maternal use of multiple drugs in the PCE group, and differences remained after adjusting for number of other drugs used during pregnancy that may exert teratogenic effects on brain development (Cortese et al. 2006; Dwyer et al. 2008; Balaraman et al. 2012).
While the exact mechanisms underlying the observed GM volume differences are unclear, our findings lend support to the hypothesis that prenatal cocaine exposure during critical periods of growth and organization impairs human brain development resulting in significant structural, and possibly functional, differences at birth. Animal research reveals direct pharmacological effects of cocaine on fetal brain activity (Benveniste et al. 2010), and reduction of cortical neurons that is not related to cocaine-induced malnutrition (Ren et al. 2004). Fetal exposure is extended due to prolonged fetal, compared with maternal, clearance (Dow-Edwards 1990), and is associated with reduction of cortical neurons (Ren et al. 2004), changes in the density of dendritic spines in striatal spiny neurons (Frankfurt et al. 2009), and alterations in dopamine receptor activity and sub-cellular expression (Stanwood and Levitt 2007). Moreover the pattern of smaller GM volumes observed with in utero exposure is similar to that reported in adults with chronic cocaine use. Cocaine-dependent adults exhibit GM loss in frontal regions including orbital frontal, dorsal lateral prefrontal cortex, inferior, medial and superior frontal gyri and premotor cortex, with greater lifetime exposure resulting in greater reductions (Franklin et al. 2002; Sim et al. 2007; Alia-Klein et al. 2011; Barros-Loscertales et al. 2011; Ersche et al. 2011; Moreno-Lopez et al. 2012). These deficits are accompanied by impairment in cognitive skills including decision-making, memory, nonverbal problem solving, response inhibition, impulsivity, spatial processing and reaction time tasks (Fein et al. 2002; Lane et al. 2010)(62). Similarly, reductions in GM density and/or cortical thickness are reported in frontal, temporal and cingulate cortex in cocaine-dependent adults, and are linked to poorer performance on decision-making and judgment tasks (Matochik et al. 2003; Hanlon et al. 2011). Alternatively, our findings may reflect a heritable pattern of structural differences in infants at risk for later drug abuse. Recent reports show that drug-free first-degree relatives of stimulant-dependent adults share a pattern of decreased GM in insula, post-central and inferior frontal gyrus, and GM enlargement in subcortical structures (amygdala, putamen). However, these reports also suggest that reduced GM volume in prefrontal cortex is more likely a consequence of drug exposure than genetic predisposition (Ersche et al. 2012; Ersche et al. 2012). Finally, the lesser GM volume we observed in PCE infants is very likely due, in part, to cocaine-related reductions in placental blood flow and gestation duration. Maternal cocaine use during pregnancy causes uterine contraction and umbilical artery vasoconstriction, reducing blood flow to the placenta and creating fetal hypoxia and impaired growth (Covert et al. 1994). PCE infants in the current sample had lower birth weights and were born earlier in gestation than CTL and NCOC infants, consistent with other reports (Gouin et al. 2011).
Mechanisms underlying our finding of enlarged CSF volume in infants with PCE, despite the fact that lateral ventricle volumes did not differ between groups (data not shown), also remain unclear. Enlarged CSF volume has been reported concurrent with WM injury in toddlers born prematurely (Tzarouchi et al. 2009). CSF enlargement is also consistent with adult cocaine or alcohol abuse and suggestive of response to neuronal injury. Cocaine-dependent adults exhibit increased cortical CSF volume (Bartzokis et al. 2000). Alcohol-related GM and WM loss is linked to enhanced CSF volumes, which decrease proportionally with longer abstinence (Demirakca et al. 2011). Moreover, enhanced CSF volume is a strong predictor of brain atrophy in neurodegenerative diseases in older adults (Fox and Schott 2004; Squitieri et al. 2009). Animal and in vitro studies indicate that CSF enlargement may be a marker of cocaine-induced neuroinflammation characterized by up-regulation of pro-inflammatory cytokines and chemokines in glial cells, and increased permeability of the blood-brain barrier which allows migration of leukocyte, monocyte and cell adhesion molecules into the CNS (Gan et al. 1999; Yao et al. 2011).
