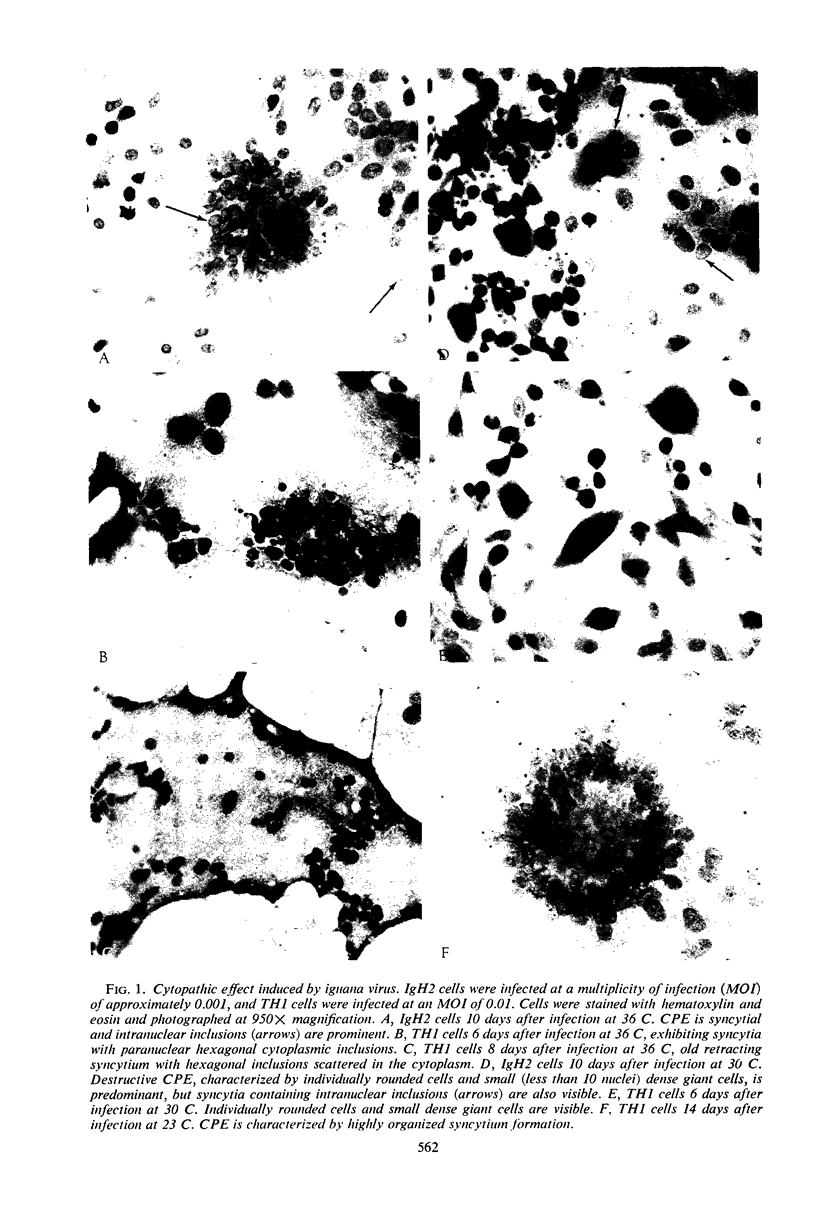
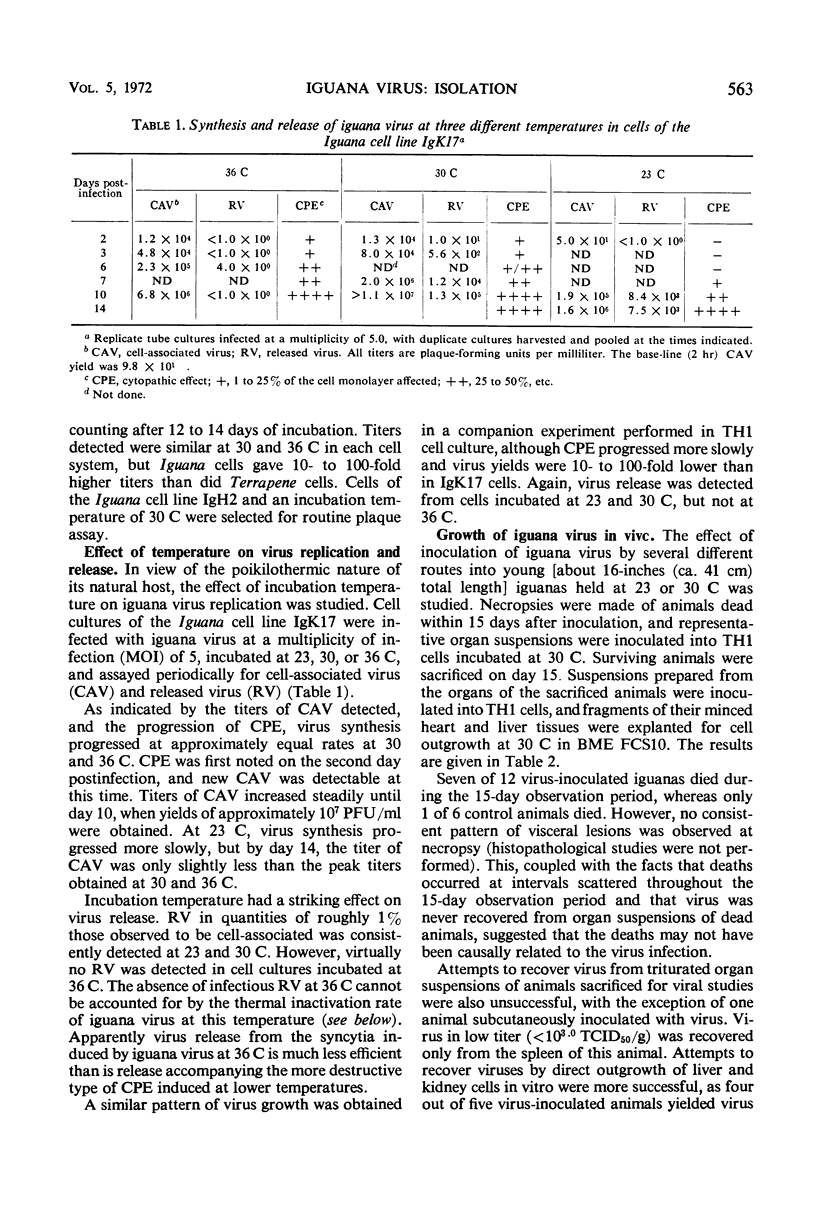
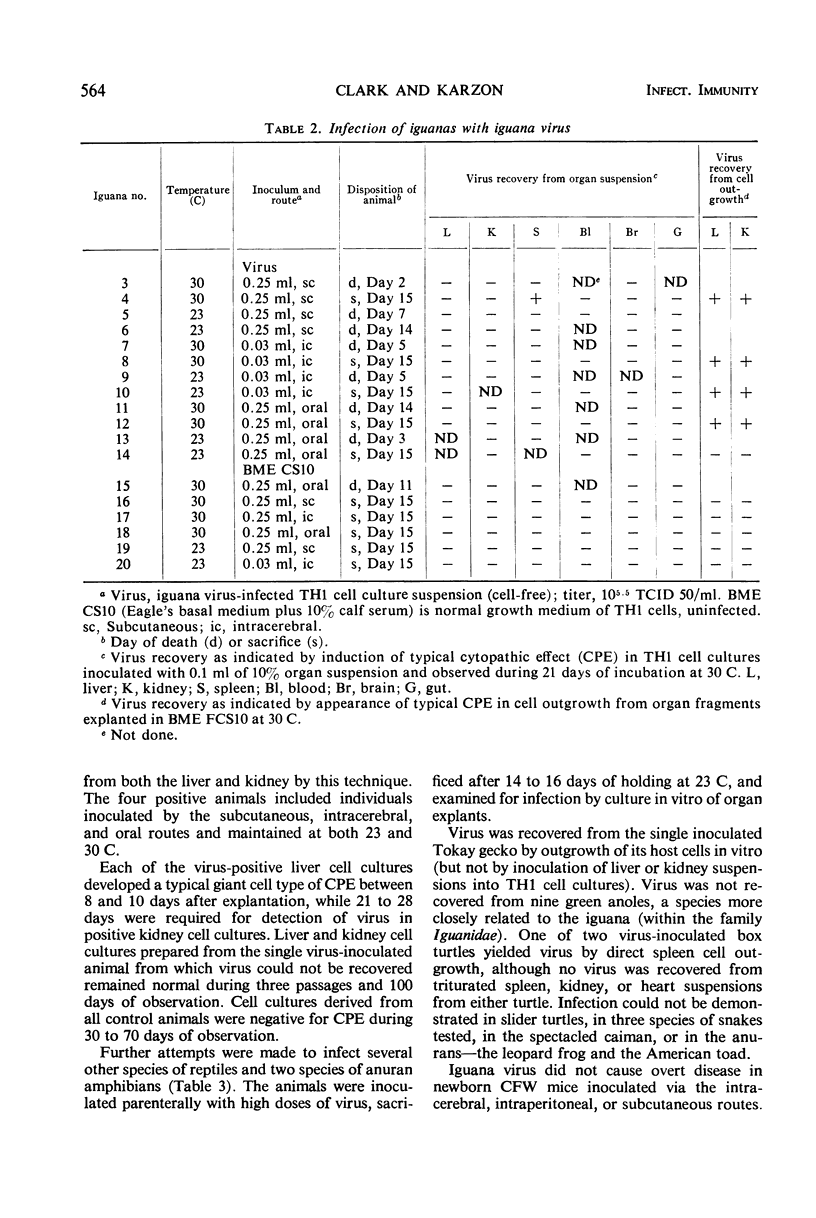
Abstract
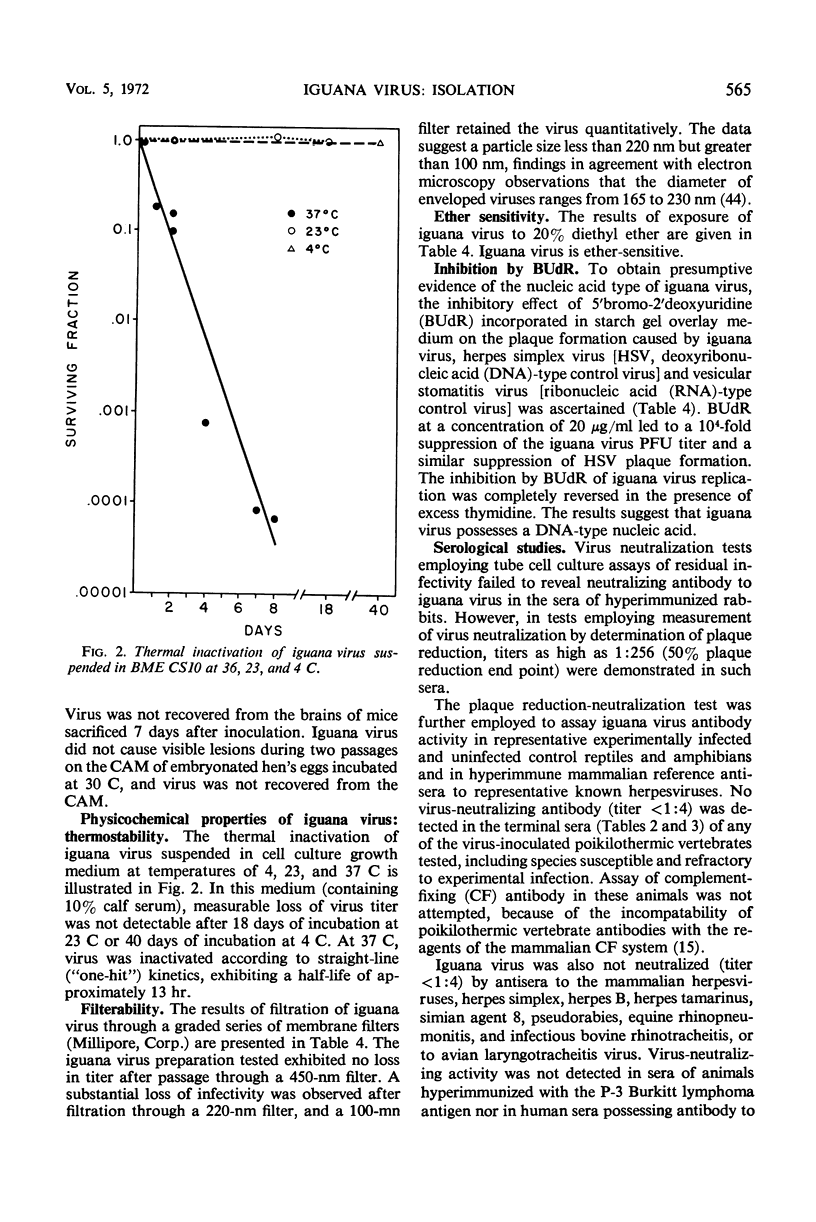
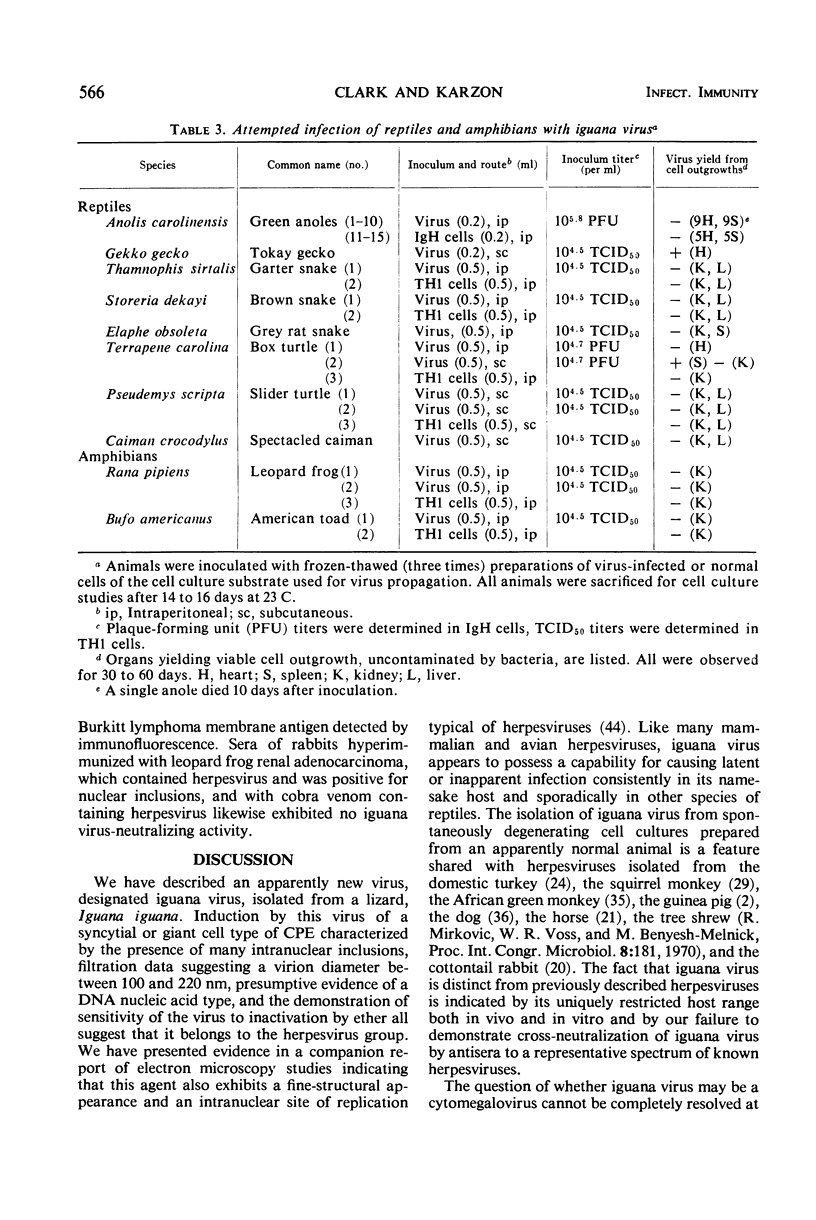
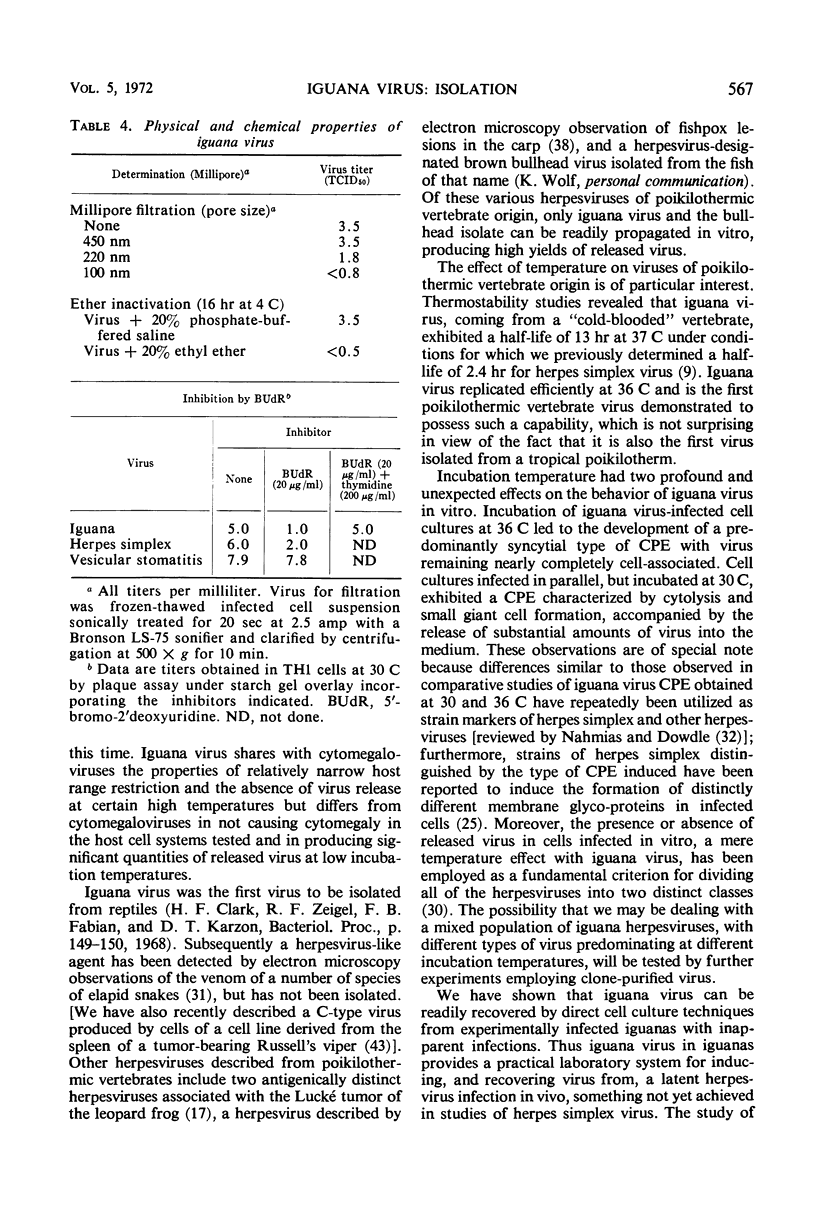
An agent cytopathic for Terrapene and Iguana cell cultures was isolated from spontaneously degenerating cell cultures prepared from a green iguana (Iguana iguana). The agent, designated iguana virus, caused a cytopathic effect (CPE) of a giant cell type, with eosinophilic inclusions commonly observed within giant cell nuclei. Incubation temperature had a marked effect on CPE and on virus release from infected cells. Within the range of 23 to 36 C, low temperatures favored CPE characterized by cytolysis and small giant cell formation, and significant virus release was observed. At warmer temperatures, a purely syncytial type of CPE and total absence of released virus were noted. A unique type of hexagonal eosinophilic cytoplasmic inclusion was observed within syncytia of infected Terrapene cell cultures incubated at 36 C. In vivo studies revealed no evidence of pathogenicity of iguana virus for suckling mice, embryonated hen's eggs, or several species of reptiles and amphibians. Inoculation of iguana virus into young iguanas consistently caused infection that was “unmasked” only when cell cultures were