Abstract
The origin of mutations under selection has been intensively studied using the Cairns-Foster system, in which cells of an Escherichia coli lac mutant are plated on lactose and give rise to 100 Lac+ revertants over several days. These revertants have been attributed variously to stress-induced mutagenesis of nongrowing cells or to selective improvement of preexisting weakly Lac+ cells with no mutagenesis. Most revertant colonies (90%) contain stably Lac+ cells, while others (10%) contain cells with an unstable amplification of the leaky mutant lac allele. Evidence is presented that both stable and unstable Lac+ revertant colonies are initiated by preexisting cells with multiple copies of the F′lac plasmid, which carries the mutant lac allele. The tetracycline analog anhydrotetracycline (AnTc) inhibits growth of cells with multiple copies of the tetA gene. Populations with tetA on their F′lac plasmid include rare cells with an elevated plasmid copy number and multiple copies of both the tetA and lac genes. Pregrowth of such populations with AnTc reduces the number of cells with multiple F′lac copies and consequently the number of Lac+ colonies appearing under selection. Revertant yield is restored rapidly by a few generations of growth without AnTc. We suggest that preexisting cells with multiple F′lac copies divide very little under selection but have enough energy to replicate their F′lac plasmids repeatedly until reversion initiates a stable Lac+ colony. Preexisting cells whose high-copy plasmid includes an internal lac duplication grow under selection and produce an unstable Lac+ colony. In this model, all revertant colonies are initiated by preexisting cells and cannot be stress induced.
Keywords: adaptive mutation, gene amplification, mutation under selection, selection, stress-induced mutagenesis
KNOWING the source of mutants that arise under selective conditions is critical to understanding evolution and therefore the origins of cancer and the progress of infectious disease. A genetic system to address this problem was designed by Cairns and Foster (Cairns et al. 1988; Cairns and Foster 1991). In this system, a population of bacterial tester cells carries a leaky lac frameshift mutation on a low-copy F′lac plasmid. Following pregrowth under permissive conditions in liquid medium, 108 of these cells are plated on selective medium containing lactose as the sole carbon source. Over 1 week, 100 Lac+ revertant colonies accumulate above the nongrowing plated population. These colonies are of two types. One includes stable Lac+ mutant cells whose mutant lac allele has been corrected by a compensating frameshift mutation (Foster and Trimarchi 1994; Rosenberg et al. 1994). The other includes cells with multiple tandem copies of the original leaky mutant allele (Andersson et al. 1998). Two general models have been proposed to explain the origin of these colonies. The evidence presented below suggests some problems with both models.
Stress-induced mutagenesis in nongrowing cells
The first explanations of the Cairns system proposed that Lac+ revertants are generated by an evolved mechanism that senses growth cessation (stress) and creates mutations in hopes of genetically resolving the physiological limitation (Cairns et al. 1988; Cairns and Foster 1991; Torkelson et al. 1997). The reversion rate of the lac mutation during nonselective growth is 10−8 per cell per division. The population of 108 cells plated on lactose does not grow but gives rise to ∼100 Lac+ revertant colonies over 6 days. The number of revertants could be explained if the 108 plated cells experienced a 100-fold increase in genome-wide frameshift mutation rate (assuming one division per week). This simple explanation was ruled out because nonrevertant starved cells in the lawn show very little evidence of chromosomal mutagenesis (Foster 1994; Torkelson et al. 1997). The absence of new mutants in the lawn has been explained in two ways—“directed mutation” and “hypermutable states.”
The term directed mutation suggests that induced mutagenesis is focused preferentially on the lac region (Foster and Cairns 1992). This could explain why so few mutations are seen in the genome at large. Direction of mutations to functionally relevant targets is mechanistically difficult to imagine (Stahl 1988), but it seems clear that the number of Lac+ revertants under selection increases much more than the number of associated mutations (Torkelson et al. 1997). Furthermore, most of the recovered Lac+ revertants show little or no general mutagenesis (Rosche and Foster 1999). It seems likely that if any mutagenesis occurs in this system, it is somehow focused preferentially on the F′lac plasmid, which carries the lac mutation that is under selection (Foster 1997).
The term hypermutable states suggests that starvation causes an increase in the genome-wide mutation rate but that affects only ∼1 in 1000 of the starved population (105 of the plated 108 cells) (Hall 1990; Torkelson et al. 1997). Focusing general mutagenesis on a few cells (instead of a small genomic region) can also explain why unselected mutants were hard to detect in the starved population as a whole. If all lac revertants were caused by genome-wide mutagenesis of a small subset of the population, the general rate of frameshift mutations (which correct lac) in the chosen cells would need to increase 105-fold over that seen in growing cells. That is, ∼100 lac reversion events would have to occur in the mutagenized subpopulation (105 cells) in one division—a rate of 10−3 per cell per division. This rate would be a 105-fold increase over that measured during nonselective growth (10−8 per cell per division). Increasing the genome-wide mutation rate 105-fold seems impossibly costly in associated lethal mutations (Roth et al. 2003). Not surprisingly, demonstrable genome-wide mutagenesis (a 200-fold rate increase) is experienced by only ∼10% of Lac+ revertants, while 90% of revertants appear to form without general mutagenesis (Rosche and Foster 1999).
Thus the Cairns system shows very little evidence of an increase in general mutation rate. The mutagenesis seen in a few revertants has been attributed to the error-prone polymerase DinB, whose removal reduces overall revertant number only ∼4-fold (McKenzie et al. 2001). When DinB is eliminated, the number of stable revertants drops ∼10-fold, while unstable revertant number is unaffected. Without DinB, the residual number of lac+ revertant colonies is equally distributed between stable and unstable types. The contribution of DinB to the number of stable revertants and to associated chromosomal mutations requires that the dinB gene be located on the same F′ plasmid that carries the mutant lac allele (I. Roush, S. Maisnier-Patin, and J. R. Roth, unpublished results; Slechta et al. 2003). This requirement is consistent with the possibility that general mutagenesis requires coamplification of dinB with lac as described below.
Selective amplification in growing cells
If growth limitation acts purely as a selective agent, the behavior of the Cairns system can be explained with no increase in mutation rate. That is, partially revertant cells can arise at standard rates during growth prior to selection and grow slowly after plating on lactose medium. Cells within these clones can improve their growth rate by acquiring common mutations at unenhanced rates. That is, the number of mutation targets within a clone may increase by growth and amplification while the mutation rate per act of replication remains unchanged. Initially, the critical preexisting cells were thought to carry a common duplication of the leaky lac allele (Andersson et al. 1998). Under selection, these cells were thought to grow and improve by further amplification steps, which occur at a rate of 0.01 per cell per division (Reams et al. 2010). Each colony growing with an amplified lac allele has an exponentially increasing opportunity to acquire a spontaneous lac frameshift mutation—more lac copies per cell and more cells per colony (Hendrickson et al. 2002). The yield of revertant cells under selection is thus stimulated by increases in target copy number within colonies rather than an increase in mutation rate per target. When a frameshift mutation generates a lac+ allele, growth improves, allowing loss of amplified nonrevertant lac alleles and overgrowth of the revertant colony by haploid lac+ cells.
There is considerable evidence that this amplification-under-selection model operates in many situations (Pranting and Andersson 2011; Roth 2011; Elde et al. 2012; Näsvall et al. 2012; Quinones-Soto et al. 2012), including the Salmonella version of the Cairns system. Amplification under selection can also explain the selective evolution of new genes (Bergthorsson et al. 2007), a process that has been experimentally demonstrated (Näsvall et al. 2012). However, in the Cairns system of Escherichia coli, plated cells show so little growth that it has been hard to demonstrate selective improvement and eliminate stress-induced mutagenesis.
To help decide this issue, a fundamental difference between induced mutagenesis and selection-only models is tested here. Stress-induced mutagenesis models propose that growth limitation induces mutagenesis and thereby produces new mutants that initiate the colonies appearing under selection. In contrast, selective improvement models propose that revertant colonies are initiated by cells that arise during nonselective pregrowth. These cells arise before exposure to selective conditions. Under selection, these preexisting cells develop into visible colonies with no change in mutation rate. It is already clear that the unstable Lac+ revertants are initiated by cells formed before selection. Short duplications that underlie the selected lac amplifications are found in the unselected pregrowth culture at sufficient frequency to explain unstable revertants (Reams et al. 2012). In a few cases, particular amplification junction sequences found in unstable revertants have been directly associated with duplication junctions present in the pregrowth population (Kugelberg et al. 2006). It was not previously known whether the stable Lac+ revertant clones are also initiated prior to exposure to selective conditions.
Are all revertants initiated prior to exposure to selection?
So far, the strongest support for initiation of Lac+ colonies during selection (regardless of mechanism) is the demonstration that revertant number is not subject to Luria–Delbrück fluctuation (Cairns and Foster 1991). These experiments are very clear and have been repeated in our laboratory with the same results (E. Sano, unpublished results). Luria and Delbrück used this test to demonstrate that mutants detected by stringent bacterial laboratory selections arise prior to plating. In their classic experiments, the frequency of selectively detected mutants varied from one parallel culture to the next due to chance timing in the occurrence of the first mutation. This frequency fluctuation showed that selection detects preexisting mutants and does not stimulate their formation (Luria and Delbruck 1943). In the Cairns system, the failure to see fluctuation was reasonably interpreted as evidence that in this system, unlike in standard laboratory selections, new mutants do not preexist but are initiated only after cells are immobilized on the selection plate. Such mutants could, at least in principle, be caused by “stress-induced mutagenesis.” Alternatively, the absence of fluctuation could reflect a problem detecting the responsible preexisting mutants. For reasons described below, duplications and amplifications cannot be detected by a fluctuation test. This calls into question the strongest previous evidence for stress-induced mutagenesis.
Gene duplications are carried at a steady-state frequency during nonselective growth (Reams et al. 2010). This steady state results from a balance between a high formation rate, on one hand, and an even higher loss rate and fitness costs, on the other hand. The forces that dictate this steady state obscure frequency fluctuations due to timing of mutation events. That is, these forces bring back to steady state any high or low duplication frequency caused by early or late duplication events. Fluctuation tests cannot reveal whether revertants are initiated by duplications or amplifications that arose prior to plating. If copy-number variants initiate lac revertants in the Cairns system, some other test is required to determine whether these cells arise prior to plating on selective medium.
