Abstract
Facilitated by recent advances using CRISPR/Cas9, genome editing technologies now permit custom genetic modifications in a wide variety of organisms. Ideally, modified animals could be both efficiently made and easily identified with minimal initial screening and without introducing exogenous sequence at the locus of interest or marker mutations elsewhere. To this end, we describe a coconversion strategy, using CRISPR/Cas9 in which screening for a dominant phenotypic oligonucleotide-templated conversion event at one locus can be used to enrich for custom modifications at another unlinked locus. After the desired mutation is identified among the F1 progeny heterozygous for the dominant marker mutation, F2 animals that have lost the marker mutation are picked to obtain the desired mutation in an unmarked genetic background. We have developed such a coconversion strategy for Caenorhabditis elegans, using a number of dominant phenotypic markers. Examining the coconversion at a second (unselected) locus of interest in the marked F1 animals, we observed that 14–84% of screened animals showed homologous recombination. By reconstituting the unmarked background through segregation of the dominant marker mutation at each step, we show that custom modification events can be carried out recursively, enabling multiple mutant animals to be made. While our initial choice of a coconversion marker [rol-6(su1006)] was readily applicable in a single round of coconversion, the genetic properties of this locus were not optimal in that CRISPR-mediated deletion mutations at the unselected rol-6 locus can render a fraction of coconverted strains recalcitrant to further rounds of similar mutagenesis. An optimal marker in this sense would provide phenotypic distinctions between the desired mutant/+ class and alternative +/+, mutant/null, null/null, and null/+ genotypes. Reviewing dominant alleles from classical C. elegans genetics, we identified one mutation in dpy-10 and one mutation in sqt-1 that meet these criteria and demonstrate that these too can be used as effective conversion markers. Coconversion was observed using a variety of donor molecules at the second (unselected) locus, including oligonucleotides, PCR products, and plasmids. We note that the coconversion approach described here could be applied in any of the variety of systems where suitable coconversion markers can be identified from previous intensive genetic analyses of gain-of-function alleles.
Keywords: CRISPR/Cas9, oligonucleotide-mediated homologous recombination, coconversion, dpy-10, rde-1
TYPE II CRISPR/Cas9 bacterial immunity systems provide programmable DNA endonuclease activities that have recently revolutionized genome editing in a wide range of organisms (Wang et al. 1999; Chiu et al. 2013; Cho et al. 2013; Dicarlo et al. 2013; Friedland et al. 2013; Gratz et al. 2013; Hwang et al. 2013; Jiang et al. 2013; Katic and Großhans 2013; Li et al. 2013; Lo et al. 2013; Nekrasov et al. 2013; Kim et al. 2014; Zhao et al. 2014). Recognition by the Cas9 protein entails two sequence elements in the target: a protospacer adjacent motif (PAM) (NGG for Streptococcus pyogenes Cas9) and a region of ∼20 bp of complementarity to its guide RNA (gRNA) (Jinek et al. 2012). Following cleavage by CRISPR/Cas9 in vivo, the double-strand break site can be repaired to generate mutations, including insertions and deletions via endogenous pathways such as nonhomologous end joining (NHEJ) or targeted base mutations via homologous repair (HR) from a template or donor DNA. The ease of use of CRISPR/Cas9 for genome editing has led to its widespread adoption and promises to usher in a new era of biology.
In our application of CRISPR/Cas9 to edit the Caenorhabditis elegans genome, we sought a conversion system that met the following criteria: (1) It should be possible to make any mutation in a gene, without extraneous marker sequences, and with no constraint on the genetic background; (2) edited animals should be efficiently made and easily identifiable, so that multiple independent isolates are recovered with minimal downstream screening; and (3) the system should be fast, enabling isolation of the mutation in as few generations as possible, and require minimal plasmid construction. The ease and versatility of such a strain construction system would lessen the technical barriers of genome editing, empowering researchers and facilitating the analysis of gene function. We set out to devise such a system.
Among the techniques available to edit the C. elegans genome, the oligonucleotide-mediated conversion strategy reported by (Zhao et al. 2014) appealed to us for its relative simplicity and efficiency. In the oligonucleotide-mediated conversion strategy, an ∼100-nt single-stranded oligonucleotide bearing a desired mutation is co-injected with Cas9 and a gRNA specific for the wild-type locus of interest into the C. elegans germline. The oligonucleotide-based conversion approach has several desirable features of a genome editing system. In particular, it is relatively simple, with a single gRNA plasmid, a commercially prepared single-stranded DNA oligonucleotide, and a pair of PCR assay primers sufficient to initiate each editing experiment. Upon microinjection of these components and a plasmid bearing Cas9 into the C. elegans germline, the efficiency for this process is moderate, with 0.5–3.5% of F1 animals bearing the mutation of interest. This frequency of conversion has allowed direct (albeit somewhat labor intensive) screening for mutations of interest through a PCR-based assay on individual progeny of injected animals. Our goal in this work is a mutation system with the flexibility of the Zhao et al. (2014) approach, but with minimized screening.
For CRISPR/Cas9 to facilitate homologous repair at a locus, several steps must occur, including (i) successful injection of DNA constructs into a worm germline; (ii) expression of gRNA(s), Cas9, and assembly together; (iii) finding, binding, and cleavage of target DNA by CRISPR/Cas9; (iv) repair of the double-strand break from the template DNA; and (v) survival of the resultant egg/embryo to a stage at which it can reproduce and be screened.