prepared directly from the infected animal. Filtration studies revealed a virion size of >100 nm and <220 nm. Iguana virus is ether-sensitive and, as presumptively indicated by studies of inhibition by bromodeoxyuridine, possesses a deoxyribonucleic type of nucleic acid. The virus characteristics described, as well as electron microscopy observations described in a separate report, indicate that iguana virus is a member of the herpesvirus group.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balls M., Ruben L. N. Cultivation in vitro of normal and neoplastic cells of Xenopus laevis. Exp Cell Res. 1966 Oct;43(3):694–695. doi: 10.1016/0014-4827(66)90048-6. [DOI] [PubMed] [Google Scholar]
- Bhatt P. N., Percy D. H., Craft J. L., Jonas A. M. Isolation and characterization of a herpeslike (Hsiung-Kaplow) virus from guinea pigs. J Infect Dis. 1971 Feb;123(2):178–189. doi: 10.1093/infdis/123.2.178. [DOI] [PubMed] [Google Scholar]
- COLEMAN V., JAWETZ E. A persistent herpes simplex infection in antibody-free cell culture. Virology. 1961 Mar;13:375–377. doi: 10.1016/0042-6822(61)90162-3. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Cohen M. M., Karzon D. T. Characterization of reptilian cell lines established at incubation temperatures of 23 to 36 degrees. Proc Soc Exp Biol Med. 1970 Mar;133(3):1039–1047. doi: 10.3181/00379727-133-34622. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Diamond L. Comparative studies on the interaction of benzpyrene with cells derived from poikilothermic and homeothermic vertebrates. II. Effect of temperature on benzpyrene metabolism and cell multiplication. J Cell Physiol. 1971 Jun;77(3):385–392. doi: 10.1002/jcp.1040770313. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Kaminski F., Karzon D. T. Thermoelectrically cooled temperature-gradient apparatus for comparative cell and virus temperature studies. Appl Microbiol. 1970 May;19(5):848–854. doi: 10.1128/am.19.5.848-854.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Karzon D. T. Acquired tolerance to elevated temperatures in a poikilothermic cell line (terrapene heart, TH-1). Exp Cell Res. 1967 Nov;48(2):269–275. doi: 10.1016/0014-4827(67)90352-7. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Karzon D. T. Temperature optima of mammalian and amphibian viruses in cell cultures of homeothermic and poikilothermic origin. Arch Gesamte Virusforsch. 1968;23(3):270–279. doi: 10.1007/BF01241899. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Karzon D. T. Terrapene heart (TH-1), a continuous cell line from the heart of the box turtle Terrapene carolina. Exp Cell Res. 1967 Nov;48(2):263–268. doi: 10.1016/0014-4827(67)90351-5. [DOI] [PubMed] [Google Scholar]