Evidence reported here suggests that Lac+ revertant colonies are initiated by preexisting cells with multiple copies of the whole F′lac plasmid. Reducing the number of such cells in the pregrowth population reduces the number of both stable and unstable Lac+ revertant colonies appearing under selection. It was initially proposed that the preexisting lac gene copy number increases were tandem duplications of the local lac region of the plasmid (Andersson et al. 1998; Hendrickson et al. 2002; Kugelberg et al. 2006). Contrary to this expectation, the critical cells seem to have more copies of the entire F′lac plasmid. This finding directs attention to the biology of the F′ plasmid as a way to explain the behavior of the Cairns system. Early experiments on the Cairns system showed that appearance of revertants under selection depends on the DNA replication origin specific to conjugative transfer (Galitski and Roth 1995; Radicella et al. 1995; Peters et al. 1996; Godoy and Fox 2000). This suggested that mutants might arise during conjugative mating, but direct tests showed that the frequency of whole-plasmid transfer is low (Foster and Trimarchi 1995a,b; S. Maisnier-Patin, unpublished results). The importance of the transfer replication origin has not been explained. We propose that transfer replication produces cells with multiple plasmid copies (a form of amplification). Under selective conditions, these cells have insufficient energy to initiate chromosome replication and divide, but they can replicate their plasmid copies repeatedly until a mutation arises. In this model, F′lac plasmid overreplication, rather than an increased mutation rate, is responsible for the mutations that restore the ability to use lactose.
Materials and Methods
Bacterial strains and plasmids
All experiments were done with E. coli K12 strains whose genotypes are listed in Table 1. Salmonella typhimurium LT2 strains and plasmids used in this study are also described in Table 1.
Table 1. Bacterial strains and plasmids.
| Strain nos. | Genotypes/description | Reference or source |
|---|---|---|
| E. coli K12 | ||
| TR7177 | ara thiA ∆(lac-proB)XIII/F′128 proAB+ ∆(lacI-Z), same as FC29 | Cairns and Foster (1991) |
| TR7178 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω), same as FC40 | Cairns and Foster (1991) |
| TT26180 | F− ara thiA rifR ∆(lac-proB)XIII, same as FC36 | Cairns and Foster (1991) |
| TT26323 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) yebB11::Tn10dTc | This study |
| TT26324 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) yagU211::Tn10dTc | This study |
| TT26325 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) ychA11::Tn10dTc | This study |
| TT26326 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) tnpR11::Tn10dTc | This study |
| TT26327 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) lacA4659::Tn10dTc | This study |
| TT26328 | ara thiA rifR ∆(lac-proB)XIII btuR482::Tn10dTc/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) | This study |
| TT26900 | FC40/pSMP71 (pOU71::tetRA-gfp-cat) | This study |
| TT26905 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) zzf-1831(mhpC)::Tn10dTc-gfp-cat yebB11::Tn10dTc | This study |
| TT26911 | FC40/pSMP73 (pGB2:: tetRA-gfp-cat) | This study |
| TT26916 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) zzf-1831(mhpC)::Tn10dTc-gfp-cat | Laboratory collection |
| TT26932a | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) yebB11::Tn10dTc | This study |
| TT26933a | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) lacA4659::Tn10dTc | This study |
| TT26935 | ara thiA rifR ∆(lac-proB)XIII/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) lacA4659::Tn10dTc yebB11::Tn10dTc | This study |
| S. typhimurium LT2 | ||
| TT18784 | hisG10175::Tn10dTc hisC9955::MudJ | Laboratory collection |
| TT25387 | leuD21 proAB670::Spec | Laboratory collection |
| TT26310 | leuD21 proAB670::cam recA651::rif/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω) | Laboratory collection |
| TT26311 | leuD21 proAB670::cam recA651::rif/F′128 proAB+ lacIq lacI33(fs) lacIZ(Ω)/pNK2881 | This study |
| Plasmid | ||
| pNK2881 | Tn10 transposase under tac promoter, ApR | Way et al. (1984) |
| pGB2 | pSC101 origin, SpR | Churchward et al. (1984) |
| pOU71 | Temperature-dependent R1 origin, ApR | Larsen et al. (1984) |
| pSMP71 | pOU71::tetRA-gfp-cat, ApR, TcR, CmR | This study |
| pSMP73 | pGB2::tetRA-gfp-cat, SpR, TcR, CmR | This study |
Reconstructed duplicates.
Growth media and chemicals
Minimum medium was no-citrate E (NCE) medium described previously (Berkowitz et al. 1968). Rich medium was Luria–Bertani (LB) or nutrient broth (NB). Antibiotics were added at the following concentrations: tetracycline, 20 μg/ml; and chloramphenicol, 20 μg/ml. Anhydrotetracycline (AnTc) was used at concentrations described in the text. Plates were solidified with 1.5% agar. Nonselective NCE medium was supplemented with 0.2% glycerol, thiamine, and other nutrients at concentrations described previously (Davis et al. 1980). For lactose selection plates, NCE agar was supplemented with 0.1% lactose and thiamine. The chromogenic β-galactosidase substrate 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used at a concentration of 25 μg/ml (minimal medium) or 40 μg/ml (rich medium).
Growth rates
Growth of the strains with and without AnTc was measured in a 96-well plate, using a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments). Each strain was grown to saturation in 2.0 ml NCE with 0.2% glycerol and thiamine. To determine growth rates, an aliquot of 0.002 ml from each overnight culture was dispensed into 0.2 ml NCE glycerol (thiamine) contained in a well of the 96-well microplate. Cells were placed in the microplate reader for incubation at 37° for 20 hr. The microplate reader measured the optical density of the cell cultures at a 650-nm wavelength every 15 min. Doubling times (Dt) were calculated using Equation 1, where (N1) is the number of cells at the initial time point (t1) and (N2) is the number of cells at the final time point (t2):
| (1) |
Competitions
Each strain was grown to saturation in 2.0 ml NCE with 0.2% glycerol and thiamine. After overnight growth, cells were washed in NCE salts. The two competing strains were inoculated at an ∼1:1 ratio in 10 ml NCE glycerol (thiamine) medium with or without AnTc. After 24 hr of growth, cultures were diluted 1:1000 into fresh medium and allowed to compete for an additional 24 hr. Samples were taken at 0, 24, and 48 hr. Three independent replicates were performed for each competition. Viable cell titer was determined on NB agar plates and NB agar plates containing tetracycline.
Reversion tests
All reversion experiments were performed as previously described (Andersson et al. 1998). One day prior to plating the tester cells, lactose minimum selection plates were seeded with ∼2 × 109 Lac− cells (scavenger strain TR7177). All tester cultures were inoculated with cells from an independent single colony. All preselection cultures were grown to saturation at 37° in glycerol minimal medium with aeration by shaking. Each reversion experiment was done with at least five replicates for each strain tested. When used, AnTc (216 nM) was included in the pregrowth medium. Cells were sedimented and resuspended twice in NCE minimal medium prior to plating. This protocol was used for all reversion assays performed.
Construction of random Tn10dTc insertional mutants on F′128
Generalized transduction with P22 was used for random mutagenesis. The P22 lysate used for delivery of Tn10dTc was obtained by growing P22 (mutant HT105/1 int-201) on a Salmonella strain carrying a defective Tn10 transposon (lacking transposase function). The F′128 strain TT26311 used as recipient was first transformed with the plasmid pNK2881, which carries the Tn10 transposase gene under control of the tac promoter (Way et al. 1984; Kleckner et al. 1991). The resulting tetracycline-resistant transductants were pooled and conjugated to an F− strain of Salmonella (TT25387) to screen for transposition events that specifically occurred on F′128. Subsequent crosses consisted of a generalized transduction by P22 to move each Tn10dTc element to a new F′128 that had not been subjected to transposition events. Finally the whole plasmid was conjugated to a F− strain of E. coli (TT26180). The insertion points of Tn10dTc were determined by direct sequencing of a single-primer PCR product from one end of the Tn10dTc element, using TP93 (5′-ACCTTTGGTCACCAACGCTTTTCC-3′). The sequencing primer was TP133 (5′-CAAGATGTGTATCCACCTTAACTTAATG-3′).
Cloning of the “tetRA-gfp-cat” cassette and determination of copy number
Plasmids pOU71 (Larsen et al. 1984) and pGB2 (Churchward et al. 1984) were cleaved at a unique restriction site with the restriction enzymes EcoRI and SmaI, respectively. The linearized plasmids were made blunt ended with Klenow enzyme (New England Biolabs, Beverly, MA) and dephosphorylated by calf intestinal alkaline phosphatase (New England Biolabs), prior to purification with the Qiaquick PCR Purification Kit (QIAGEN, Valencia, CA). The cleaved plasmids were then ligated with a “tetRA-gfp-cat” DNA fragment, which was amplified by PCR from template DNA prepared from strain TT26916. This strain carries an engineered mini-Tn10dTc transposon where gfp and cat genes have been inserted at the 3′ end Tn10dTc (Sun et al. 2009). The primers used were TP2889 (5′-TTAAGACCCACTTTCACATTT-3′, binds end of tetR) and TP2891 (5′- GTCATTTCTGCCATTCATCC-3′, binds downstream of the cat gene). Prior to ligation the PCR fragments were purified using the Qiaquick PCR Purification Kit and phosphorylated using T4 kinase (New England Biolabs). All CmR transformants were also tetR and the new plasmids (pSMP71 and pSMP73) purified using a QIAGEN plasmid prep kit were introduced by transformation into TR7178. The relative copy number of the tetRA-gfp-cat cassette was determined by quantitative PCR, using an Applied Biosystems (Foster City, CA) 7900HT real-time PCR platform. The reactions were performed using SYBR Green PCR Master Mix (Applied Biosystems) and primers TP2874 (5′-CTTTCCGTCCGTTTCATCAC-3′) and TP2875 (5′-TTCTTTCCACTGCGGGTTAG-3′) to amplify 150 bp of pck used as the reference chromosomal gene and primers TP2829 (5′-GACGGTGAGCTGGTGATATG -3′) and TP2830 (5′-CGGAAATCGTCGTGGTATTC-3′) to amplify 107 bp of the cat gene located on the plasmid.