In practice, any or a number of these steps may be inefficient or fail, and thus markers for one or more steps in an intended manipulation can be useful in obtaining engineered strains (Stinchcomb et al. 1985; Mello et al. 1991; Jinek et al. 2012; Dickinson et al. 2013; Kim et al. 2014). For the purposes of introducing custom mutations, it would be ideal if animals that had experienced all steps required for a functional HR event could be easily discerned from animals that had not. In the absence of a readily identifiable phenotype of the desired mutation, a coconversion strategy could be employed: a second unlinked marker locus where CRISPR/Cas9-facilitated HR yielded an easily discernable phenotype would, by definition, enable identification of animals that had been exposed to Cas9, guide RNA, and donor DNA populations sufficient for HR, with a goal being that this might enrich for the desired (unmarked) mutation event. The validity of such a coselection approach is supported by a recently described co-CRISPR strategy, in which animals selected for CRISPR/Cas9-induced NHEJ mutations at the unc-22 locus served as a marker and enriched for CRISPR/Cas9-induced NHEJ events at a second unlinked locus (Kim et al. 2014). Because successful oligonucleotide-templated introduction of specific mutations requires that animals have experienced an HR event, coconversion requires a more appropriate selection strategy for specific genome modification than co-CRISPR, which requires that recovered animals only be competent for NHEJ.
Here we combine the ease of genome editing afforded by CRISPR/Cas9-facilitated, oligonucleotide-templated conversion with a coconversion strategy that mitigates the amount of downstream (PCR-based) screening. We demonstrate the coconversion strategy efficiently recovers custom genetic modifications in an otherwise unmarked genetic background. We find that the tendency for CRISPR/Cas9 to induce multiple mutational events imposes nontrivial constraints on the genetic properties of phenotypic marker mutations to be used for coconversion, particularly in cases where multiple rounds of HR are to be executed. We describe several phenotypic markers and strategies useful for the coconversion method and demonstrate their utility for recursive rounds of CRISPR/Cas9 genome editing (recursive mutagenesis).
Materials and Methods
Strains
The Bristol N2 strain was used in all experiments, grown on nematode growth medium (NGM) plates seeded with OP50 (Brenner 1974). Animals were grown at 16° prior to injection and at 23° after injection.
Template DNA
Donor oligonucleotides bearing the mutation of interest and additional silent mutations were designed to ablate the gRNA cleavage site and introduce a restriction site. To design silent restriction sites, we found the SiteFind tool useful (Evans and Liu 2005). Donor oligonucleotides encompassed 50 nt of flanking homology on either side. Oligonucleotide preparations (IDT) were dissolved to a final concentration of 10 μM and used in injection mixes at ∼20 ng/μl without further purification.
The rde-1(AAA) plasmid used for integration in Figure 4 (pJP44.3.1) encompasses the entire rde-1 gene, including ∼2700 and 900 bp of upstream and downstream sequence (Pak et al. 2012). The rde-1(AAA) PCR product was generated with primers AF-JA-21/82 using pJA32, a derivative of pJP44.3.1, as a substrate. The full sequence of the PCR product is presented in Supporting Information, File S1. The lin-14::GFP plasmid (L7969) was derived from a full-length GFP-tagged version of lin-14 [VT333G (Hong et al. 2000)] by retention of a SalI to NotI fragment with consequent deletion of sequences upstream of the lin-14B coding region and of lin-14B exons 1–3.
Figure 4.
Coconversion from nonoligonucleotide substrates as the second-site donor DNA. (A) Coconversion strategy using plasmid DNA as the donor for the rde-1 mutations. The rde-1(AAA) plasmid was included in the injection mix at ∼593 ng/μl. F1 Rol animals that tested positive for rde-1(AAA) DNA were subsequently progeny tested and homozygotes isolated, to discern transgenesis from true integration events. (B) Coconversion strategy using a PCR product as the donor for the rde-1 mutations. The rde-1(AAA) PCR product was included in the injection mix at ∼287 ng/μl. The PCR product also included three silent mutations upstream of D718A (not shown). F1 Unc animals were tested for integration, using one primer inside the PCR product and another outside of it. F1 Unc animals testing positive for rde-1(AAA) DNA were progeny tested as in A. Each of the eight rde-1 conversion events occurred in an independent animal.
Single-worm PCR
Single worms were picked into 15 μl of 1× PCR buffer (10 mM Tris, 50 mM KCl, 2 mM MgCl2, pH 8.0) with Proteinase K (500 ng/μl) for single-worm genomic preparation. The solution was frozen and then incubated for 1 hr at 60°, followed by 15 min at 95°. The single-worm genomic prep was included in a PCR reaction, using Phusion polymerase (New England Biolabs, Beverly, MA) at 1/10th reaction volume. PCR reactions were treated with restriction enzyme and run on a TAE ∼1–2% agarose gel or cleaned up using Shrimp Alkaline Phosphatase (United States Biochemical, Cleveland) and Exonuclease I (United States Biochemical) and sequenced with MCLab (www.mclab.com).
In a handful of cases we encountered alleles at CRISPR/Cas9 gRNA sites that failed to amplify by single-worm PCR, likely because they contain large (>500) base pair deletions that remove one or both PCR primer binding sites. These events initially appeared to be homozygous HR or NHEJ events in the F1, although they subsequently segregated as a single allele and a nonamplifiable allele. We encountered these events at a higher frequency when using two gRNAs at a single locus where primer binding sites were contained in the intervening sequence (e.g., Figure 4). These events were also observed when using rol-6(su1006) as a marker, yet in every case some of the F2 progeny were wild type.
Additional methods are detailed in File S1.
Results and Discussion
Oligonucleotide-templated conversion generates mutations with a known phenotype
With the goal of establishing useful marker mutations for the coconversion strategy, we first selected several mutations affecting C. elegans behavior and morphology from among the well-characterized products of classical genetics (Brenner 1974; Park and Horvitz 1986; Kramer et al. 1988; Levy et al. 1993). Initially we used as our criteria that (1) the mutant phenotype should be readily identifiable in the mut/+ configuration, so that marked individuals can be found in the F1, and unmarked +/+ animals can subsequently segregate among their progeny; (2) the mutant phenotype should be distinct from the phenotype conferred by null mutations in the same gene, thus allowing rapid discrimination between CRISPR/Cas9-induced HR and NHEJ; and (3) the mutant phenotype should have a rare spontaneous frequency to minimize false positive conversion events.
