Abstract
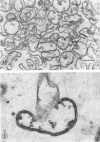
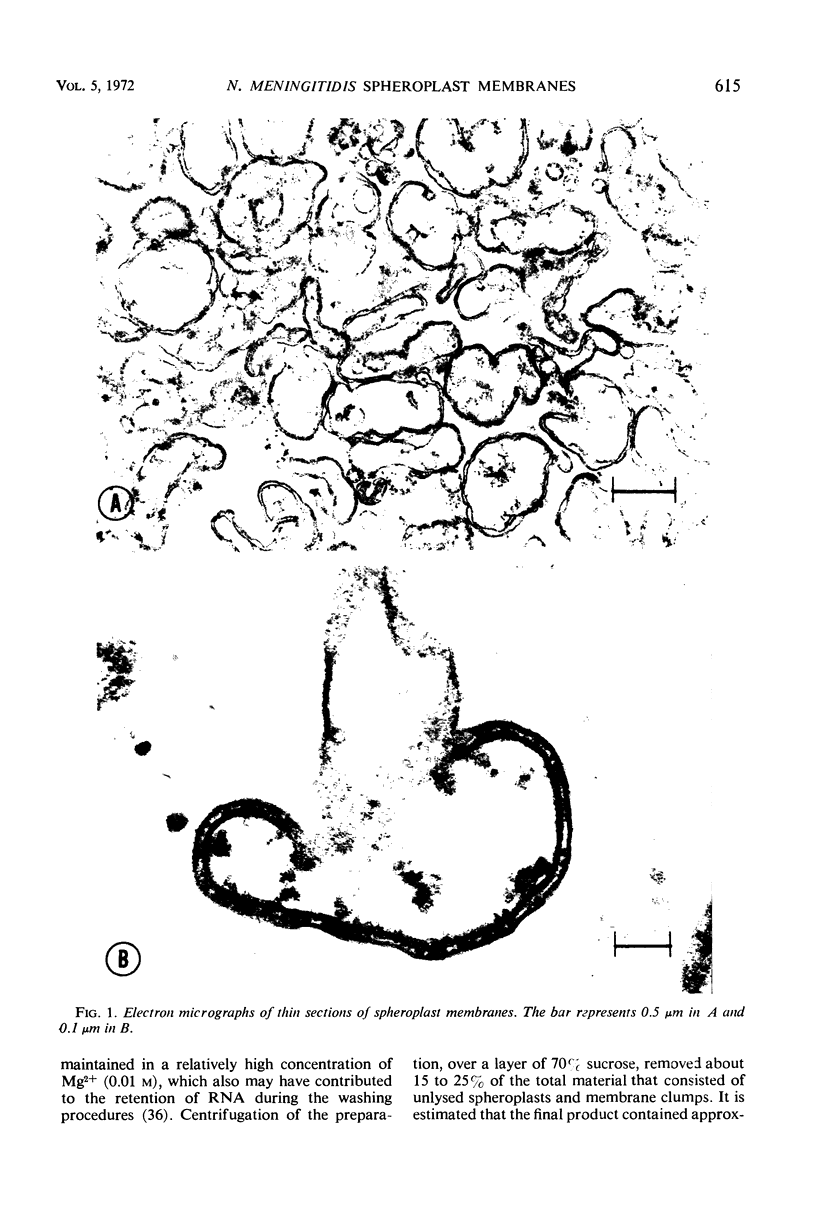
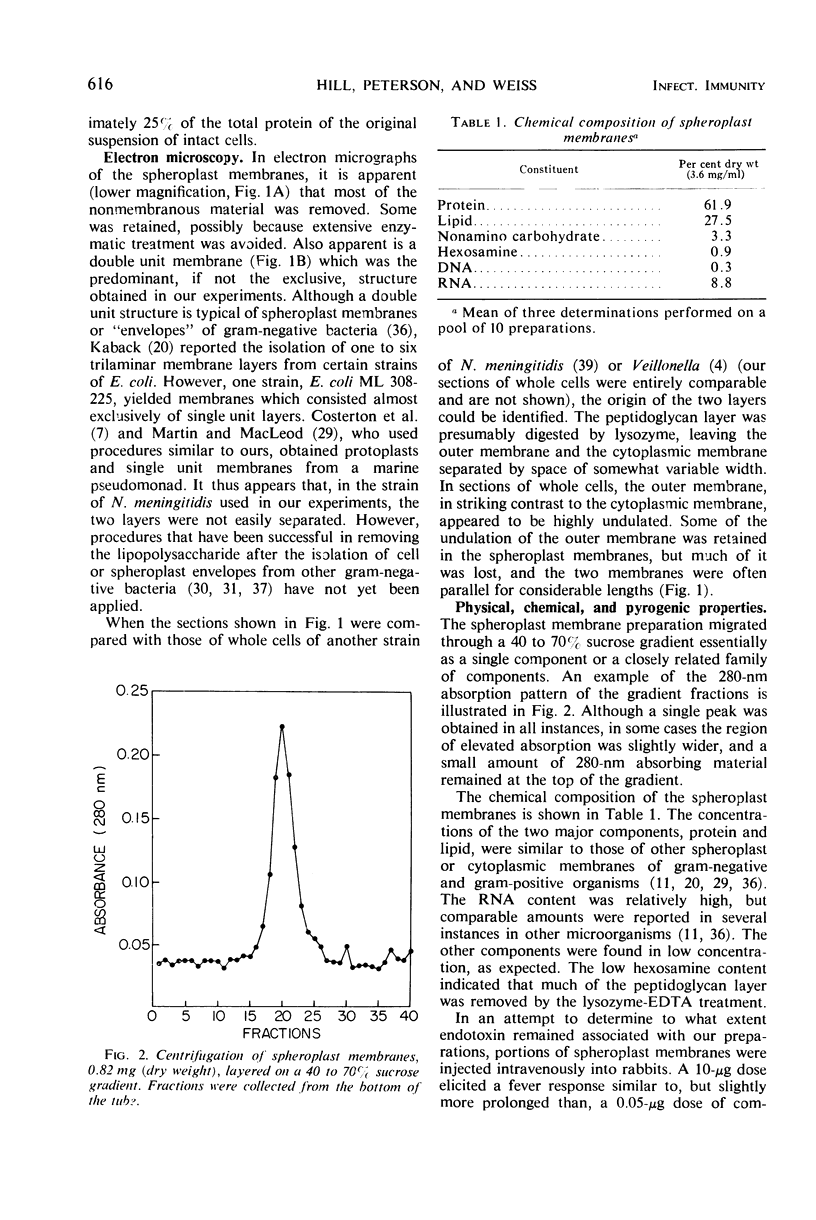
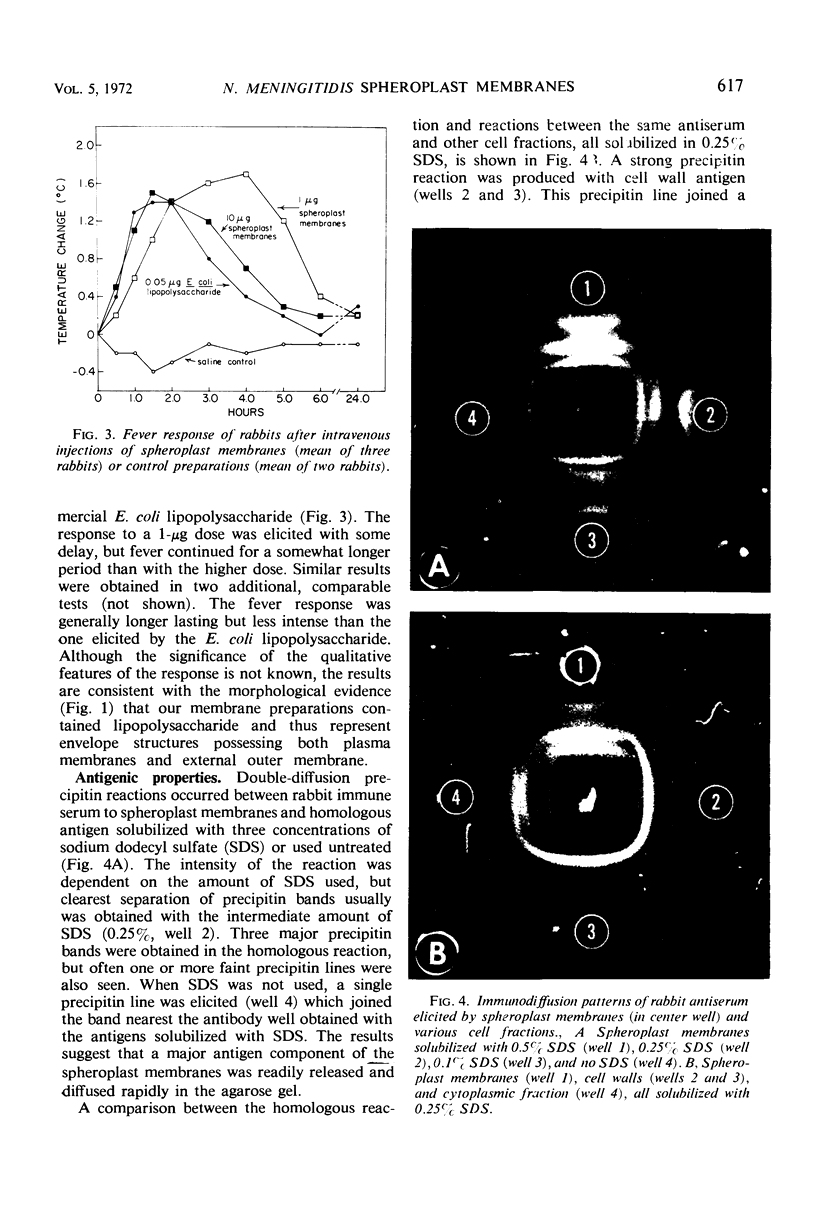
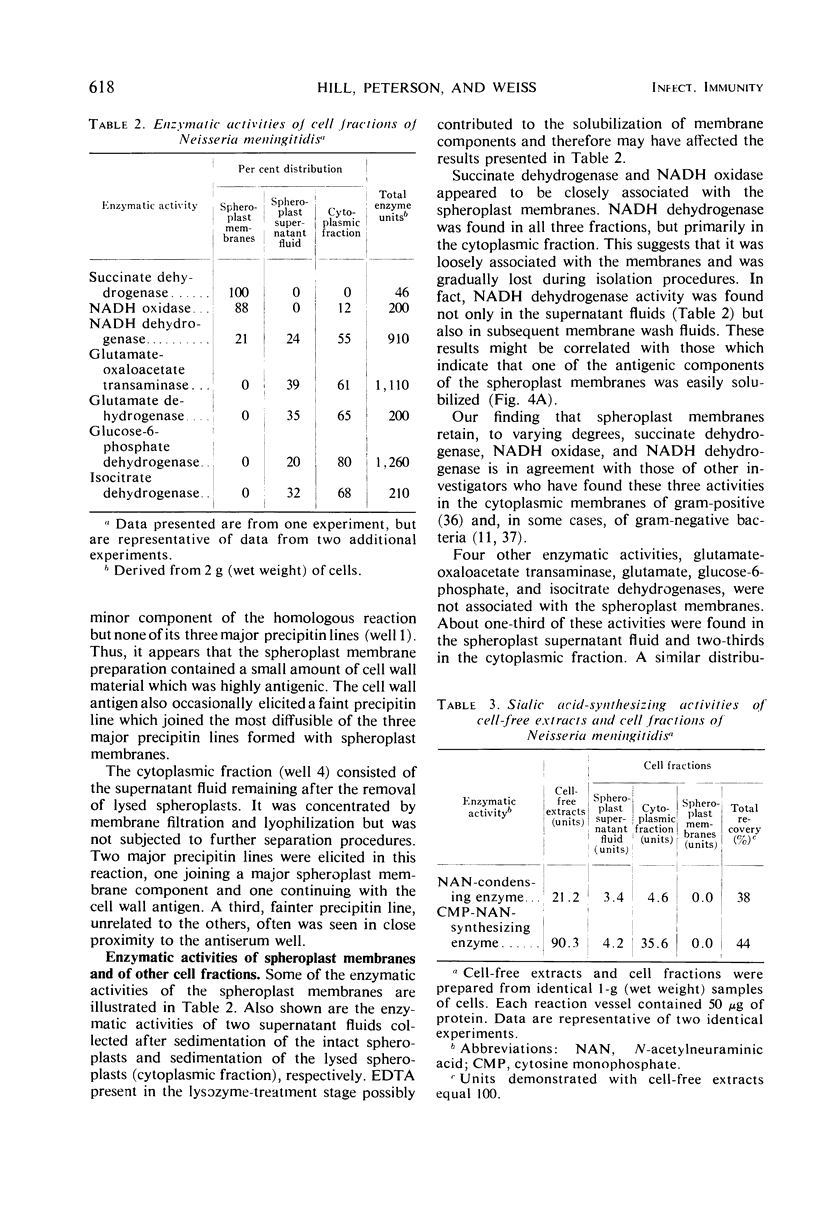
Spheroplast membranes (spheroplast envelopes) of strain 2091 of group B Neisseria meningitidis were prepared by a procedure that included lysozyme treatment of the cells and osmotic lysis of the resulting spheroplasts. Electron microscopy revealed that the membranes consisted of two unit layers, generally parallel to each other. The membrane preparation migrated as a single component in a 40 to 70% sucrose gradient and consisted of 62% protein, 28% lipid, 9% ribonucleic acid, small amounts of carbohydrate, hexosamine, and deoxyribonucleic acid. When 1 or 10 μg (dry weight) was injected intravenously into rabbits, a mild pyrogenic reaction was elicited. In immunodiffusion tests, immune rabbit serum prepared against spheroplast membranes produced three major precipitin lines, with the homologous antigen solubilized with sodium dodecyl sulfate, and a single line with untreated antigen. The immune serum also reacted with a cell wall antigen, and to a lesser extent with some of the cytoplasmic antigens. Succinate dehydrogenase and reduced nicotinamide adenine dinucleotide (NADH) oxidase activities were found to be associated with the spheroplast membranes. NADH dehydrogenase also was associated with the membranes but was gradually released and recovered in other fractions. Glutamate-oxaloacetate transaminase, glutamate, glucose-6-phosphate, and isocitrate dehydrogenase activities were not found in the membrane preparation. About one-third of these enzymatic activities were recovered in the supernatant fluid after the sedimentation of the spheroplasts and two-thirds were recovered in the cytoplasmic fraction. N-acetylneuraminic acid (NAN)-condensing enzyme and cytidine monophosphate-NAN synthesizing enzyme also were identified in this organism. These enzymes were not associated with the membranes and were recovered from extracts from whole cells, spheroplasts, or cells exposed to osmotic shock, as well as from spheroplast supernatant and shock fluids. It is concluded that the spheroplast membranes of the strain of meningococci used in these studies are typical of those recovered from gram-negative bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Schneider H., Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970 Feb 19;282(8):417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- BLACKLOW R. S., WARREN L. Biosynthesis of sialic acids by Neisseria meningitidis. J Biol Chem. 1962 Nov;237:3520–3526. [PubMed] [Google Scholar]
- BLADEN H. A., MERGENHAGEN S. E. ULTRASTRUCTURE OF VEILLONELLA AND MORPHOLOGICAL CORRELATION OF AN OUTER MEMBRANE WITH PARTICLES ASSOCIATED WITH ENDOTOXIC ACTIVITY. J Bacteriol. 1964 Nov;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The solubilization of the cytoplasmic membrane of Bacillus subtilis by sodium dodecyl sulphate. Eur J Biochem. 1967 Nov;2(4):454–459. doi: 10.1111/j.1432-1033.1967.tb00159.x. [DOI] [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine L. F., Pierce W. E., Floyd T. M., Rhode S. L., Edwards E. A., Siess E. E., Peckinpaugh R. O. Evaluation of group C meningococcal polysaccharide vaccine in marine recruits, San Diego, California. Am J Epidemiol. 1970 Jul;92(1):25–32. doi: 10.1093/oxfordjournals.aje.a121176. [DOI] [PubMed] [Google Scholar]
- Ferrandes B., Frehel C., Chaix P. Fractionment et purification des systèmes membranaires cytoplasmiques et mésosomiquees de lbacillus subtilis. Etude de quelques-unes de leurs propríetés oxydo-réductricwa associées à la chaine respiratoire. Biochim Biophys Acta. 1970 Dec 8;223(2):292–308. doi: 10.1016/0005-2728(70)90186-6. [DOI] [PubMed] [Google Scholar]