The study has several limitations. MR scanner differences may have provided non-random variability that confounded group differences in brain structure, since more PCE infants were scanned with the Trio (52%), while ~85% of CTL and NCOC infants were scanned with the Allegra. However, scanner type was included as a covariate in all statistical models. We also found that infants with PCE were born, on average, 5.1 – 5.5 days earlier and had lower mean birth weights(.75 to .83 pounds less), which may have contributed to differences in brain structure. However, although prematurity is related to neural and developmental deficits (de Kieviet et al. 2012), infants in the current sample were all delivered at ≥36 weeks gestation. Only one NCOC and two PCE infants had birth weights defined as Low Birth Weight (< 2500g), and none were classified as Very Low Birth Weight (< 1500g)(CDC 2009). We attempted to address these differences by controlling for birth weight and gestational age at MRI in statistical models, and by scanning all infants at comparable post-conception ages. Another potential limitation is our lack of information regarding maternal health, diet and obstetrical care, therefore we are unable to determine the extent to which poor or sub-optimal prenatal care and nutrition contributed to our findings. However we did measure SES indicators, which have been related to prenatal care, nutrition and birth outcome, and these were higher in drug-free CTL compared with both PCE and NCOC groups, which did not differ from one another. Therefore differences in brain structure between PCE and NCOC may be less reflective of differences in maternal care, diet and health than the comparison of PCE and CTL groups. Finally, consistent with prior reports, prenatal cocaine-using mothers in our sample engaged in a heterogeneous pattern of polydrug use in addition to cocaine. Since it is neither ethical nor possible to randomize human subjects to drug use groups, or to limit drug use to cocaine or any other single drug, this heterogeneity is unavoidable and reflects drug use in the community. Mothers in the PCE group smoked more cigarettes per day during pregnancy, however in contrast to some studies (Singer et al. 2005; Eiden et al. 2011), rates of prenatal use of other drugs (alcohol, marijuana, opiates) were not greater in PCE than NCOC groups, and significant differences in brain structure were retained after adding the number of other drugs used to statistical models.
Based on our current findings, a critical question for future study is whether in utero exposure to cocaine interrupts or alters important growth, organization and connectivity to the extent that it cannot be completely normalized after birth. Although smaller at birth, infants with prenatal cocaine exposure reportedly ‘catch-up’ in physical growth by 6 months of age, with no clear or consistent differences from non-exposed children at preschool age, even in those with heavy exposure (Lumeng et al. 2007). It remains to be determined whether this pattern holds true for brain development. Imaging data from non-exposed children born prematurely suggest that prematurity-related deficits in brain structure persist and underlie developmental impairments at school age (Lowe et al. 2012; Rogers et al. 2012), with delay in some, but not all, processes of cortical maturation (Phillips et al. 2011). Adolescents with PCE show reductions in frontal cortical volumes, however this finding is not consistent across all imaging studies of exposed children and adolescents (Smith et al. 2001; Dow-Edwards et al. 2006; Avants et al. 2007; Rao et al. 2007; Rivkin et al. 2008; Roussotte et al. 2010). The cocaine-related GM deficits we observed appear similar to those seen in cocaine-abusing adults, who show partial recovery of brain volume and cognitive function with cocaine abstinence including increases in GM density in frontal and temporal cortices that are related to better performance on executive function tasks (Hanlon et al. 2011). However, unlike adult use, in utero exposure occurs during a critical time of extraordinary brain development characterized by massive growth, neurogenesis, neuronal migration, synaptogenesis and organization of cytoarchitecture (Knowles and Penn 2012). These processes lay the foundation for the subsequent dramatic and concurrent development of brain structure and function in the months following birth. MRI studies of healthy unexposed infants report that total brain size increases by over 100% in the first 12 months (Knickmeyer et al. 2008), reaching approximately 72% of adult size in the first year (Gilmore et al. 2007) and 80–90% of adult size by two years of age (Pfefferbaum et al. 1994). This rapid growth is driven primarily by increases in cortical gray matter, which increases, on average, by 108% in the first year, however this growth is quite variable, ranging from 62–154%. Findings in healthy neonates and children show that frontal gyrus is one of the fastest growing regions of GM growth during this time (Gilmore et al. 2012). These anterior brain regions are integral to multiple developing brain networks that are fundamentally involved in essential cognitive functions including attention, perception, memory, inhibition, as well as development of default mode network activity (Lin et al. 2008; Gao et al. 2009; Yap et al. 2011). Our data represent only a single time point embedded within this rapidly changing period, and do not shed light on whether the cocaine-related GM and CSF differences may be normalized by an accelerated postnatal pace of growth. Moreover, imaging studies in non-exposed children and young adults suggest that cognitive deficits are often better related to variations in the rate of change rather than to variations in anatomy at any single time point, and that differences in cortical volume may not be as meaningfully predictive as other measures including cortical thickness and surface area (Raznahan et al. 2011).