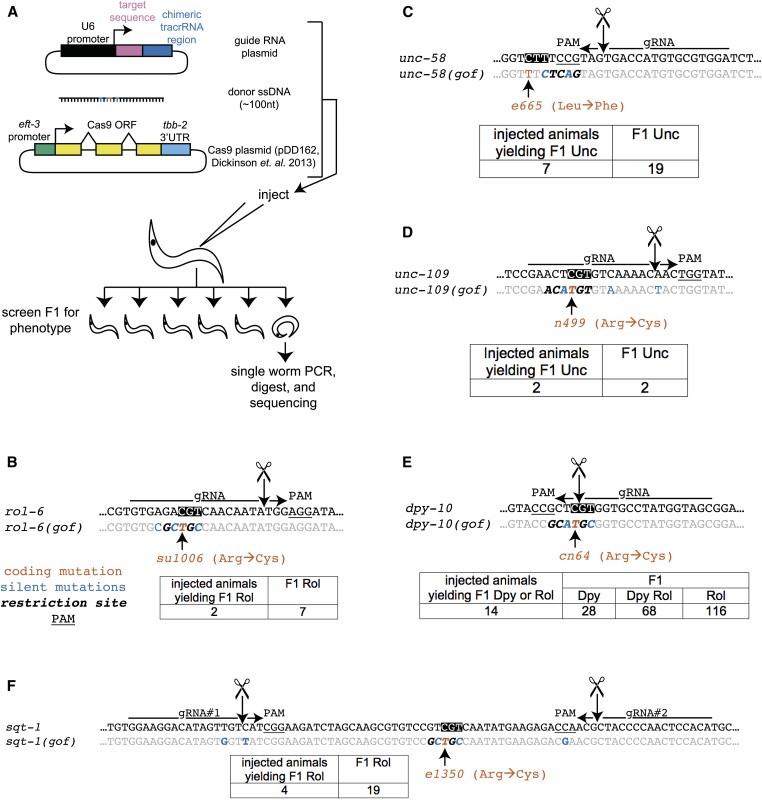
We selected six dominant alleles that meet these criteria: rol-6(su1006), sqt-1(e1350), and dpy-10(cn64) confer dominant right, right, and left rolling (Rol) phenotypes, respectively; unc-58(e665) causes paralysis of most of the body (except for the head) and a peculiar shaker phenotype; unc-109(n499) causes paralysis, including a pharyngeal pumping defect; and unc-43(n498) confers paralysis and egg-laying defects. For each mutation, we designed a gRNA to cleave the wild-type locus, a donor DNA oligonucleotide with both the dominant mutation and additional silent mutations to create a restriction site, and a pair of PCR primers for screening (see Figure S3 for gRNA plasmid design and cloning). Here and throughout this study, we included additional silent mutations in many of the donor oligonucleotides to unambiguously define HR events (e.g., distinguishing from DNA or strain contamination) and for ease of screening by restriction digest. In principle the restriction site is not required and can be eliminated; no evidence for contamination was observed in these experiments and screening can be performed (albeit somewhat more labor intensively) by single-worm PCR and sequencing alone.
As shown in Figure 1, for five of six candidate marker mutations we obtained animals with the expected phenotypes in the F1 progeny of the injected animal. Initially we included an mCherry marker (pCFJ104, Pmyo-3::mCherry) in the rol-6 injections, although none of the F1 Rol animals recovered expressed body wall mCherry. This result suggests that heritable transgenesis is not required for oligonucleotide-based conversion per se and is consistent with previous observations (Zhao et al. 2014) (also see below). For each mutation, phenotypically affected animals were verified to contain a conversion event by single-worm PCR, restriction digest, and sequencing. Each conversion event segregated in a Mendelian manner in follow-up experiments. In all cases, some of the progeny from affected F1 individuals were phenotypically wild type, consistent with the known dominant nature of these alleles. The combination of heritability, dominance, and molecular verification for the majority of affected F1 animals confirms both the specificity and low background for the phenotypic screens employed.
Figure 1.
Strategy to track effective gene conversion using dominant point mutations. (A) A Cas9 expression plasmid [pDD162 (Dickinson et al. 2013)] is co-injected with a target-specific gRNA plasmid and a template oligonucleotide bearing the desired mutations. The F1 of the injected animal is screened for phenotypically affected animals. (B) Schematic of the coding strand of the rol-6(su1006) locus. The su1006 mutation (Arg→Cys) is indicated in red, with wild-type Arg boxed (Kramer and Johnson 1993). The gRNA sequence is indicated with an arrow, with PAM underlined. Additional silent mutations conferred by the donor single-stranded DNA (ssDNA) creating a BbvI restriction site (italics) are highlighted in blue. The expected Cas9 cleavage site is indicated with arrow and scissors. Twenty-eight animals were injected. (C) Schematic for unc-58(e665) DdeI site in italics and causative lesion (e665, Leu→Phe) noted (Phil S. Hartman, James Barry, Whitney Finstad, Numan Khan, S. Sato, Naoaki Ishii, and Kayo Yasuda, unpublished results). Twenty-one animals were injected. (D) Schematic for unc-109(n499). PciI site is in italics (n499 sequence from Chen and Jorgensen 2013). Twenty-one animals were injected. (E) Schematic for dpy-10(cn64). SphI site is in italics. See text for description of Dpy, Dpy Rol, and Rol animals. Twenty-one animals were injected. (F) Schematic for sqt-1(e1350), with BbvI site in italics, using injection of two gRNAs. Thirty animals were injected. Injections were performed with day-to-day variability in injection efficiency, and the number of phenotypically affected progeny should not be interpreted as a statement on the efficiency of HR at that locus. All plasmids are 50 ng/μl, and ssDNA is ∼20 ng/μl.
Not all guide/template combinations have been successful in obtaining the desired phenotypes. We failed to recover Rol progeny with two additional gRNAs for sqt-1 and one additional rol-6 gRNA, likewise observing two gRNA failures for unc-58 and one for unc-43 (Figure S1). We do not know why some of the gRNAs failed, although possibilities are presented in File S1.