Results
Inhibiting growth of cells that carry a gene amplification
The tetA gene of transposon Tn10 encodes an efflux pump whose expression provides resistance to the antibiotic tetracycline (Tet). Overexpression of this pump inhibits cell growth (Moyed et al. 1983; Eckert and Beck 1989), perhaps by compromising proton motive force. Because of this inhibition, cells with multiple (5–10) copies of tetA are less resistant to tetracycline than cells with one copy (Coleman et al. 1983). Even cells with a single induced copy of tetA can be counterselected, but this requires the presence of lipophilic metal chelators, which allow even low levels of TetA to compromise membrane integrity perhaps by extracting metal ions (Bochner et al. 1980). In the absence of these chelators, the nontoxic analogs that induce tetA expression do not impair growth of cells with a single Tn10 copy, but can inhibit cells that overexpress TetA from many gene copies.
Here lac tester strains with tetA near lac on the F′lac plasmid are pregrown with AnTc, which induces expression of tetA, but is not directly toxic to cells (Moyed et al. 1983; Eckert and Beck 1989). Induction of tetA preferentially inhibits growth of cells with multiple copies of the Tn10 element. When the amplified region includes both tetA and the lac genes, growth with the inducer reduces the frequency of cells with many lac copies. These inducers were used to test the importance of lac gene amplification for reversion in the Cairns system (Hendrickson et al. 2002; Stumpf et al. 2007).
In the previous tests, Tn10 (including tetA) was inserted at various positions on the F′lac plasmid and an inducer of tetA [chlortetracycline (ChTc] was included in the selective plate. Presence of ChTc reduced revertant yield if the tetA gene was located near lac, but (in our hands) caused a progressively smaller reduction when tetA was inserted farther from lac on the same plasmid (Hendrickson et al. 2002). These results were interpreted as evidence that the process of lac reversion on the selective plate included amplification of a small region of the F′lac plasmid that included both tetA and lac. Such amplifications of lac were thought to improve growth on lactose, as suggested by the amplification–selection model described above. Cells in which tetA was coamplified with lac became sensitive to inhibition by the analog, which reduced the number of revertant colonies. The reduction was greatest when tet was placed near lac on the plasmid, favoring coamplification. This result supported the initial selection model in which selective amplification of lac under selection permitted point mutations and stable Lac+ revertants. Stumpf and Foster later confirmed that ChTc in the selection medium caused a drop in revertant yield, but they did not confirm the effect of tetA position vis-à-vis lac. They concluded that local lac amplifications within the F′ plasmid did not contribute to origin of revertants on the plate (Stumpf et al. 2007).
In revisiting these experiments, we found that results are sensitive to slight variations in protocol that we have not been able to control. Especially important is how cells are handled during the pregrowth period and whether pregrowth cultures enter stationary phase. Some experiments duplicated our previous results and some duplicated those of Stumpf and Foster and some differed from both. It seems possible that both published results are correct under some conditions that we cannot yet duplicate reliably. We suspect that part of the problem is that when the inducer ChTc is present in the selective lactose medium, cells face two opposite selections—for and against amplification. That is, amplification of the lac tetA region favors growth on lactose by giving more copies of the limiting lac allele, but the same amplification increases cell sensitivity to ChTc by producing more of the toxic TetA protein. The balance of these opposite selections may make the results sensitive to slight variations in conditions. In the experiments reported here, cells are exposed to the tetA inducer (AnTc) during nonselective growth prior to plating on lactose. Results are more robust when cells are exposed to a single selection. The presence of AnTc in the pregrowth culture removes cells with multiple lac tet copies prior to plating.
We used this inducer to test whether preexisting cells with multiple copies of the lac region are critical for initiating revertant colonies. Before exposure to the lactose selection, cultures were grown with AnTc to reduce the number of cells with many copies of tetA (and coamplified lac). Cultures grown in this way showed fewer Lac+ revertants after plating on selective medium. We expected decreases in revertant number only in strains with Tn10dTc near lac as seen previously (Hendrickson et al. 2002), but instead we observed a drop in revertant number regardless of the relative positions of lac and Tn10dTc in the plasmid. This agrees with observations of Stumpf and Foster (Stumpf et al. 2007) and suggests that the preexisting lac amplification critical to revertant initiation is provided by increased copy number of the entire F′lac plasmid.
Copy-number variation in bacterial populations
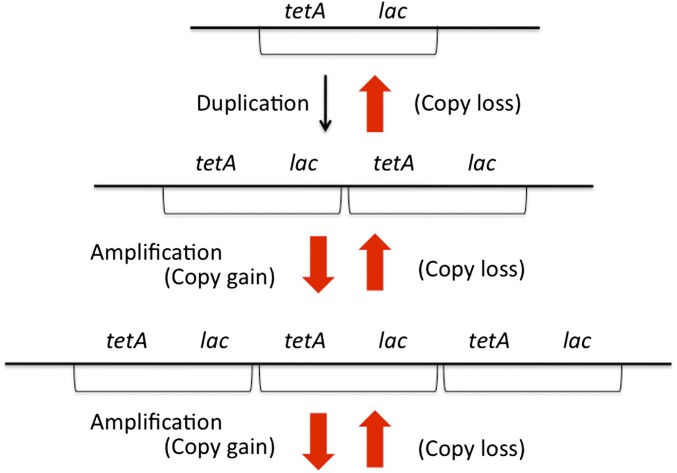
The frequency of cells with a duplication comes to a high steady state (10−4) in an unselected population (Reams et al. 2010). The process of tandem duplication and amplification is diagrammed in Figure 1.
Figure 1.
The process of gene amplification by tandem duplication. The sequence of events is essentially a random walk with two boundary conditions—amplification initiation is limited by a low initial duplication rate and expansion is limited by increasing fitness cost and copy loss rate in higher amplifications. Only single-copy changes are depicted, but exchanges between more distant copies can cause larger copy-number shifts. Each amplification and loss step (red arrows) depends on recombination between a pair of long repeats. These recombination-dependent events are expected to occur at the same rate, which is higher than that of RecA-independent initial duplication formation (black arrow). The whole system (frequency of all copy-number variants) comes to a steady state as a unit due to the high loss rate and increasing fitness cost (S. Maisnier-Patin, M. Savageau, and J. R. Roth, unpublished results; Reams et al. 2010). Fitness cost can be offset (and steady-state frequencies can rise) when growth is stimulated by the increased copy number of a gene within the amplified region.
The frequency of duplications comes to a steady state due to a balance between the initial duplication formation rate and the higher loss rate combined with the fitness cost of the duplication. This has been demonstrated experimentally and modeled mathematically (Reams et al. 2010). Each further increase in copy number is expected to occur at a fixed rate similar to that of duplication loss. Higher amplification is expected to cause higher fitness cost. As a result, the entire system of copy-number variants comes to a steady state with higher copy-number variants present at lower steady-state frequencies. The steady-state frequency of higher copy-number variants has been modeled mathematically and demonstrated experimentally (S. Maisnier-Patin, M. Savageau, and J. R. Roth, unpublished results). Thus, growth of rare high copy-number variants is expected to be inhibited by AnTc even in a population whose overall growth rate is not strongly affected. In effect, the analog gives a higher fitness cost to those cells with a sufficiently high copy number of any region that includes tetA, causing a decrease in their steady-state frequency, but having very little effect on the frequency of cells with lower copy number.
The same steady-state considerations are expected to apply to any system of highly reversible variants in lac copy number. This includes not only the tandem amplifications described above, but also cells with differences in plasmid copy number, plasmid multimer size (Projan et al. 1983; Pinkel et al. 1998), or rolling-circle replication (Cohen and Clark 1986; Mann and Slauch 1997). That is, steady-state frequencies of copy-number variants are expected whenever the array of copy-number variants expands and contracts at characteristic fixed rates and causes characteristic fitness costs.
The effect of AnTc on growth of cells with Tn10dTc (tetA)
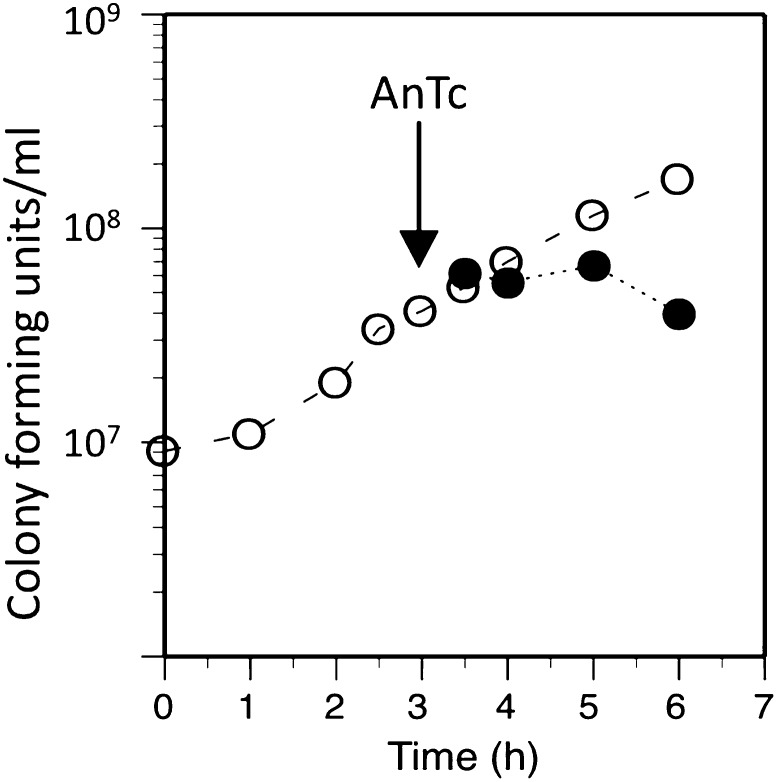
A normally regulated single tetA gene has very little effect on fitness in the absence of induction (Nguyen et al. 1989). Overexpression of multiple tetA copies from a foreign promoter can be lethal (Eckert and Beck 1989), but induced expression of multiple tetA copies by tetracycline or its analogs seems to inhibit growth without killing (Moyed et al. 1983). A demonstration of this sensitivity is in Figure 2, which shows the AnTc-sensitivity of an E. coli strain carrying an R1 plasmid with a temperature-sensitive copy-number control. The strain described was grown at 39° where it showed a tetA gene copy number of 15, measured by quantitative PCR. The same strain is resistant to AnTc at 37° when its copy number is ∼3. We estimate that 10 copies of tetA are needed for the analog AnTc to completely inhibit cell growth in the minimal glycerol medium used here.
Figure 2.