Conclusion
We report that neonates with prenatal cocaine exposure had smaller gray matter volumes in prefrontal and frontal cortical regions important for later executive functions and inhibitory control, and also had CSF enlargement indicative of neuronal abnormality. Based on these findings, it is not clear whether the cocaine-related gray matter growth is merely delayed or if the trajectory of development may be more permanently altered. It is also unclear from our data whether and when CSF enlargement may normalize during early life. It will be important to determine whether the observed early GM differences, and the altered timing of GM development that may occur with accelerated postnatal growth, are associated with aberrations in anatomical and functional connectivity. If so, study of whether such aberrations substantively impair cognitive function would be warranted. Future longitudinal imaging studies that include measures of anatomical structure, function and connectivity are needed to determine how these early differences might influence brain development. Incorporation of imaging findings with developmental assessments and careful quantification of prenatal exposures, as well as pre- and postnatal environmental risk factors, will advance understanding of the effects of cocaine on early brain structure and function, and will inform potential early interventions to remediate impairments of brain and cognitive development.
Supplementary Material
Highlights.
Prenatal cocaine exposure is linked to reduced neonatal total cortical gray matter
Prenatal cocaine exposure is linked to enlarged neonatal total CSF volume
Cocaine-induced GM loss is greatest in neonatal prefrontal cortex
Acknowledgments
This research was supported by funding from the National Institutes of Health: 1P01DA022446-01A1 (J.J.); K01DA019949-01A1 (K.G.); R01MH070890 (J.H.G.); Conte Center MH064065 (J.H.G); NA-MIC Project U54EB005149 (G.G.); and Utah Science and Technology Initiative, University of Utah (G.G.). We thank the staff at the UNC Biomedical Research Imaging Center for technical assistance and indispensable advice, and Dianne Evans for invaluable help with implementation of the infant imaging protocol. We also thank participating families who have made this research possible.
Footnotes
Financial Disclosures
Dr. Grewen, Dr. Gerig, Dr. Gilmore, Dr. Johns, Dr. Lin, Mr. Vachet, Mr. Gouttard, and Ms Elam reported no biomedical financial interests or potential conflicts of interest. Dr. Burchinal reports receiving honoraria from Mathematica Policy Institute, Child Trends, First 5 LA, American Institute of Research, and the University of Lisbon.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accornero VH, Amado AJ, et al. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28(3):195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, et al. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011;68(3):283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Hurt H, et al. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol. 2007;37(4):275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Balaraman S, Winzer-Serhan UH, et al. Opposing actions of ethanol and nicotine on microRNAs are mediated by nicotinic acetylcholine receptors in fetal cerebral cortical-derived neural progenitor cells. Alcohol Clin Exp Res. 2012;36(10):1669–1677. doi: 10.1111/j.1530-0277.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, et al. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23(6):545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, et al. Prenatal drug exposure: infant and toddler outcomes. J Addict Dis. 2010;29(2):245–258. doi: 10.1080/10550881003684871. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Garavan H, et al. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56(3):1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, et al. Increased CSF volumes are associated with diminished subjective responses to cocaine infusion. Neuropsychopharmacology. 2000;23(4):468–473. doi: 10.1016/S0893-133X(00)00122-6. [DOI] [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Dev Psychol. 1998;34:555–564. [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing with Independent Statistics. Journal of Educational and Behavioral Statistics. 2000;25:60–83. [Google Scholar]
- Benveniste H, Fowler JS, et al. Cocaine is pharmacologically active in the nonhuman primate fetal brain. Proc Natl Acad Sci U S A. 2010;107(4):1582–1587. doi: 10.1073/pnas.0909585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt LM, Yang W, et al. Adolescents with and without gestational cocaine exposure: Longitudinal analysis of inhibitory control, memory and receptive language. Neurotoxicol Teratol. 2011;33(1):36–46. doi: 10.1016/j.ntt.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: The effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33(1):47–60. doi: 10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Pediatric and Pregnancy Nutrition Surveillance System. 2009 from http://www.cdc.gov/pednss/what_is/pednss_health_indicators.htm.