Simultaneous tracking of sperm- and oocyte-derived alleles during marker conversion: evidence for dual chromosome NHEJ/HR events in single F1 animals
In addition to the aforementioned dominant phenotypes, three additional features of the gain-of-function dpy-10(cn64) and sqt-1(e1350) alleles have been very useful to deduce the genotype at the corresponding loci from phenotype. First, dpy-10(cn64) and sqt-1(e1350) confer a different phenotype as mut/mut homozygotes than as mut/+ heterozygotes, with both mutations conferring recessive dumpy (Dpy) phenotypes (Kramer et al. 1988; Levy et al. 1993). This allows the desired marker heterozygote to be identified and subsequently segregated away, while avoiding any homozygous mutants for which segregation of the marker locus would be much more cumbersome. As a second advantage of these two loci, heteroallelic combinations mut/o confer a different phenotype from the desired mut/+ heterozygote [sqt-1(e1350)/sqt-1(o) are dumpy and dpy-10(cn64)/dpy-10(o) are dumpy roller]. Finally, loss-of-function alleles in dpy-10 or sqt-1 confer a recessive Dpy phenotype, again allowing a distinction from the desired mut/+ heterozygotes.
These properties of sqt-1 and dpy-10 lead to an ability to detect diverse combinations of CRISPR/Cas9 events, with a simple (non-Dumpy) roller phenotype being the desired HR/+ heterozygote for either gene. Injections with either system indeed yielded simple rollers as the majority of affected animals, with sequencing confirming the expected genotype in all but rare cases. For dpy-10, we examined populations in more detail, observing additional phenotypes consistent with dpy-10(cn64)/dpy-10(o) or dpy-10(o)/dpy-10(o) (Figure 1E), and confirmed several of these by molecular analysis. In principle, F1 dumpy animals may arise from dpy-10(cn64/cn64), but we have yet to observe such homozygous HR animals (see below).
Coconversion at two loci occurs at a high frequency
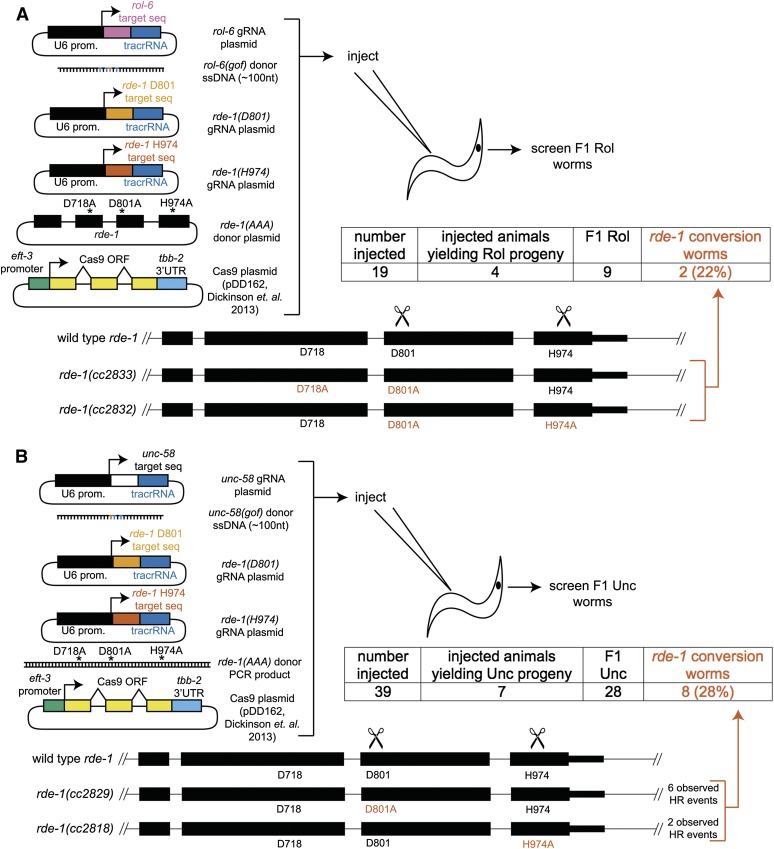
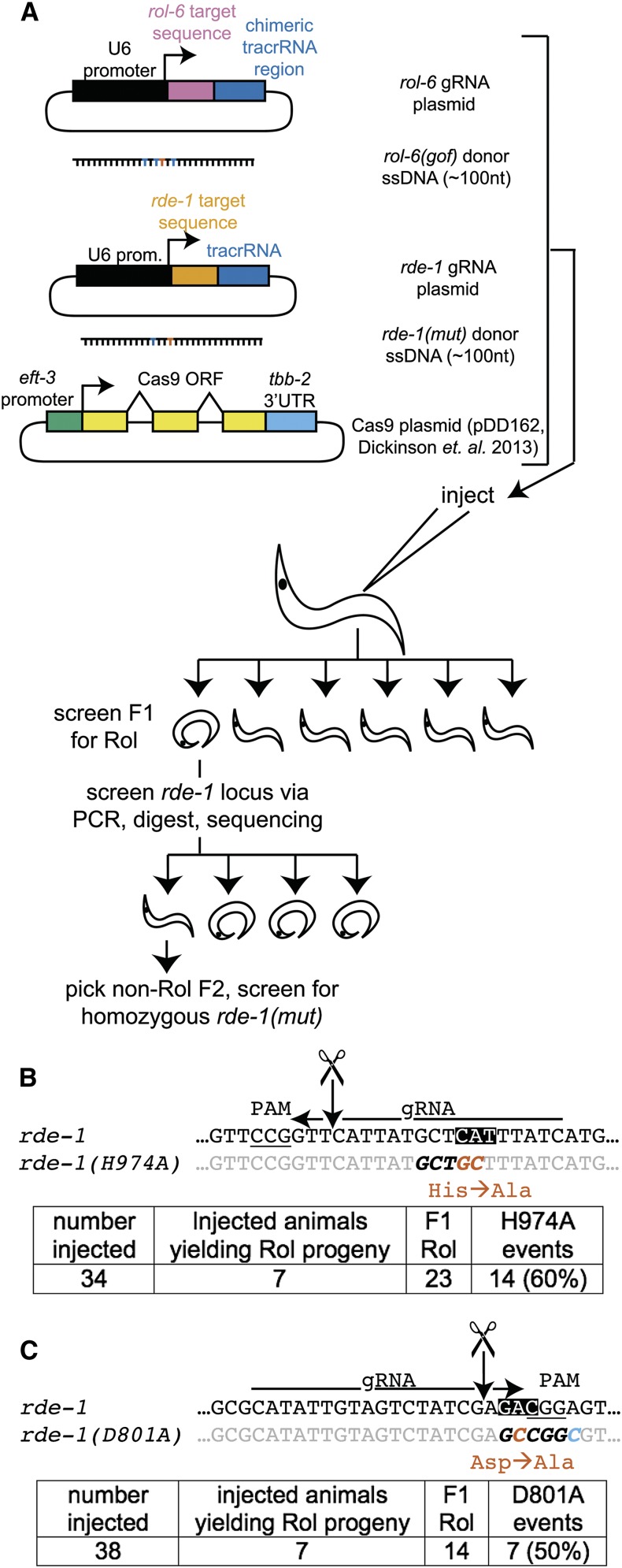
Having established dominant phenotypic markers for the coconversion strategy, we next set out to assess to what extent coconversion occurred. First we attempted to convert rol-6(su1006) and simultaneously mutate a catalytic residue H974 of the Argonaute RDE-1 by injection of the cognate gRNAs and repair templates (Figure 2, A and B). Of a total of 34 injected animals, we recovered 25 Rol progeny from 7 injected animals. When screened by single-worm PCR, digest, and sequencing, 14 of 23 (60%) animals also contained the H974A mutation. After the allele of interest was identified among the F1 Rol animals, we screened the rde-1 locus of the non-Rol progeny of that animal by single-worm PCR, digest, and sequencing for homozygosity of the H974A mutation. We isolated homozygous rde-1(H974A) animals in an otherwise unmarked [rol-6(+)] background (see also Figure S2). Thus the coconversion strategy enabled recovery of multiple independent rde-1(H974A) isolates while minimizing the number of animals screened.
Figure 2.