Inhibition by AnTc of growth of a strain with high tetA copy number. The strain used (TT26900) is a derivative of the standard reversion tester FC40 (TR7178) carrying a nonconjugative R1 plasmid having a tetracycline resistance cassette cloned from Tn10. The cassette includes the tetR tetA genes and their regulatory sequences. Cells were grown in NCE glycerol (0.1%) (open circles), with anhydrotetracycline (AnTc) added at 216 nM where indicated (solid circles). Samples were withdrawn, diluted, and plated on LB medium.
Thus the bulk of cells in a population with Tn10dTc on the F′ lac plasmid may not be sensitive to AnTc, but rare cells with more copies of the plasmid or its tetA region will be inhibited. When Tn10dTc (with tetA) is inserted near lac, the steady-state frequency of cells with a duplication of the tetA–lac region is ∼10−4, based on a trapping assay that detects any cell with two or more heritable copies of lac (Reams et al. 2010). Since the F′128 lac plasmid is normally carried at one to two copies per cell, duplication-bearing cells are likely to have about four copies of lac and tetA. Cells with a plasmid dimer may also have about four copies of this region if their copy number is similarly controlled. Alternatively, if plasmid copy-number control breaks down or conjugative plasmid replication switches to rolling-circle replication, occasional cells might have multiple copies of the entire plasmid. Any of these situations is likely to be unstable and subject to the shifts in copy number shown in Figure 1. Since so few cells are expected to be sensitive to growth inhibition, one does not expect a major effect of AnTc on overall strain growth rate. However, the steady-state plasmid copy number will be affected since impaired copy-number variants are continuously replaced at a high rate. Growth rates are presented in Figure 3.
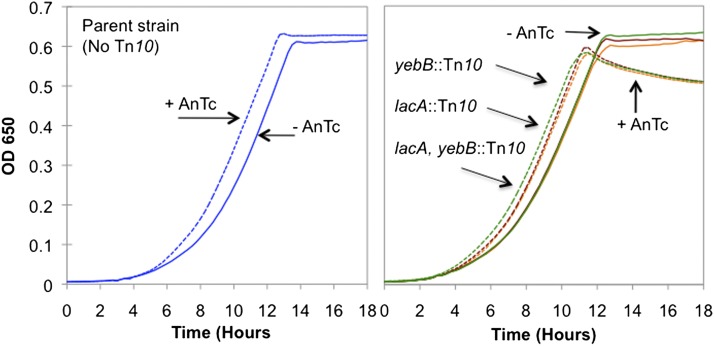
Figure 3.
Effect of AnTc on growth of strains with Tn10dTc on the F′ plasmid. The strain without Tn10 (left) is the standard tester strain (TR7178). Strains with Tn10dTc (right) have an insertion in lac (TT26933), in the yebB gene, far from lac (TT26932), or at both positions (TT26935). Growth was measured in minimal NCE medium with glycerol using an automated plate reader.
As expected, AnTc in the medium had very little effect on the overall doubling time whether or not Tn10 is present in the plasmid. While growth rates of most cells are insensitive to inhibition by AnTc, one expects that growth of rare cells with more tetA copies would be inhibited. This inhibition is expected to remove cells with high tetA copy number from the equilibrium system diagrammed in Figure 1. Growth results are presented in Figure 3, where culture OD650 is plotted linearly to make differences in final density more obvious. The growth rates are indistinguishable during exponential phase, but strains with Tn10dTc on the F′lac showed a slightly decreased number of cells (OD650) at stationary phase following growth with AnTc. This drop was also seen in viable cell assays. It should be noted the Tn10dTc-containing strains grown with AnTc reached a maximum cell density earlier and their cell number (and viable counts) dropped slightly with time in stationary phase. Results similar to these were seen for several different single and double Tn10dTc insertions at various positions in the F′ plasmid. Strains with two Tn10dTc insertions in F′lac did not show a significant increase in their sensitivity to AnTc when tested directly.
Growth competitions allowed better visualization of small effects of AnTc. Strains with and without Tn10dTc in their F′lac plasmid were grown together for two passages (see Figure 4). Cultures were inoculated with a 1:1 mixture of the two strains, grown in minimal glycerol medium with or without AnTc, and then diluted 1000-fold in the same medium at each passage. Strains with Tn10dTc on the F′ plasmid showed a competitive disadvantage when grown in the presence of AnTc over two cycles. The competitive disadvantage could be due to slightly slower overall growth rate, to occasional cell death, or to slower emergence from stationary phase between cycles. The strain with two Tn10dTc insertions in F seemed to show a slightly clearer competitive disadvantage, an effect noted for other strains.
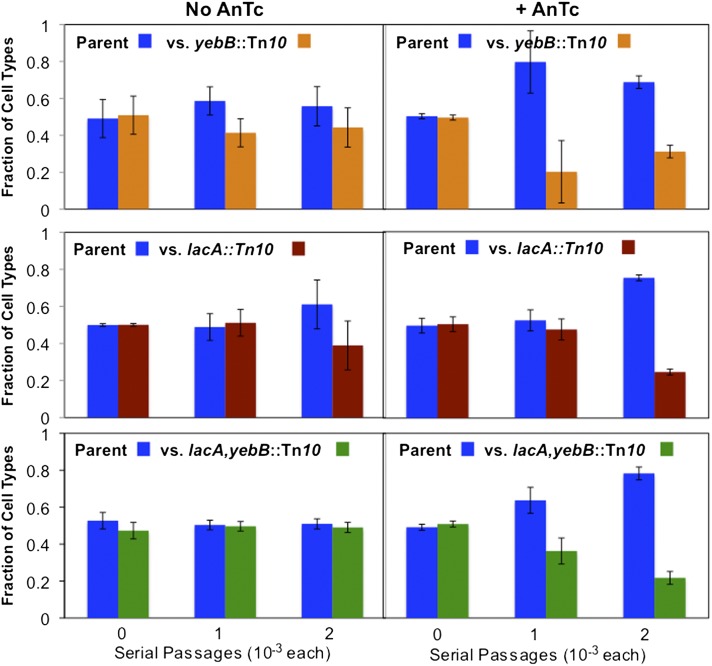
Figure 4.
Competition between strains with and without Tn10dTc. To better observe effects of AnTc on growth, pairs of strains with and without a Tn10dTc insertion in F′lac were mixed and grown together in minimal glycerol medium with or without AnTc. The cultures were grown for two passages and the ratio of cells with and without Tn10dTc was scored by plating diluted cultures on rich medium with and without tetracycline. Strains: parent with no Tn10 (TR7178); one insertion, yebB::Tn10dTc (TT26932); one insertion of Tn10dTc in lacA (TT26933); and two Tn10dTc insertions, one in lacA and the other in yebB (TT26935).
One of the two Tn10dTc insertions used here (lacA) is very close to lacZ, ∼1 kb away from lacZ, while the other (yebB) is at the opposite side of the plasmid, near the origin of replication. The two insertions had essentially the same effect on the sensitivity of growth with AnTc. Effects of AnTc on general growth in these strains are small as expected if inhibition bears only on the few cells with multiple copies of tetA. The similar sensitivity of strains with Tn10dTc at different positions suggests that different points on the plasmid have the same probability of amplification either individually or as part of a general increase in plasmid copy number. Whenever such an amplification happens to include lac as well as Tn10dTc(tetA), one expects growth with AnTc to also reduce the number of cells in the population with multiple lac copies. We had expected that tet–lac amplification would primarily occur by tandem arrays of small regions of the plasmid and the effect of AnTc on lac copy number (reduction in revertant yield) would be seen only in strains with Tn10dTc near lac. Contrary to this expectation, tests below show that position of tetA on the plasmid made little difference in revertant yield, suggesting that sensitivity to AnTc may be due to occasional amplification of the entire plasmid.
Effect of AnTc on revertant yield in the Cairns system
The tester strain used in the Cairns system carries an F′lac plasmid with a lac frameshift mutation (Cairns and Foster 1991). When 108 cells of this strain were pregrown on glycerol and plated on lactose, ∼100 revertant colonies appeared over the course of 6 days. The selective amplification model proposes that these revertants are initiated by preexisting cells with multiple copies of lac. These duplications and amplifications arise during the pregrowth period and their frequency approaches a steady state (Reams et al. 2010).
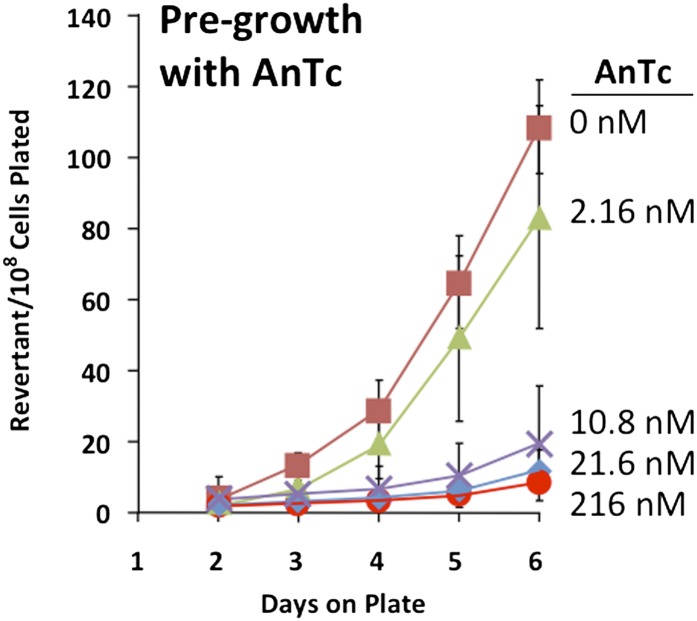
To test the effect of pregrowth with AnTc on revertant yield, cells with Tn10dTc on the F′lac plasmid were pregrown in glycerol medium containing various concentrations of AnTc and were then plated on lactose plates to detect revertants. Following growth to stationary phase, cells were washed to remove AnTc and plated on lactose medium without AnTc. Figure 5 shows that pregrowth with AnTc concentrations >10 nM reduced the number of revertant colonies that accumulated over the following 6 days on selective lactose plates. Revertant yields typically dropped between 5- and 10-fold following growth with sufficient concentrations of AnTc.
Figure 5.
Concentrations of AnTc in pregrowth medium that reduce revertant yield. Reversion was tested as described in Materials and Methods, using strain TT26327 with a Tn10dTc insertion in the lacA gene of plasmid F′128lac. The revertant colony number is presented per viable cell of the tester strain plated.