- DEMAEYER E., SCHONNE E. STARCH GEL AS AN OVERLAY FOR THE PLAQUE ASSAY OF ANIMAL VIRUSES. Virology. 1964 Sep;24:13–18. doi: 10.1016/0042-6822(64)90142-4. [DOI] [PubMed] [Google Scholar]
- GEBHARDT L. P., STANTON G. J., HILL D. W., COLLETT G. C. NATURAL OVERWINTERING HOSTS OF THE VIRUS OF WESTERN EQUINE ENCEPHALITIS. N Engl J Med. 1964 Jul 23;271:172–177. doi: 10.1056/NEJM196407232710402. [DOI] [PubMed] [Google Scholar]
- Granoff A. Viruses of amphibia. Curr Top Microbiol Immunol. 1969;50:107–137. doi: 10.1007/978-3-642-46169-9_4. [DOI] [PubMed] [Google Scholar]
- Gravell M., Malsberger R. G. A permanent cell line from the fathead minnow (Pimephales promelas). Ann N Y Acad Sci. 1965 Aug 10;126(1):555–565. doi: 10.1111/j.1749-6632.1965.tb14302.x. [DOI] [PubMed] [Google Scholar]
- Gravell M. Viruses and renal carcinoma of Rana pipiens. X. Comparison of herpes-type viruses associated with Lucké tumor-bearing frogs. Virology. 1971 Mar;43(3):730–733. doi: 10.1016/0042-6822(71)90301-1. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze H. C. New member of the herpesvirus group isolated from wild cottontail rabbits. Infect Immun. 1971 Feb;3(2):350–354. doi: 10.1128/iai.3.2.350-354.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D., Fischman H. R., Fong C. K., Green R. H. Characterization of a cytomegalo-like virus isolated from spontaneously degenerated equine kidney cell culture. Proc Soc Exp Biol Med. 1969 Jan;130(1):80–84. doi: 10.3181/00379727-130-33492. [DOI] [PubMed] [Google Scholar]