Coconversion strategy for induction of designated point mutations. (A) The coconversion strategy. A gRNA and donor oligonucleotide to create the rol-6(su1006) mutation are co-injected with a gRNA and donor oligonucleotide to create a point mutation in the desired gene (in this case rde-1). F1 progeny were screened for the Rol phenotype. Rol animals were singled and, after laying eggs, were screened by single-worm PCR and characterization of the designated mutational target (rde-1 for this experiment). Nonroller F2 progeny of appropriate F1 animals were singled and after laying eggs were screened for homozygosity of the rde-1 mutation. (B) Schematic of the rde-1(H974A) locus. Ala mutations are shown in red and the BbvI site in italics. All plasmids were 50 ng/μl. Two additional animals at this stage rolled but failed single-worm PCR; these were not included in the 23 count. (C) Schematic for rde-1(D801A) locus. NaeI site is in italics. Two of the seven D801A events contained only the D801A mutation and lacked a complete NaeI site (blue C). gRNA plasmids were 25 ng/μl. An additional animal rolled but failed single-worm PCR and was not included in the 14 count.
We sought to determine whether picking F1 broods with high marker HR frequency could enrich for the desired (unselected) mutation event, among both marked and unmarked F1 animals. As we see a dramatic difference among broods in the frequency of HR events, focusing on broods with a moderately high frequency of events might be sufficient to enrich for desired HR events, even without choosing animals that have themselves been subject to marker conversion. We thus compared brood-level and individual-level selection for the degree of HR enrichment at the unselected locus. As a reference, 12 nonroller animals from broods with no roller animals each failed to show conversion at rde-1 (assayed by single-worm PCR and restriction digest). From broods with roller animals, we screened 26 Rol animals, with coconversion observed in 22 animals (84%). Among age-matched non-Rol siblings of F1 Rol animals, 14 of 25 (56%) also contained the rde-1(H974A) mutation. The frequency of the rde-1(H974A) mutation among F1 Rol animals was higher than among non-Rol siblings (P = 0.033, two-tailed Fisher’s exact test), yet the frequency of HR in both groups was quite high. We conclude that our screening efforts were maximally rewarded by focusing on phenotypically marked animals, although the number of isolates of rde-1(H974A) was quite workable upon screening their phenotypically wild-type siblings. Picking of unmarked siblings may prove useful when the marker mutation would be undesirable or incompatible with a genetic background (e.g., when the marker locus is linked to a desired mutation or a balancer chromosome is present).
Because the coconversion strategy resets to the initial genetic background, recursive coconversion is theoretically possible. To demonstrate the capability for the coconversion strategy to generate multiply mutant animals and to test its efficiency with other gRNAs, we attempted to mutate additional catalytic residues at the rde-1 locus in the rde-1(H974A) background. Using rol-6(su1006) as a marker and the rde-1(H974A) host, we mutated D801 to Ala and recovered multiple isolates of rde-1(D801A, H974A) (Figure 2C). We performed an additional round of CRISPR/Cas9, using rol-6(su1006), unc-58(e665), or dpy-10(cn64) as the marker and D718 as the second site to generate the complete catalytic triad mutant rde-1(D718A, D801A, H974A) (Figure 3). Thus the coconversion strategy can be applied recursively to generate multiply mutant animals, each round taking ∼2 weeks, with most of that time spent waiting for animals to grow.
Figure 3.