In Figure 5 and most of the reversion tests described below, the number of viable cells plated is used to normalize results of the reversion test. That is, plotted revertant numbers are expressed per viable cell plated. This normalization has been done in much of the analysis of the Cairns system because many recombination mutants reduce viability and normalizing to viable cells plated ensures that the number of revertants scored is derived from the same number of cells. We have done this normalization here to make our results comparable to those of early experiments. However, it should be noted that in these experiments, the population grown with AnTc has been enriched for cells that lack the amplification, and thus normalization understates the effect of AnTc on revertant number. Viability decreases following growth with AnTc did not exceed 2-fold. With this normalization, revertant numbers were typically reduced 7-fold by AnTc, and without normalization 10-fold decreases were typical. Similarly, lawn viability on the selection plate was tested for cells pregrown with and without AnTc and found to be essentially constant over 5 days.
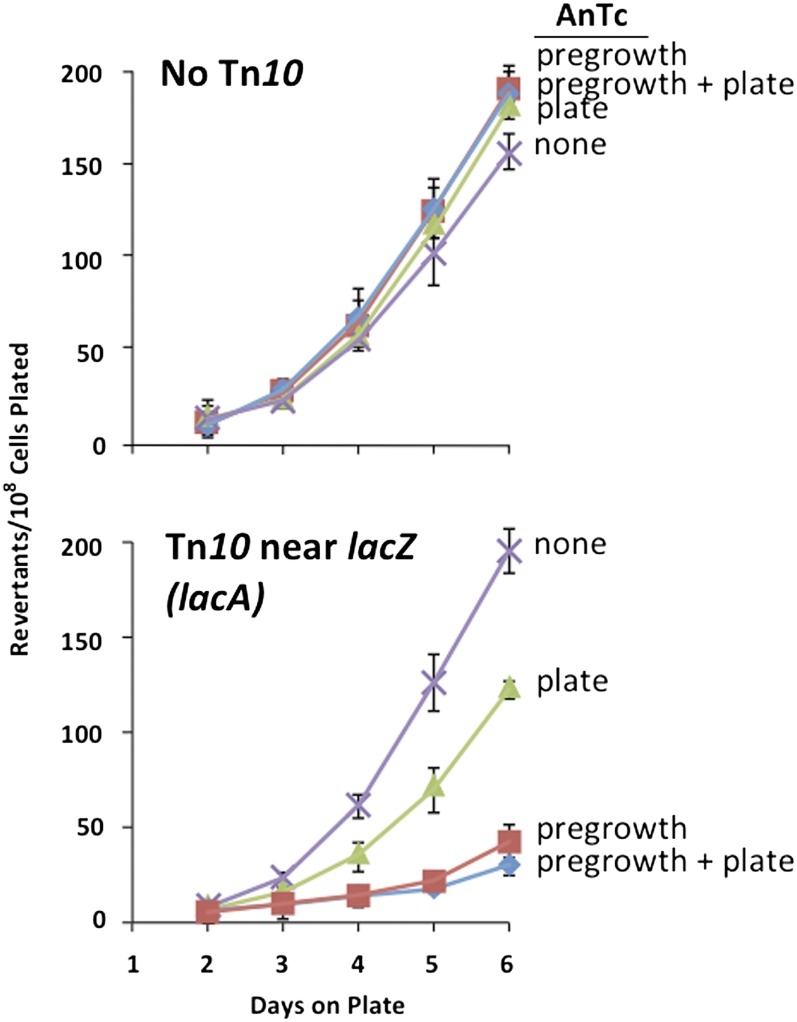
The major effect of AnTc is seen during nonselective pregrowth
The reduction in revertant yield in strains with Tn10dTc was greatest when AnTc was included in otherwise nonselective pregrowth medium. This is shown in Figure 6, which describes the effects of exposure to AnTc before selection, during selection, or both. In strains lacking Tn10dTc (Figure 6, top), revertant yield was not affected by AnTc added either before or during selection (in lactose plates). In strains with Tn10dTc on the F′lac plasmid, addition of AnTc to the selective lactose plates caused only a slight decrease in revertant number, as reported previously (Hendrickson et al. 2002; Stumpf et al. 2007). However, pregrowth of these strains with AnTc prior to plating caused strong reduction in the number of revertants produced later on selective medium. This effect of AnTc added during nonselective pregrowth in glycerol routinely caused a 5- to 10-fold reduction in revertant yield (Figure 6, bottom). This is recorded following normalization for viability, which understates the AnTc effect.
Figure 6.
Effect of AnTc on revertant yield requires Tn10dTc and exposure during pregrowth. The strain without Tn10dTc (TR7178) is the original tester strain of Cairns and Foster (1991) and the strain with Tn10dTc in lac (TT26327) carried an insertion at the distal end of the lac operon of the F′128 plasmid. Cells were pregrown for 20 generations in minimal glycerol with and without AnTc (216 nM) and plated on lactose medium with or without AnTc.
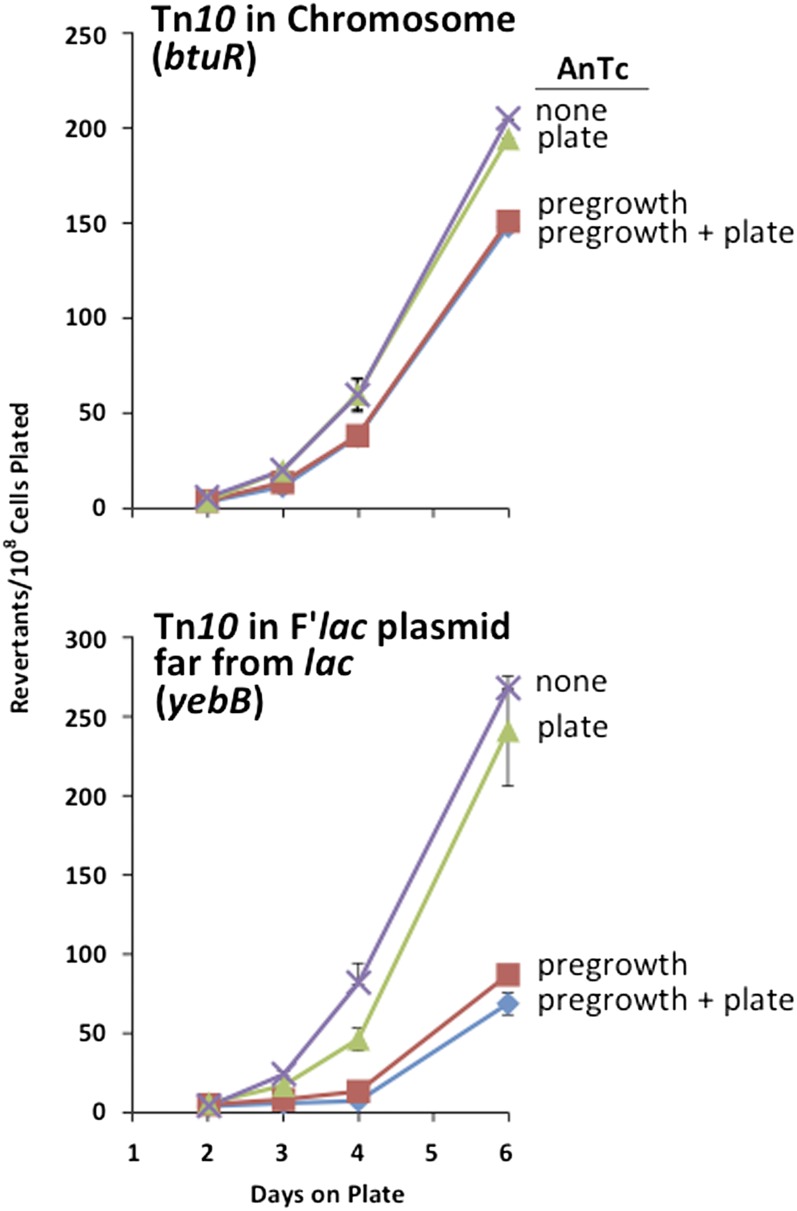
The effect of AnTc on lac reversion requires having Tn10dTc on the F′lac plasmid
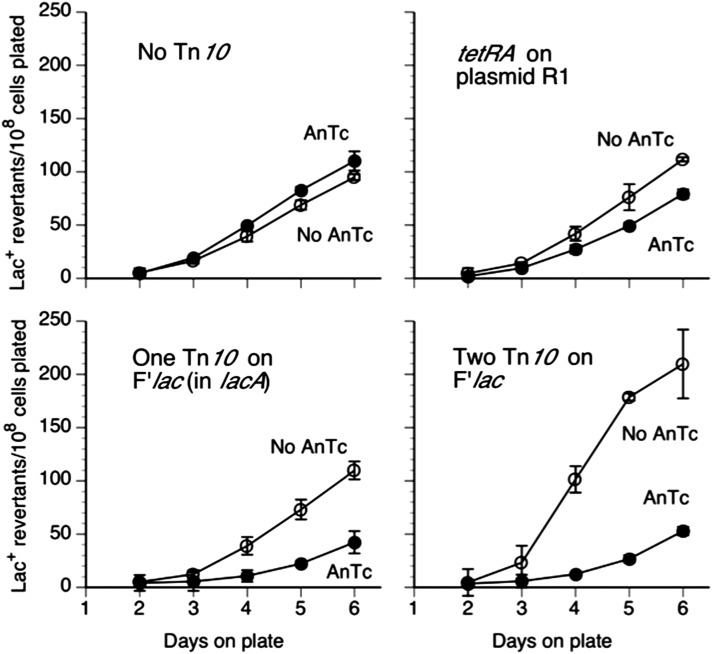
The effect of AnTc on revertant yield seen above was attributed to reducing the number of cells in the pregrowth culture that have coamplified with lac and with tetA genes. Initially it was expected that amplification was due to multiple tandem copies of the mutant lac region. This expectation predicts that Tn10dTc (tetA) would have to be located on the F′lac plasmid close enough to lac to be frequently coamplified. Figure 7 (top) shows that AnTc has little effect on revertant yield when Tn10dTc is inserted in the chromosome (btuR) rather than on F.
Figure 7.