- Fink M. A., Feller W. F., Sibal L. R. Methods for detection of antibody to the mammary tumor virus. J Natl Cancer Inst. 1968 Dec;41(6):1395–1400. [PubMed] [Google Scholar]
- Franklin R. M., Datta A., Dahlberg J. E., Braunstein S. N. The cell membranes of a marine pseudomonad, Pseudomonas BAL-31; physical, chemical, and biochemical properties. Biochim Biophys Acta. 1971 Jun 1;233(3):521–537. doi: 10.1016/0005-2736(71)90152-0. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Nachbar M. S., Salton M. R. Immunological properties of Micrococcus lysodeikticus membranes. J Bacteriol. 1971 Jan;105(1):86–92. doi: 10.1128/jb.105.1.86-92.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. V. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med. 1969 Jun 1;129(6):1385–1395. doi: 10.1084/jem.129.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hill J. C. Effect of glutamate on exogenous citrate catabolism of Neisseria meningitidis and of other species of Neisseria. J Bacteriol. 1971 Jun;106(3):819–823. doi: 10.1128/jb.106.3.819-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Koke J. R., Gupta P. D., Malhotra S. K. A succinic dehydrogenase activity in "mesosomes" of Neurospora crassa. Biochem Biophys Res Commun. 1971 Feb 5;42(3):576–582. doi: 10.1016/0006-291x(71)90410-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Dunne F. T., Jonssen E. K. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J Biol Chem. 1971 Aug 10;246(15):4703–4712. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Jonssen E. K., Wysocki J. R. Studies on the meningococcal polysaccharides. I. Composition and chemical properties of the group A polysaccharide. J Biol Chem. 1971 May 10;246(9):2849–2858. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Martin E. L., MacLeod R. A. Isolation and chemical composition of the cytoplasmic membrane of a gram-negative bacterium. J Bacteriol. 1971 Mar;105(3):1160–1167. doi: 10.1128/jb.105.3.1160-1167.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Goldschneider I. The serum bactericidal system: ultrastructural changes in Neisseria meningitidis exposed to normal rat serum. J Exp Med. 1969 Jan 1;129(1):51–79. doi: 10.1084/jem.129.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedros N. A., Hill P. R. Chemical and antigenic analysis of the cell walls of Neisseria meningitidis group B. J Bacteriol. 1966 May;91(5):1992–1997. doi: 10.1128/jb.91.5.1992-1997.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L., BLACKLOW R. S. The biosynthesis of cytidine 5'-monophospho-n-acetylneuraminic acid by an enzyme from Neisseria meningitidis. J Biol Chem. 1962 Nov;237:3527–3534. [PubMed] [Google Scholar]