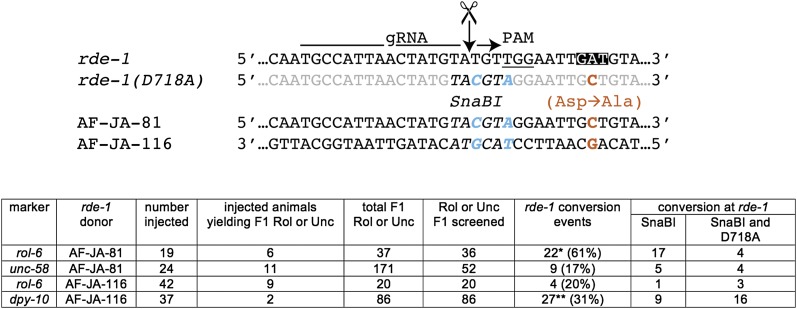
Oligonucleotide-mediated homologous recombination is local. Schematic of the rde-1(D718A) locus is shown. Silent mutations are in blue, the SnaBI site is in italics, and the D718A mutation is in red. For all injections, both gRNA plasmids were 25 ng/μl. For both rol-6 injections, the rde-1(D718A) donor DNA was 20 ng/μl. For unc-58 and dpy-10 the rde-1(D718A) donor DNA was ∼17.25 ng/μl. *From the sequencing trace it was unclear whether an additional animal was full or partial HR. **From the sequencing trace it was unclear whether two additional animals were full or partial HR.
Oligonucleotide-templated conversion is local
During the course of screening, we noted several animals with incomplete copying of sequences from the donor oligonucleotide. Of the Rol animals recovered from sqt-1(e1350) construction using two gRNAs whose cut sites span 50 nt, a single animal contained partial conversion off the donor oligonucleotide, yielding mutation at the first gRNA site and the e1350 locus, but not at the second gRNA site. At D718 in rde-1, many of the events recovered exhibited introduction of the linked restriction site (SnaBI) only, with several failing to convert the D718A mutation 7 bases away (Figure 3). For D801A, among the seven animals recovered with a D801A mutation, two lacked a complete version of the template-induced site (NaeI), showing partial incorporation of mutant bases only 3 nt apart [partial conversion is GCCGGA, wild type (wt) is GACGGA]. Based on these observations, we conclude that proximity of bases in the donor oligonucleotide does not guarantee their faithful copying into the target locus.
To determine whether different coconversion conditions gave rise to more complete conversion from the donor oligonucleotide, we repeated coconversion at D718A. With all three markers tested (rol-6, unc-58, and dpy-10), we obtained a significant fraction of partial conversion events. We also obtained partial conversion events using either strand of DNA as the repair template. We conclude that although copying sequence from the donor oligonucleotide can occur over distances as far as 22 bases (sqt-1), it is often highly local.
Homozygous oligonucleotide-templated conversion is rare
Given the high frequency of coconversion between independent loci (14–84%), one might expect some fraction of F1 animals to be homozygous for the oligonucleotide-templated mutation, either at the marker (rol-6, unc-58, dpy-10) or at the second (rde-1) loci. It is noteworthy that in the course of our experiments, despite >400 F1 animals screened, each with at least one conversion event, we never observed a bona fide event where both alleles in an F1 animal had been subjected to HR (Figure 1, Figure 2, and Figure 3, see also Materials and Methods). While failure to observe homozygous conversion does not preclude rare occurrence, such events must be much less frequent than would be expected if the second allele were subject to the same frequency of conversion as an unlinked locus.
The dearth of homozygous oligonucleotide-templated mutations may prove informative for understanding the timing and mechanisms of genome editing by CRISPR/Cas9 in C. elegans. Failure to observe homozygous convertants is not due to a lack of CRISPR/Cas9 activity: animals bearing presumed NHEJ mutations in both copies of dpy-10 were recovered (Figure 1E). From this we conclude that while both oocyte and sperm-derived alleles can be targeted by CRISPR/Cas9, only one copy of a given locus is apparently receptive to introduction of oligonucleotide-templated mutations. We consider it likely the genomic copy subject to HR is the oocyte-resident genomic copy for two reasons: (1) injected DNA constructs are delivered to the developing oocytes, while the sperm genomic copy is unavailable for editing until hours later upon oocyte fertilization, and (2) the meiotically developing oocyte exhibits a bias in repair pathways toward HR over NHEJ (Lemmens et al. 2013). Given that both germ cells and the embryo appear competent for homologous repair pathways (Clejan et al. 2006), Cas9-induced lesions in the early embryo may be refractory to templated repair simply because the injected (single-stranded) donor DNA is unstable and/or unavailable (as proposed by Kim et al. 2014). Additional work will provide further insight into the dynamics of CRISPR/Cas9 genome editing in the germline of C. elegans.
Incorporation of sequences from nonoligonucleotide substrates
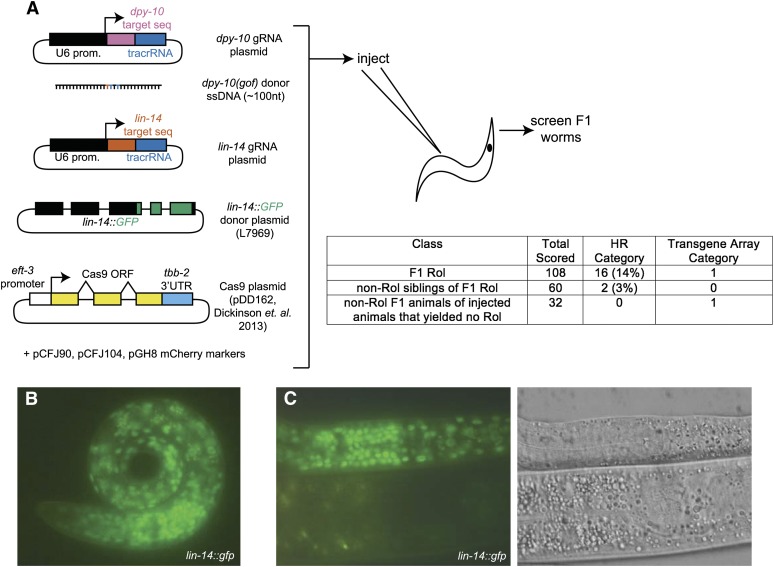
The success of coconversion from oligonucleotides prompted us to test incorporation of sequences from nonoligonucleotide substrates, such as plasmids or PCR products, at a second locus. With rol-6(su1006) or unc-58(e665) as markers, we were able to introduce mutations into rde-1 from a plasmid or PCR product, respectively (Figure 4). Interestingly, one mutant contained incorporation of the D718A mutation, which is >300 nt away from the gRNA cut site. We also attempted incorporation of GFP at a locus while using dpy-10(gof) as a marker for HR (Figure 5). Among 107 F1 Rol animals, we observed 16 incorporation events. The frequency of integration among Rol animals (16/107) was higher than in the non-Rol siblings of Rol animals (2/60) (Fisher’s exact test, two-tailed P-value = 0.02), demonstrating the dpy-10(gof) marker was useful for enriching for GFP incorporation events. (We also failed to observe integration of GFP among 32 non-Rol animals from injected animals that failed to yield Rol progeny.) Thus oligonucleotide-mediated HR at a marker locus can facilitate recovery of a diverse selection of HR events, including templated introduction of sequences from PCR and plasmid sources over hundreds of nucleotides.