Effect of Tn10dTc position on sensitivity of reversion to AnTc. Cells were pregrown to stationary phase in glycerol with or without AnTc (216 nM) and then washed and plated on selective lactose plates with or without AnTc (216 nM). The strain at the top carries Tn10dTc in the chromosomal btuR gene (TT26328). The strain at the bottom carries Tn10dTc in the yebB gene (TT26323). Revertant number is expressed relative to viable cells plated. This normalization understates the effect of pregrowth with AnTc, by including only cells that have survived the effects of AnTc and eliminating those multicopy cells lost during pregrowth.
Unexpectedly, as seen in the bottom of Figure 7, reversion was strongly reduced even when the F′lac Tn10dTc was inserted at a position far from lac (yebB::Tn10dTc). The yebB::Tn10dTc insertion is near the plasmid vegetative replication origin at the greatest possible distance from lac on the F′lac plasmid (Figure 8). In the belief that Lac+ revertants were initiated by cells with a tandem duplication of a small region of the plasmid that included lac, we expected that AnTc would reduce revertant yield only in strains whose Tn10dTc(tetA) was inserted close enough to lac to be coamplified. The observed effect of AnTc on strains with a Tn10 far from lac suggested that the cells responsible for initiating revertant colonies must carry multiple copies of the entire F′lac plasmid, not just the immediate lac region. This position-independent effect of Tn10 on revertant number was noted previously by Stumpf and Foster (Stumpf et al. 2007).
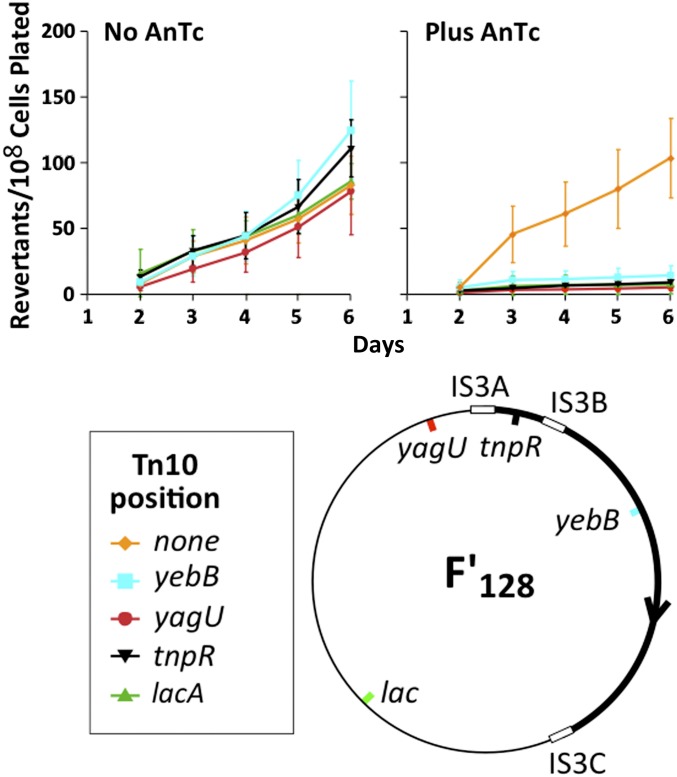
Figure 8.
Insertions of Tn10dTc at points far from lac on the F′ plasmid allowed AnTc to inhibit revertant yield. A series of tester strains with Tn10dTc inserted at various points in the F′lac plasmid were compared for their yield of Lac+ revertants following pregrowth with and without AnTc. These strains are described in Table 1. Revertant yields following pregrowth without and with AnTc are described in the top left and top right, respectively. The map positions of the Tn10dTc insertions are described in the bottom left.
Effect of Tn10dTc at various positions on the F′ plasmid
To pursue the effect of Tn10dTc position on revertant inhibition, a series of insertions at varying distances from lac were tested for their ability to make reversion sensitive to pregrowth with AnTc. Figure 8 shows a map of the F′128lac plasmid with the positions of various insertions. Each strain was pregrown either with or without AnTc and then plated without AnTc on selective lactose medium. Regardless of the position of Tn10dTc on the plasmid, pregrowth with AnTc reduced the yield of Lac+ revertants.
No AnTc effect is seen when Tn10dTc and lac are on different plasmids
This was tested by inserting a Tn10dTc(tetA) on an R1 plasmid in a strain with the lac mutation on the standard F′lac plasmid. The R1 plasmid used here (and in Figure 2) carries a temperature-sensitive replication origin (Larsen et al. 1984) and is present at copy number of three during growth at 37°, approximately the same number (one to two) as F′lac. As seen in Figure 9 (top right), AnTc has little effect on lac reversion when Tn10dTc and lac are on separate plasmids.
Figure 9.
Effect of Tn10dTc on lac reversion under selection. Strains with tetA in plasmid R1 are compared to strains with one and two copies of Tn10dTc in F′lac. The standard tester strain (TR7178) with F′lac but no Tn10 is at top left. The strain at top right (TT26900) carries plasmid R1 with a cloned tetR tetA cassette derived from Tn10. The strain at bottom left has Tn10dTc inserted in lacA and is the same strain described in Figure 6 and Figure 8. The strain at bottom right (TT26905) has two tetA genes, one within insertion yebB::Tn10dTc far from lac and the other within a derivative of zzf-1881::Tn10dTc inserted in inverse order ∼5 kb away from lac—this derivative has a Cm resistance marker and a GFP gene (Sun et al. 2009).
Effect of two Tn10dTc elements on the plasmid with lac
The bottom of Figure 9 describes the effect of two Tn10dTc insertions on the same plasmid. The strain on the bottom left has one Tn10dTc element inserted in lacA. The strain on the bottom right has two Tn10dTcs, one inserted far from lac (yebB::Tn10dTc) and the other inserted 5 kb from lacA (zzf-1831::Tn10dTc-GFP-Cm) (Sun et al. 2009). These two strains were tested together and the strain carrying both mhpC31 and yebB insertions was more sensitive to pregrowth with AnTc, as one might expect if cells with more tetA copies are more sensitive to inhibition. Both Tn10dTc insertions in the double mutant are defective for transposase and are placed in opposite orientation, so they are not expected to contribute to duplication by either transposition or recombination. The higher revertant yield in the strain with inverse-order Tn10dTc copies may reflect a previous observation that strains with inverse-order repeats are prone to dimer formation (Lyu et al. 1999).
Populations inhibited by AnTc regained revertant yield when passaged without AnTc
The effects of AnTc described above were interpreted as evidence that reversible lac copy-number variants such as tandem amplifications or plasmid multimers arise during pregrowth and initiate the revertant colonies that develop and appear during 1 week under selection. This reversibility causes copy-number variants to come to a steady-state frequency in nonselective conditions when a balance is attained between high rates of formation and loss (Reams et al. 2010). This instability of copy number was suggested to explain why revertant number, like duplication frequency, is not subject to Luria–Delbruck fluctuation (Cairns and Foster 1991; Reams et al. 2010). That is, the forces that drive frequency to steady state obscure fluctuations due to the time of initial mutant formation. An alternative possibility is that pregrowth in the presence of AnTc favors some conventional stable mutants that are impaired for reversion under selection (e.g., plasmid loss or lac deletion). Several lines of evidence argue strongly against this possibility.
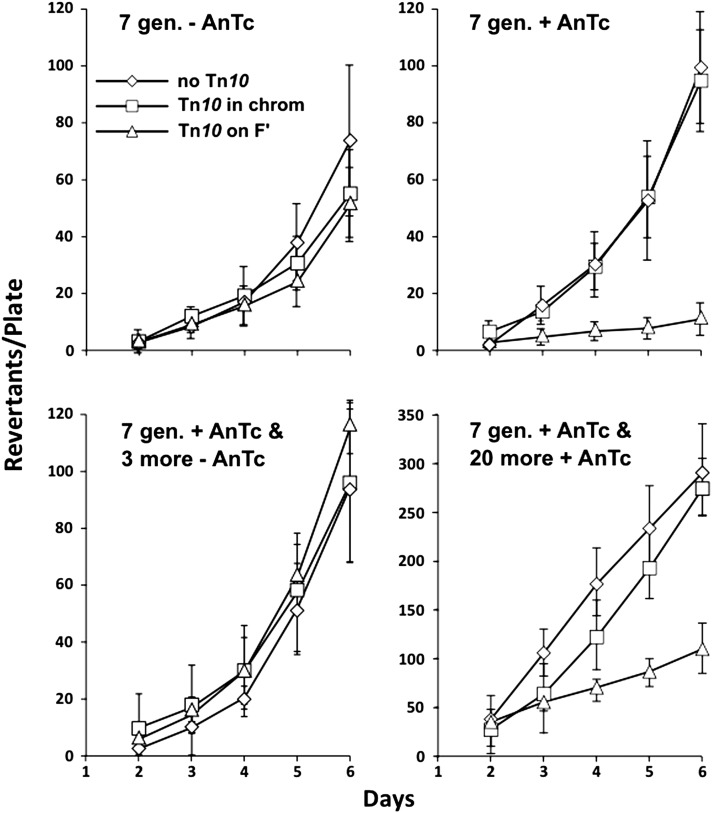
The reversibility of the AnTc-induced reduction in revertant number was shown by growing tester cells first with AnTc and then diluting them 10-fold to inoculate a second passage without AnTc. The revertant yield of the two cultures is shown in Figure 10, top. The initial tester strain was pregrown for seven generations either without (Figure 10, top left) or with (Figure 10, top right) AnTc and the revertant yield was reduced by AnTc as expected. The AnTc-grown population was then diluted 10-fold and grown for three to four generations without AnTc. During this brief period, the strain regained full ability to revert under selection (Figure 10, bottom left). We interpret this as evidence that copy-number variants form and are lost at high rates. The analog AnTc inhibits growth of the variants with highest copy number and thereby reduces the steady-state frequency of these variants in the population. However, the high steady-state frequency is restored quickly by interconversion of copy-number variants once the inhibitor is removed. The loss rate of large duplications in the chromosome and on the F′lac plasmid is ∼10−2 per cell per generation (Reams et al. 2010). We do not yet know the formation or loss rates for the plasmid copy-number variants inferred here.
Figure 10.