- Chaplin TM, Freiburger MB, et al. Prenatal cocaine exposure, gender, and adolescent stress response: a prospective longitudinal study. Neurotoxicol Teratol. 2010;32(6):595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese B, Moore G, et al. Magnetic resonance and spectroscopic imaging of prenatal alcohol-exposed children: Preliminary findings in the caudate nucleus. Neurotoxicol Teratol. 2006;28:597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Covert RF, Schreiber MD, et al. Hemodynamic and cerebral blood flow effects of cocaine, cocethylene and benzoylecgonine in consious and anesthetized fetal lambs. J Pharmacol Exp Ther. 1994;270:118–126. [PubMed] [Google Scholar]
- Coyle JT. Brain Structural Alterations Induced by Fetal Exposure to Cocaine Persist Into Adolescence and Affect Behavior. JAMA Psychiatry. 2013;167(4):348–354. doi: 10.1001/jamapsychiatry.2013.1949. [DOI] [PubMed] [Google Scholar]
- de Kieviet JF, Zoetebier L, et al. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012;54(4):313–323. doi: 10.1111/j.1469-8749.2011.04216.x. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, et al. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol Teratol. 2011;33(1):110–119. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Ende G, et al. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35(9):1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D. Fetal and maternal cocaine levels peak rapidly following intragastric administration in the rat. J Subst Abuse. 1990;2(4):427–437. doi: 10.1016/s0899-3289(12)80003-4. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D. Translational issues for prenatal cocaine studies and the role of environment. Neurotoxicol Teratol. 2011;33(1):9–16. doi: 10.1016/j.ntt.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards DL, Benveniste H, et al. Neuroimaging of prenatal drug exposure. Neurotoxicol Teratol. 2006;28:386–402. doi: 10.1016/j.ntt.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, et al. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84(1):30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Eiden RD, McAuliffe S, et al. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicol Teratol. 2009;31(1):60–68. doi: 10.1016/j.ntt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Schuetze P, et al. Maternal cocaine use and mother-infant interactions: Direct and moderated associations. Neurotoxicol Teratol. 2011;33(1):120–128. doi: 10.1016/j.ntt.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Schuetze P, et al. Cocaine Exposure and Children’s Self-Regulation: Indirect Association via Maternal Harshness. Front Psychiatry. 2011;2:31. doi: 10.3389/fpsyt.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, et al. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134(Pt 7):2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, et al. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, et al. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012;169(9):926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, et al. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68(1):87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer’s disease. Lancet. 2004;363(9406):392–394. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, et al. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285(12):1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, McCarten KM, et al. Level of in utero cocaine exposure and neonatal ultrasound findings. Pediatrics. 1999;104:1101–1105. doi: 10.1542/peds.104.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Salas-Ramirez K, et al. Cocaine alters dendritic spine density in cortical and subcortical brain regions of the postpartum and virgin female rat. Synapse. 2011;65(9):955–961. doi: 10.1002/syn.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Wang HY, et al. Prenatal cocaine increases dendritic spine density in cortical and subcortical brain regions of the rat. Dev Neurosci. 2009;31(1–2):71–75. doi: 10.1159/000207495. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51(2):134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gan X, Zhang L, et al. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol. 1999;91(1):68–76. doi: 10.1006/clim.1998.4683. [DOI] [PubMed] [Google Scholar]
- Gao W, Zhu H, et al. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Schmitt JE, et al. Genetic and environmental contributions to neonatal brain structure: A twin study. Hum Brain Mapp. 2010;31(8):1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 2012;22(11):2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Anson B, Ramsey RG. Pachygyria in a neonate with prenatal cocaine exposure: MR features. J Comput Assist Tomogr. 1994;18(4):637–639. doi: 10.1097/00004728-199407000-00023. [DOI] [PubMed] [Google Scholar]