Figure 5.
Oligonucleotide-templated HR as a marker for integration of GFP at a second locus. (A) L7969 is a derivative of VT333G (Hong et al. 2000) and encodes a C-terminal lin-14::GFP fusion (exons 4 through the C terminus of lin-14) and was included in the injection mix at 20 ng/μl. Guide RNA plasmids were 25 ng/μl, pDD162 was 50 ng/μl, and dpy-10 donor DNA was 500 nM. Transgenic-marking reporter fusions were included in the injection mix: Pmyo-2::mCherry::unc-54 (pCFJ90, 2.5 ng/μl), Pmyo-3::mCherry::unc-54 (pCFJ104, 5 ng/μl), and Prab-3::mCherry::unc-54 (pGH8, 10 ng/μl). Forty-four animals were injected and 23 yielded Rol progeny. A large number of Rol and non-Rol progeny were observed, only a fraction of which were scored here. The HR category includes F1 animals screened by a combination of single-worm PCR and examination of their young progeny for characteristic lin-14::GFP expression patterns. The transgene array category includes animals yielding heritable GFP and mCherry coexpression. Of the 16 lin-14::GFP integration events among F1 Rol, 3 were from F1 mCherry-positive parents. (B) LIN-14::GFP expression pattern in a newly hatched L1 larva, similar to that reported in Hong et al. (2000). (C) Close-up of LIN-14::GFP expression pattern showing punctate nuclear GFP signal, consistent with that in Hong et al. (2000). Also note lack of expression in L4 larvae (bottom half). The faint yellowish signal in the L4 larvae is autofluorescence from the gut.
Conclusions
Here we show that coconversion between two loci with CRISPR/Cas9 can facilitate the incorporation of marker-free mutations at a locus of interest while avoiding extensive screening. High-frequency coconversion enables independent isolation of multiple strains with the desired mutation, facilitating adherence to best practices of analyzing two to three independently derived animal lines. Modifications of the approaches used here may be useful for testing gRNA functionality, efficiently integrating larger portions of DNA, and identifying more sensitive reporters of CRISPR/Cas9 activity, HR, and/or NHEJ. We provide evidence that CRISPR/Cas9 can persist in the zygote in C. elegans long enough to cleave both genomic copies of a locus, giving rise to heteroallelism, yet homozygous HR events are extremely rare. The approach we demonstrate may prove useful for other organisms where gain-of-function alleles satisfying the criteria outlined can be identified. Perhaps most importantly, the coconversion strategy should diminish the technical hurdles of the genome editing process at almost all steps and empower researchers to apply CRISPR/Cas9 to understanding their favorite biological process.
While conceptually similar to the recently reported co-CRISPR approach (Kim et al. 2014), the coconversion strategy described here has three differences. Namely,
-
1.
The coconversion strategy described here does not necessitate selection for transgenesis, whereas co-CRISPR does. There are substantial disadvantages that can have important consequences for strain construction and subsequent experimental interpretation in needing to make a transgenic for each strain. In experiments where we included mCherry markers for transgenesis (Figure 1B and Figure 5), only a subset of Rol animals was red. This suggests that CRISPR/Cas9-facilitated HR is not contingent on transgenesis, consistent with the observations of Zhao et al. (2014).
-
2.
The coconversion strategy described here allows straightforward multiround mutant construction. While the use of unc-22 as a marker in co-CRISPR (Kim et al. 2014) also allows multiround construction, such a process would necessitate floating each generation of animals in levamisole to first select heterozygote unc-22 animals and then verify the unc-22 lesion has segregated away. Among the markers described here, dpy-10(cn64) and sqt-1(e1350) allow phenotypic discrimination between mut/+, mut/null, +/+, and mut/mut or null/null animals, enabling facile identification of the desired genotype at each stage.
-
3.
The coconversion strategy described here yields specific HR events while minimizing the number of animals that require singling and PCR screening. While efficient for gene knockouts, co-CRISPR requires singling many F1 animals for HR events, with only a fraction of them suitable for subsequent HR screening among the F2. Candidate HR events are identified in the F1 with our coconversion, sparing screening efforts with the earlier identification of candidates.
As presently applied in our laboratory, we have standardized our protocols to make use of dpy-10(cn64) coconversion for a broad set of applications. While we initiated construction of the rde-1(D718A, D801A, H974A) strain with rol-6(su1006) as a marker, rol-6(su1006) [and unc-58(e665)] have two undesirable genetic properties: (1) mut/+ and mut/o are phenotypically similar, if not identical, and (2) the null (o/o) and wild-type (+/+) phenotypes differ very subtly, if at all. The genetic property of a wild-type null phenotype (Greenwald and Horvitz 1980), while useful under some circumstances, requires additional screening work to ensure that the original unmarked genetic background has indeed been recovered after a round of coconversion. For these reasons, we favor the use of marker mutations [e.g., dpy-10(cn64) and sqt-1(e1350)] that are phenotypically distinct in trans to a wild-type allele (the desired F1 configuration) compared to in trans to a null allele (the configuration from any event where the homologous chromosome has been subject to additional mutations). When dpy-10(cn64) and sqt-1(e1350) are used as coconversion markers, the distinct phenotypes of mut/+ and mut/o should enable exclusion of animals bearing additional marker locus mutations during the initial F1 screening. Indeed, in all but rare Rol animals analyzed in dpy-10(cn64) and sqt-1(e1350) experiments we have observed a wild-type allele opposite an HR allele at the marker locus. Careful selection of genetic markers provides another option (along with lowering gRNA plasmid levels) to mitigate recovery of nontemplated mutations at CRISPR/Cas9-targeted loci. Our current working recipe for the C. elegans germline injection mix is 50 ng/μl Cas9 (pDD162), 25 ng/μl dpy-10 gRNA plasmid, and 500 nM dpy-10(cn64) oligonucleotide (AF-ZF-827), along with a gRNA plasmid (25 ng/μl) and oligonucleotide (500 nM) for the mutation of interest.