The effect of AnTc on revertant yield is reversed by growth without AnTc. Preexisting cells responsible for initiating revertants appearing under selection are thought to have multiple copies of the F′lac plasmid (including the tetA gene). The stability of such cells was tested using a strain with Tn10dTc in lacA (TT26327), a strain with Tn10dTc inserted into the chromosomal btuR gene (TT26328), and a strain with no Tn10 (TR7178). All strains were pregrown for 7 generations without (top left) or with (top right) AnTc and then were used in a standard reversion experiment. Immediately following growth with AnTc all three mutants were diluted 10-fold into fresh medium lacking AnTc, grown for 3 generations, and retested for reversion (bottom left). The same strain was passaged six times by 10-fold dilution into glycerol medium containing AnTc (20 additional generations with AnTc) (bottom right).
Irreversible changes in behavior are seen after longer growth in the presence of AnTc
When cells with Tn10dTc near lac (in lacA) on the F′lac plasmid were passaged six additional times (each a 10-fold dilution) in the presence of AnTc, they began to regain ability to generate Lac+ revertants (see Figure 10, bottom right). This restoration of revertant yield is accompanied by loss of Tn10dTc (TetR) from the selectively held F′lac plasmid by ∼10% of cells. Cells that lost TetR retained the leaky Lac+ phenotype characteristic of the parent mutation. The loss of Tn10dTc from F′lac renders cells insensitive to growth inhibition by AnTc and allows the lac copy number to increase in the population. Thus, induction of the tetA gene by AnTc appears to impose a small growth inhibition on the entire population. We infer that AnTc acts primarily on the high-copy subpopulation, increasing their fitness cost and reducing their frequency in the population. Since these disadvantaged cells are continually replaced by new amplifications, the effect of AnTc is ultimately felt by the whole population. That is, any cell sublineage that loses Tn10dTc from the plasmid can produce cells with multiple F′lac copies that escape inhibition by AnTc.
Preexisting cells with a lac amplification initiate both stable and unstable Lac+ revertants
The Lac+ revertant colonies that accumulate under selection in the Cairns system are of two types. About 90% of colonies are composed primarily of stable lac+ revertant cells that have acquired a point mutation that corrects the parental +1 frameshift. About 10% of revertant colonies contain cells with an unstable amplification of the original leaky lac allele. The initial growth-under-selection model proposed that cells with a tandem duplication of the parent lac region grow under selection and develop in one of two ways. They either acquire a point mutation and form a stable revertant or acquire a deletion that reduces amplification repeat size and fitness cost, allowing higher amplification and formation of an unstable revertant (Kugelberg et al. 2010). The results presented here suggest that revertant colonies are initiated by cells with more copies of the whole plasmid rather than a tandem amplification of the lac region. This raises the question of whether whole-plasmid amplification is required for both stable and unstable revertant types.
The yield of Lac+ revertants drops (typically about sevenfold) following pregrowth with AnTc to reduce the frequency of cells carrying F′lac at high copy number. In standard reversion experiments, ∼2–10% of total revertants appearing on days 5 and 6 are unstably Lac+ (Andersson et al. 1998; Hastings et al. 2000). To determine whether pregrowth with AnTc reduces the number of unstable revertants, we tested 156 total revertant colonies following pregrowth in the presence of AnTc of strains with yebB::Tn10dTc or lacA::Tn10dTc insertions on their F′lac plasmids. Of 156 revertants tested (66 from yebB and 99 from lacA), unstably Lac+ cells were found in 17 (6 from yebB and 11 from lacA). This is just over 10% of the total revertants. Thus while AnTc reduced total revertant number about sevenfold, it had no effect on the fraction that contains unstably Lac+ cells. This suggests that presence of AnTc in the pregrowth culture reduces the yield of both stable and unstable revertants to the same extent and both types of revertants have a common starting point prior to imposition of selection.
Discussion
These results suggest that both stable and unstable Lac+ revertants appearing under selection in the Cairns system are initiated by cells that form during nonselective pregrowth and carry a high copy number of the F′lac plasmid. This suggests shortfalls in both stress and -induced mutation and selective amplification models for the origin of mutants. First, revertants are not initiated under selection as proposed by stress-induced mutagenesis. Second, the initiating cells appear to carry multiple copies of the entire F′lac plasmid, not a tandem amplification of just the lac region, as proposed by the amplification-under-selection model.
The results shed light on two aspects of the Cairns system that have been used to explain the paucity of evidence for general mutagenesis. The amplification of the whole F′lac plasmid suggests that directed mutation may mean increased likelihood of mutations on this plasmid due to repeated replication and copy-number increase and not to mutagenesis. The subset of plated cells suggested to enter the “hypermutable state” may correspond to the preexisting cells with extra copies of the F′lac plasmid. The results presented are consistent with early evidence that the F′ plasmid plays a central role in the reversion process. We discuss these results in the context of a new model for the process of reversion under selection.
There is a caveat in our interpretation of the effects of AnTc. The results are interpreted as evidence that growth with AnTc reduces the number of cells able to initiate a revertant. This interpretation is based on evidence that AnTc inhibits growth of cells with high TetA levels (Moyed et al. 1983; Eckert and Beck 1989) and this inhibition increases with added tetA gene copy number (S. Maisnier-Patin, M. Savageau, and J. R. Roth, unpublished results). An alternative interpretation, which we cannot eliminate, is that all cells grown with AnTc, regardless of their TetA level, are placed in a shared physiological state that reduces the probability of their surviving or reverting when placed under selective conditions. We know that pregrowth with AnTc does not impair survival of starved cells, but we cannot eliminate the possibility that all AnTc-grown cells have a stochastically reduced probability of reversion. We think this alternative is unlikely because it requires that AnTc have two independent effects—one in inhibiting growth of cells with high TetA protein levels and a second in reducing the probability of reversion under lactose selection. These alternative particulate and stochastic interpretations of population behavior are common to many biological situations (e.g., assays of phage titer, bacterial colony-forming ability, and cancer stem cell transplantation).
The interpretation of these experiments depends on AnTc impairing growth only of cells with multiple copies of tetA or F′lac tetA. That is, the bulk of the tester population retains the F′pro+ lac tetA+ plasmid during growth with AnTc. A simple alternative would be to suggest that AnTc causes plasmid loss and leads to more cells with no lac allele capable of reversion. Several lines of evidence presented here support the idea that plated cells still carry the F′pro+ lac tetA+ plasmid: (a) The population is grown on minimal glycerol medium, which selects for retention of the plasmid pro+ allele; (b) the plated population demonstrably retains the plasmid-encoded Pro+ TetR phenotypes and the leaky Lac+ phenotype of the mutant lac allele; and (c) following revertant loss due to pregrowth with AnTc, the tester population regains full ability to produce revertants within only three generations of additional growth without AnTc. This rapid recovery is inconsistent with complete loss of the lac allele.
The nature of the preexisting cells that initiate revertant colonies remains uncertain. It is inferred that these cells have multiple copies of the entire F′lac because reversion was sensitive to AnTc, regardless of the position of Tn10dTc vis-à-vis the plasmid lac operon. Cells might acquire multiple plasmid copies in several ways. Stochastic loss of plasmid copy-number control could lead to occasional cells with multiple independent plasmid copies. Stochastic failure of dimer resolution mechanisms could lead to accumulation of plasmid multimers formed by plasmid–plasmid recombination or sister strand exchange during replication. Multiple plasmid copies could be generated by temporary rolling-circle replication. Many plasmids are known to occasionally switch into rolling-circle mode in which a single replication fork proceeds repeatedly around the basic circular plasmid, producing a linear double-stranded concatamer of repeated plasmid genome copies (Viret et al. 1991). Such replication underlies growth of many viruses and is known to occur stochastically in cells carrying circular plasmids. On the F′ plasmid, rolling-circle replication forks could be derived from forks initiated at F′ plasmid transfer origin, whose forks normally produce a 5′-ended single strand for conjugation. Conversion to a full stable replication fork could allow this origin to produce nontransferable double-stranded linear products. Cells with a stable rolling-circle fork could form reversibly and incur a fitness cost, leading to a steady-state frequency of such cells in the preselection population. A final possibility is copy addition by repeated acts of plasmid transfer.
Early work on the Cairns/Foster system revealed that revertant yield depends heavily on presence of a functional F′lac conjugation replication origin (oriT) (Galitski and Roth 1995; Radicella et al. 1995; Peters et al. 1996; Godoy and Fox 2000). Essentially all Lac+ reversion is eliminated in cells that lack the plasmid TraI protein, an endonuclease that nicks plasmid DNA at the plasmid oriT site, displacing a 5′-ended single-stranded plasmid copy for transfer into the recipient cell. Reversion also depends heavily on other plasmid-encoded proteins needed to signal readiness to mate and to assemble the transfer replication complex—e.g., the TraD proteins, pilus structure (Foster and Trimarchi 1995a,b). These dependencies demonstrate the importance of conjugation abilities of the F′ plasmid. However, direct tests of mating suggested that actual DNA transfer is not required on the selection plate for formation of the majority of revertants (Foster and Trimarchi 1995a,b). An increase in plasmid copy number due to successive mating between F′ cells was suggested previously (Peters et al. 1996), but not pursued in the light of evidence against transfer. This evidence did not eliminate mating events prior to plating, transfer between immediate siblings, or exceptional establishment of a transfer replication fork within a single cell or cell filament.
A new model for the origin of mutations under selection in the Cairns system
This model accounts for the results described here in terms of generally accepted properties of the Cairns system:
-
1.
The bulk of the plated population with a standard number of lac copies is unable to grow under lactose selection, due to insufficient energy to trigger initiation of standard chromosome replication.
-
2.
Rare variants with multiple copies of the whole F′lac plasmid have enough energy to divide once, but produce daughter cells without sufficient extra lac copies to grow exponentially. However, nondividing cells with multiple F′lac plasmid copies may have enough energy to continuously replicate their F′ plasmid from unregulated origins. The cells that do this may be any or all of the forms discussed above. These cells replicate their F′lac repeatedly with little or no cell division until a reversion event occurs at any time within several days. As soon as reversion occurs, energy is resupplied by the lac+ allele, cell division starts, and the clone expands exponentially, maintaining its revertant allele under selection.
-
3.
Occasional preexisting cells have multiple copies of a plasmid that already carries an internal tandem duplication of lac. Cells with a short duplication on F′lac are present at ∼10−4 in an unselected population (Reams et al. 2012). The join points of these duplications are like those of the amplifications found in unstable revertants (Kugelberg et al. 2006, 2010; Slack et al. 2006). The extra lac copies provided by multiple copies of a plasmid with an internal lac amplification are sufficient to allow immediate exponential growth under selection and development of an unstable revertant colony with an expanded tandem lac amplification. The frequency of short lac duplications prior to selection is sufficient to account for the observed number of unstable Lac+ revertants.