- Jensen M. H. Research on the virus of Egtved disease. Ann N Y Acad Sci. 1965 Aug 10;126(1):422–426. doi: 10.1111/j.1749-6632.1965.tb14292.x. [DOI] [PubMed] [Google Scholar]
- KARZON D. T., BUSSELL R. H. Cytopathic effect of canine distemper virus in tissue culture. Science. 1959 Dec 18;130(3390):1708–1709. doi: 10.1126/science.130.3390.1708. [DOI] [PubMed] [Google Scholar]
- KARZON D. T., POLLOCK B. F., BARRON A. L. Phase variation in ECHO virus type 6. Virology. 1959 Dec;9:564–576. doi: 10.1016/0042-6822(59)90149-7. [DOI] [PubMed] [Google Scholar]
- Kawamura H., King D. J., Jr, Anderson D. P. A herpesvirus isolated from kidney cell culture of normal turkeys. Avian Dis. 1969 Nov;13(4):853–863. [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane D. E., Jr, Clark H. F., Karzon D. T. A plaque method for titration of frog viruses using starch gel overlay. Proc Soc Exp Biol Med. 1967 May;125(1):50–54. doi: 10.3181/00379727-125-32010. [DOI] [PubMed] [Google Scholar]
- Lehane D. E., Jr, Clark H. F., Karzon D. T. Antigenic relationships among frog viruses demonstrated by the plaque reduction and neutralization kinetics tests. Virology. 1968 Apr;34(4):590–595. doi: 10.1016/0042-6822(68)90080-9. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Melnick J. L., McCombs R. M. Classification and nomenclature of animal viruses, 1966. Prog Med Virol. 1966;8:400–409. [PubMed] [Google Scholar]