- Gouin K, Murphy K, et al. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am J Obstet Gynecol. 2011;204(4):340 e341–312. doi: 10.1016/j.ajog.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, et al. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011;218(4):681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indiana Natality Report. Indiana State Department of Health; 1999–2002. http://www.in.gov/isdh/19598.htm. [Google Scholar]
- Irner TB. Substance exposure in utero and developmental consequences in adolescence: a systematic review. Child Neuropsychol. 2012;18(6):521–549. doi: 10.1080/09297049.2011.628309. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Kang C, et al. Twin-singleton differences in neonatal brain structure. Twin Res Hum Genet. 2011;14(3):268–276. doi: 10.1375/twin.14.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JK, Penn AA. In: Perinatal Brain, Development, Malformation, and Injury. McCarthy MM, editor. Morgan Claypool Life Sciences; 2012. [Google Scholar]
- Landi N, Crowley MJ, et al. Deviant ERP response to spoken non-words among adolescents exposed to cocaine in utero. Brain Lang. 2012;120(3):209–216. doi: 10.1016/j.bandl.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, et al. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5(7):e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16(6):233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lagasse L, et al. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr. 2003;142(3):279–285. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Minnes S, et al. The effects of prenatal cocaine on language development at 10 years of age. Neurotoxicol Teratol. 2011;33(1):17–24. doi: 10.1016/j.ntt.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, et al. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicol Teratol. 2009;31(6):342–348. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, et al. Increased “default mode” activity in adolescents prenatally exposed to cocaine. Hum Brain Mapp. 2011;32(5):759–770. doi: 10.1002/hbm.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhu Q, et al. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol. 2008;29(10):1883–1889. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link EA, Weese-Mayer DW, et al. Magnetic resonance imaging in infants exposed to cocaine prenatally: a preliminary report. Clin Pediatr (Phila) 1991;30(8):506–508. doi: 10.1177/000992289103000810. [DOI] [PubMed] [Google Scholar]
- Liu J, Lester BM, et al. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. 2013;167(4):348–354. doi: 10.1001/jamapediatrics.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JR, Maclean PC, et al. Comparison of cerebral volume in children aged 18–22 and 36–47 months born preterm and term. J Child Neurol. 2012;27(2):172–177. doi: 10.1177/0883073811415409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Cabral HJ, et al. Pre-natal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29(4):446–457. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, et al. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19(3):1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Mayes L, Snyder PJ, et al. Visuospatial working memory in school-aged children exposed in utero to cocaine. Child Neuropsychol. 2007;13(3):205–218. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Quenzer LF. Psychopharmacology: Drugs, the Brain and Behavior. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Minnes S, Singer L, et al. Effects of prenatal cocaine/polydrug use on maternal-infant feeding interactions during the first year of life. Developmental and Behavioral Pediatrics. 2005;26(3):194–200. doi: 10.1097/00004703-200506000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, et al. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Morrow CE, Culbertson JL, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905–931. doi: 10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland JS, Singer LT, et al. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27(3):429–438. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Phillips JP, Montague EQ, et al. Prematurity affects cortical maturation in early childhood. Pediatr Neurol. 2011;45(4):213–219. doi: 10.1016/j.pediatrneurol.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolo M, McCarthy DM, et al. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26(2–4):229–244. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, et al. Automatic segmentation of MR images of the developing newborn brain. Med Image Anal. 2005;9(5):457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Rao H, Wang J, et al. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120(5):e1245–1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, et al. How does your cortex grow? J Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JQ, Malanga CJ, et al. Neuropathological consequences of prenatal cocaine exposure in the mouse. Int J Dev Neurosci. 2004;22(5–6):309–320. doi: 10.1016/j.ijdevneu.2004.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, Hurni N, et al. Alpha2-adrenergic receptor activation regulates cortical interneuron migration. Eur J Neurosci. 2012;36(7):2879–2887. doi: 10.1111/j.1460-9568.2012.08231.x. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, et al. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121(4):741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CE, Anderson PJ, et al. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. J Am Acad Child Adolesc Psychiatry. 2012;51(2):181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte F, Soderberg L, et al. Structural, metabolic, and functional brain abnormalities as a result of prenatal exposure to drugs of abuse: evidence from neuroimaging. Neuropsychol Rev. 2010;20(4):376–397. doi: 10.1007/s11065-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Brodsky NL, et al. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26(1):42–47. [PubMed] [Google Scholar]
- Schenker S, Yang Y, et al. The transfer of cocaine and its metabolites across the term human placenta. Clin Pharmacol Ther. 1993;53(3):329–339. doi: 10.1038/clpt.1993.29. [DOI] [PubMed] [Google Scholar]
- Scher MS, Richardson GA, et al. Effects of prenatal cocaine/crack and other drug exposure on electroencephalographic sleep studies at birth and one year. Pediatrics. 2000;105(1 Pt 1):39–48. doi: 10.1542/peds.105.1.39. [DOI] [PubMed] [Google Scholar]
- Schroder MD, Snyder PJ, et al. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain Cogn. 2004;55(2):409–412. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32(10):2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, et al. Developing language skills of cocaine-exposed infants. Pediatrics. 2001;107(5):1057–1064. doi: 10.1542/peds.107.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Eisengart LJ, et al. Prenatal cocaine exposure and infant cognition. Infant Behav Dev. 2005;28(4):431–444. doi: 10.1016/j.infbeh.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Chang L, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. A. R. Foundation. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- Song ZM, Abou-Zeid O, et al. alpha2a adrenoceptors regulate phosphorylation of microtubule-associated protein-2 in cultured cortical neurons. Neuroscience. 2004;123(2):405–418. doi: 10.1016/j.neuroscience.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Song ZM, Undie AS, et al. D1 dopamine receptor regulation of microtubule-associated protein-2 phosphorylation in developing cerebral cortical neurons. J Neurosci. 2002;22(14):6092–6105. doi: 10.1523/JNEUROSCI.22-14-06092.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squitieri F, Cannella M, et al. Distinct brain volume changes correlating with clinical stage, disease progression rate, mutation size, and age at onset prediction as early biomarkers of brain atrophy in Huntington’s disease. CNS Neurosci Ther. 2009;15(1):1–11. doi: 10.1111/j.1755-5949.2008.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Prenatal exposure to cocaine produces unique developmental and long-term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci. 2007;27(1):152–157. doi: 10.1523/JNEUROSCI.4591-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick EZ, Messinger DS, et al. Cocaine exposure is associated with subtle compromises of infants’ and mothers’ social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Dev Psychol. 2005;41(5):711–722. doi: 10.1037/0012-1649.41.5.711. [DOI] [PubMed] [Google Scholar]
- Tzarouchi LC, Astrakas LG, et al. Periventricular leukomalacia in preterm children: assessment of grey and white matter and cerebrospinal fluid changes by MRI. Pediatr Radiol. 2009;39(12):1327–1332. doi: 10.1007/s00247-009-1389-0. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev Neurosci. 2003;25(2–4):245–256. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, et al. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine–exposed children. Pediatrics. 2006;118(5):2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, et al. Serotonin as a developmental signal. Behav Brain Res. 1996;73(1–2):19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Yao H, Kim K, et al. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31(16):5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap PT, Fan Y, et al. Development trends of white matter connectivity in the first years of life. PLoS One. 2011;6(9):e24678. doi: 10.1371/journal.pone.0024678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.