Supplementary Material
Acknowledgments
We thank Alexandre Paix and Geraldine Seydoux for first drawing our attention to the oligonucleotide-templated approach. We are grateful to Craig Mello, Victor Ambros, and their colleagues at the University of Massachusetts for sharing of plasmids. We thank Michael Nonet for helpful discussions and critical reading of the manuscript; Pin An Chen and Erik Jorgensen for detailed information on the nature of unc-109(n499); and Christian Frøkjær-Jensen, Julia Pak, and Nimit Jain for discussions, help with injection, and critical reading of the manuscript. This work was supported by National Institutes of Health grants R01GM37706, T32GM007790, and T32HG000044.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.169730/-/DC1.
Communicating editor: O. Hobert
Literature Cited
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P. A., and E. Jorgensen, 2013 Trimeric G-proteins converge on a novel ion channel. Ph.D. Thesis, University of Utah, Salt Lake City [Google Scholar]
- Chiu H., Schwartz H. T., Antoshechkin I., Sternberg P. W., 2013. Transgene-free genome editing in Caenorhabditis elegans using CRISPR-Cas. Genetics 195: 1167–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. W., Lee J., Carroll D., Kim J.-S., Lee J., 2013. Heritable gene knockout in Caenorhabditis elegans by direct injection of Cas9-sgRNA ribonucleoproteins. Genetics 195: 1177–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clejan I., Boerckel J., Ahmed S., 2006. Developmental modulation of nonhomologous end joining in Caenorhabditis elegans. Genetics 173: 1301–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo J. E., Norville J. E., Mali P., Rios X., Aach J., et al. , 2013. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41: 4336–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Ward J. D., Reiner D. J., Goldstein B., 2013. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. M., Liu C., 2005. SiteFind: a software tool for introducing a restriction site as a marker for successful site-directed mutagenesis. BMC Mol. Biol. 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland A. E., Tzur Y. B., Esvelt K. M., Colaiácovo M. P., Church G. M., et al. , 2013. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 10: 741–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Cummings A. M., Nguyen J. N., Hamm D. C., Donohue L. K., et al. , 2013. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194: 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. S., Horvitz H. R., 1980. unc-93(e1500): a behavioral mutant of Caenorhabditis elegans that defines a gene with a wild-type null phenotype. Genetics 96: 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., R. C. Lee, and V. Ambros, 2000. Structure and function analysis of LIN-14, a temporal regulator of postembryonic developmental events in Caenorhabditis elegans. Mol. Cell. Biol. 20: 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., et al. , 2013. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31: 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Bikard D., Cox D., Zhang F., Marraffini L. A., 2013. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., et al. , 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic I., Großhans H., 2013. Targeted heritable mutation and gene conversion by Cas9-CRISPR in Caenorhabditis elegans. Genetics 195: 1173–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Ishidate T., Ghanta K. S., Seth M., Conte D., et al. , 2014. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics 197: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Johnson J. J., 1993. Analysis of mutations in the sqt-1 and rol-6 collagen genes of Caenorhabditis elegans. Genetics 135: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Johnson J. J., Edgar R. S., Basch C., Roberts S., 1988. The sqt-1 gene of C. elegans encodes a collagen critical for organismal morphogenesis. Cell 55: 555–565. [DOI] [PubMed] [Google Scholar]
- Lemmens B. B. L. G., Johnson N. M., Tijsterman M., 2013. COM-1 promotes homologous recombination during Caenorhabditis elegans meiosis by antagonizing Ku-mediated non-homologous end joining. PLoS Genet. 9: e1003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. D., Yang J., Kramer J. M., 1993. Molecular and genetic analyses of the Caenorhabditis elegans dpy-2 and dpy-10 collagen genes: a variety of molecular alterations affect organismal morphology. Mol. Biol. Cell 4: 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-F., Norville J. E., Aach J., McCormack M., Zhang D., et al. , 2013. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31: 688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T.-W., Pickle C. S., Lin S., Ralston E. J., Gurling M., et al. , 2013. Precise and heritable genome editing in evolutionarily diverse nematodes using TALENs and CRISPR/Cas9 to engineer insertions and deletions. Genetics 195: 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov V., Staskawicz B., Weigel D., Jones J. D. G., Kamoun S., 2013. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31: 691–693. [DOI] [PubMed] [Google Scholar]
- Pak J., Maniar J. M., Mello C. C., Fire A., 2012. Protection from feed-forward amplification in an amplified RNAi mechanism. Cell 151: 885–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. C., Horvitz H. R., 1986. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics 113: 821–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Shaw J. E., Carr S. H., Hirsh D., 1985. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol. Cell. Biol. 5: 3484–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Gaba A., Sachs M. S., 1999. A highly conserved mechanism of regulated ribosome stalling mediated by fungal arginine attenuator peptides that appears independent of the charging status of arginyl-tRNAs. J. Biol. Chem. 274: 37565–37574. [DOI] [PubMed] [Google Scholar]
- Zhao P., Zhang Z., Ke H., Yue Y., Xue D., 2014. Oligonucleotide-based targeted gene editing in C. elegans via the CRISPR/Cas9 system. Cell Res. 24: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.