- Monroe J. H., Shibley G. P., Schidlovsky G., Nakai T., Howatson A. F., Wivel N. W., O'Connor T. E. Action of snake venom on Rauscher virus. J Natl Cancer Inst. 1968 Jan;40(1):135–145. [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R. Antigenic and biologic differences in herpesvirus hominis. Prog Med Virol. 1968;10:110–159. [PubMed] [Google Scholar]
- Rafferty K. A., Jr The cultivation of inclusion-associated viruses from Lucké tumor frogs. Ann N Y Acad Sci. 1965 Aug 10;126(1):3–21. doi: 10.1111/j.1749-6632.1965.tb14266.x. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Thiel J. F., Newman J. T., Harvey E., Trousdale M. D., Gehle W. D., Clark G. Cytomegaloviruses as common adventitious contaminants in primary African green monkey kidney cell cultures. J Natl Cancer Inst. 1969 Mar;42(3):489–496. [PubMed] [Google Scholar]
- Spertzel R. O., Huxsoll D. L., McConnell S. J., Binn L. N., Yager R. H. Recovery and characterization of a herpes-like virus from dog kidney cell cultures. Proc Soc Exp Biol Med. 1965 Dec;120(3):651–655. doi: 10.3181/00379727-120-30615. [DOI] [PubMed] [Google Scholar]