-
4.
Cells with multiple copies of the F′lac plasmid also have multiple copies of the dinB gene, which encodes an error-prone polymerase and happens to lie on that plasmid. Thus DNA replication in both the stable and unstable lineages occurs in the presence of elevated levels of the mutagenic DinB polymerase. In lineages leading to stable revertants, the chromosome does not replicate extensively and mutagenesis is focused on the replicating F′ plasmid. In unstable lineages (10% of total), many of the lac duplications coamplify the nearby dinB gene. These cells grow and replicate their entire chromosome in the presence of elevated DinB. Thus mutagenesis affects the entire chromosome. While DinB mutagenesis contributes to the yield of stable Lac+ revertants, its effect is essentially an artifact due to the chance location of dinB on F′lac where it can be coamplified with lac during replication under selection.
Summary
The history of work on the Cairns system is remarkable in that a large body of excellent data has accumulated and been interpreted in two diametrically opposed ways—mutagenesis and selection. This body of information is largely agreed to by all participants in the discussion and contains very few internal contradictions. However, most experiments have been directed at verifying one model or the other rather than discriminating between alternatives. The results and model described above may help refocus the discussion.
Acknowledgments
We thank laboratory co-workers Mahtab Danai, Natalie Duleba, Manjot Grewel, Douglas Huseby, Eric Kofoid, Andrew Reams, and Ivy Roush for discussion and advice on the manuscript. We also thank faculty colleagues Wolf Heyer and Steven Kowalczykowski for helpful suggestions. This work was supported in part by National Institutes of Health grant GM27068.
Footnotes
Available freely online through the author-supported open access option.
Communicating editor: S. Sandler
Literature Cited
- Andersson D. I., Slechta E. S., Roth J. R., 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282: 1133–1135. [DOI] [PubMed] [Google Scholar]
- Bergthorsson U., Andersson D. I., Roth J. R., 2007. Ohno’s dilemma: evolution of new genes under continuous selection. Proc. Natl. Acad. Sci. USA 104: 17004–17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Roth J. R., Ames B. N., 1968. Procedures for identifying nonsense mutations. J. Bacteriol. 96: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N., 1980. Positive selection for loss of tetracycline resistance. J. Bacteriol. 143: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J., Foster P. L., 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S., 1988. The origin of mutants. Nature 335: 142–145. [DOI] [PubMed] [Google Scholar]
- Churchward G., Belin D., Nagamine Y., 1984. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene 31: 165–171. [DOI] [PubMed] [Google Scholar]
- Cohen A., Clark A. J., 1986. Synthesis of linear plasmid multimers in Escherichia coli K-12. J. Bacteriol. 167: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Chopra I., Shales S. W., Howe T. G., Foster T. J., 1983. Analysis of tetracycline resistance encoded by transposon Tn10: deletion mapping of tetracycline-sensitive point mutations and identification of two structural genes. J. Bacteriol. 153: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Botstein D., Roth J. R., 1980. Advanced Bacterial Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Eckert B., Beck C. F., 1989. Overproduction of transposon Tn10-encoded tetracycline resistance protein results in cell death and loss of membrane potential. J. Bacteriol. 171: 3557–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elde N. C., Child S. J., Eickbush M. T., Kitzman J. O., Rogers K. S., et al. , 2012. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 150: 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., 1994. Population dynamics of a Lac− strain of Escherichia coli during selection for lactose utilization. Genetics 138: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., 1997. Nonadaptive mutations occur on the F’ episome during adaptive mutation conditions in Escherichia coli. J. Bacteriol. 179: 1550–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Cairns J., 1992. Mechanisms of directed mutation. Genetics 131: 783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Trimarchi J. M., 1994. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science 265: 407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Trimarchi J. M., 1995a Adaptive reversion of an episomal frameshift mutation in Escherichia coli requires conjugal functions but not actual conjugation. Proc. Natl. Acad. Sci. USA 92: 5487–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Trimarchi J. M., 1995b Conjugation is not required for adaptive reversion of an episomal frameshift mutation in Escherichia coli. J. Bacteriol. 177: 6670–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galitski T., Roth J. R., 1995. Evidence that F plasmid transfer replication underlies apparent adaptive mutation. Science 268: 421–423. [DOI] [PubMed] [Google Scholar]
- Godoy V. G., Fox M. S., 2000. Transposon stability and a role for conjugational transfer in adaptive mutability. Proc. Natl. Acad. Sci. USA 97: 7393–7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G., 1990. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics 126: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings P. J., Bull H. J., Klump J. R., Rosenberg S. M., 2000. Adaptive amplification: an inducible chromosomal instability mechanism. Cell 103: 723–731. [DOI] [PubMed] [Google Scholar]
- Hendrickson H., Slechta E. S., Bergthorsson U., Andersson D. I., Roth J. R., 2002. Amplification-mutagenesis: evidence that “directed” adaptive mutation and general hypermutability result from growth with a selected gene amplification. Proc. Natl. Acad. Sci. USA 99: 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Bender J., Gottesman S., 1991. Uses of transposons with emphasis on Tn10. Methods Enzymol. 204: 139–180. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Kofoid E., Reams A. B., Andersson D. I., Roth J. R., 2006. Multiple pathways of selected gene amplification during adaptive mutation. Proc. Natl. Acad. Sci. USA 103: 17319–17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E., Kofoid E., Andersson D. I., Lu Y., Mellor J., et al. , 2010. The tandem inversion duplication in Salmonella enterica: selection drives unstable precursors to final mutation types. Genetics 185: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S., 1984. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene 28: 45–54. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbruck M., 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28: 491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y. L., Lin C. T., Liu L. F., 1999. Inversion/dimerization of plasmids mediated by inverted repeats. J. Mol. Biol. 285: 1485–1501. [DOI] [PubMed] [Google Scholar]
- Mann B. A., Slauch J. M., 1997. Transduction of low-copy number plasmids by bacteriophage P22. Genetics 146: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie G., Lee P., Lombardo M.-J., Hastings P., Rosenberg S., 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7: 571–579. [DOI] [PubMed] [Google Scholar]
- Moyed H. S., Nguyen T. T., Bertrand K. P., 1983. Multicopy Tn10 tet plasmids confer sensitivity to induction of tet gene expression. J. Bacteriol. 155: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näsvall J., Sun L., Roth J. R., Andersson D. I., 2012. Real-time evolution of new genes by innovation, amplification, and divergence. Science 338: 384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. N., Phan Q. G., Duong L. P., Bertrand K. P., Lenski R. E., 1989. Effects of carriage and expression of the Tn10 tetracycline-resistance operon on the fitness of Escherichia coli K12. Mol. Biol. Evol. 6: 213–225. [DOI] [PubMed] [Google Scholar]
- Peters J. E., Bartoszyk I. M., Dheer S., Benson S. A., 1996. Redundant homosexual F transfer facilitates selection-induced reversion of plasmid mutations. J. Bacteriol. 178: 3037–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Segraves R., Sudar D., Clark S., Poole I., et al. , 1998. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat. Genet. 20: 207–211. [DOI] [PubMed] [Google Scholar]
- Pranting M., Andersson D. I., 2011. Escape from growth restriction in small colony variants of Salmonella typhimurium by gene amplification and mutation. Mol. Microbiol. 79: 305–315. [DOI] [PubMed] [Google Scholar]
- Projan S. J., Carleton S., Novick R. P., 1983. Determination of plasmid copy number by fluorescence densitometry. Plasmid 9: 182–190. [DOI] [PubMed] [Google Scholar]
- Quinones-Soto S., Reams A. B., Roth J. R., 2012. Pathways of genetic adaptation: multistep origin of mutants under selection without induced mutagenesis in Salmonella enterica. Genetics 192: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicella J. P., Park P. U., Fox M. S., 1995. Adaptive mutation in Escherichia coli: a role for conjugation. Science 268: 418–420. [DOI] [PubMed] [Google Scholar]
- Reams A. B., Kofoid E., Kugelberg E., Roth J. R., 2012. Multiple pathways of duplication formation with and without recombination (RecA) in Salmonella enterica. Genetics 192: 397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reams A. B., Kofoid E., Savageau M., Roth J. R., 2010. Duplication frequency in a population of Salmonella enterica rapidly approaches steady state with or without recombination. Genetics 184: 1077–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosche W. A., Foster P. L., 1999. The role of transient hypermutators in adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 96: 6862–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. M., Longerich S., Gee P., Harris R. S., 1994. Adaptive mutation by deletions in small mononucleotide repeats. Science 265: 405–407. [DOI] [PubMed] [Google Scholar]
- Roth J. R., 2011. The joys and terrors of fast adaptation: new findings elucidate antibiotic resistance and natural selection. Mol. Microbiol. 79: 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Kofoid E., Roth F. P., Berg O. G., Seger J., et al. , 2003. Regulating general mutation rates: examination of the hypermutable state model for Cairnsian adaptive mutation. Genetics 163: 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack A., Thornton P. C., Magner D. B., Rosenberg S. M., Hastings P. J., 2006. On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet. 2: e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slechta E. S., Bunny K. L., Kugelberg E., Kofoid E., Andersson D. I., et al. , 2003. Adaptive mutation: general mutagenesis is not a programmed response to stress but results from rare coamplification of dinB with lac. Proc. Natl. Acad. Sci. USA 100: 12847–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., 1988. News and views: a unicorn in the garden. Nature 355: 112–113. [DOI] [PubMed] [Google Scholar]
- Stumpf J. D., Poteete A. R., Foster P. L., 2007. Amplification of lac cannot account for adaptive mutation to Lac+ in Escherichia coli. J. Bacteriol. 189: 2291–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Berg O. G., Roth J. R., Andersson D. I., 2009. Contribution of gene amplification to evolution of increased antibiotic resistance in Salmonella typhimurium. Genetics 182: 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkelson J., Harris R. S., Lombardo M.-J., Nagendran J., Thulin C., et al. , 1997. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J. 16: 3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret J. F., Bravo A., Alonso J. C., 1991. Recombination-dependent concatemeric plasmid replication. Microbiol. Rev. 55: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N., 1984. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene 32: 369–379. [DOI] [PubMed] [Google Scholar]