- THOMAS L. A., EKLUND C. M. Overwintering of western equine encephalomyelitis virus in experimentally infected garter snakes and transmission to mosquitoes. Proc Soc Exp Biol Med. 1960 Oct;105:52–55. doi: 10.3181/00379727-105-26006. [DOI] [PubMed] [Google Scholar]
- WOLF K., DUNBAR C. E. Cultivation of adult teleost tissues in vitro. Proc Soc Exp Biol Med. 1957 Jul;95(3):455–458. doi: 10.3181/00379727-95-23250. [DOI] [PubMed] [Google Scholar]
- WOLF K., SNIESZKO S. F., DUNBAR C. E., PYLE E. Virus nature of infectious pancreatic necrosis in trout. Proc Soc Exp Biol Med. 1960 May;104:105–108. doi: 10.3181/00379727-104-25743. [DOI] [PubMed] [Google Scholar]
- Wolf K., Gravell M., Malsberger R. G. Lymphocystis virus: isolation and propagation in centrarchid fish cell lines. Science. 1966 Feb 25;151(3713):1004–1005. doi: 10.1126/science.151.3713.1004. [DOI] [PubMed] [Google Scholar]
- Wolf K. The fish viruses. Adv Virus Res. 1966;12:35–101. doi: 10.1016/s0065-3527(08)60846-5. [DOI] [PubMed] [Google Scholar]
- Zeigel R. F., Clark H. F. Electron microscopic observations on a "C"-type virus in cell cultures derived from a tumor-bearing viper. J Natl Cancer Inst. 1969 Nov;43(5):1097–1102. [PubMed] [Google Scholar]
- Zeigel R. F., Clark H. F. Electron microscopy observations on a new herpes-type virus isolated from Iguana iguana and propagated in reptilian cells in vitro. Infect Immun. 1972 Apr;5(4):570–582. doi: 10.1128/iai.5.4.570-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]