Abstract
Thyroid hormones (TH) cross the plasma membrane with the help of transporter proteins. As charged amino acid derivatives, TH cannot simply diffuse across a lipid bilayer membrane, despite their notorious hydrophobicity. The identification of monocarboxylate transporter 8 (MCT8, SLC16A2) as a specific and very active TH transporter paved the way to the finding that mutations in the MCT8 gene cause a syndrome of psychomotor retardation in humans. The purpose of this review is to introduce the current model of transmembrane transport and highlight the diversity of TH transmembrane transporters. The interactions of TH with plasma transfer proteins, T3 receptors, and deiodinase are summarized. It is shown that proteins may bind TH owing to their hydrophobic character in hydrophobic cavities and/or by specific polar interaction with the phenolic hydroxyl, the aminopropionic acid moiety, and by weak polar interactions with the iodine atoms. These findings are compared with our understanding of how TH transporters interact with substrate. The presumed effects of mutations in MCT8 on protein folding and transport function are explained in light of the available homology model.
Key Words: Thyroid hormone, Transporter, Mechanism, Rocker switch, Thyroid hormone-binding globulin, Albumin, Transfer protein transthyretin, Deiodinase, T3 receptor, Mutation, Membrane insertion
Discovery of Thyroid Hormone Transmembrane Transporters
The concept of plasma membrane transport of thyroid hormones (TH) was first approached in the 1970s by Govind Rao and Heinz Breuer in Germany [1] and Georg Hennemann in the Netherlands [2], followed by Jack Robbins, Jacques Blondeau and several others. The unspecific association of radiolabeled TH with cells always represented an issue, but several laboratories showed saturable and stereo-specific uptake indicative of plasma membrane transporter action. Initially only few transporters, and none of them TH transporters, were cloned (e.g. LacY, the Escherichia coli lactose permease). It constantly remained an issue in the field whether TH uptake into cells was energy dependent, could be competed with bile acids and amino acids, or had Km values in the nanomole or micromole range. From the 1990s, expression cloning of transporters led to the identification of several transporter gene families and subsequent screening of cloned transporters for TH uptake led to the identification of several additional TH transporters [3]. The identification of monocarboxylate transporter 8 (MCT8, SLC16A2) as a specific and very active TH transporter [4] paved the way to the finding that mutations in the MCT8 gene cause a syndrome of psychomotor retardation in humans [5,6]. Only then was it realized that the Allan-Herndon-Dudley syndrome, an X-linked mental retardation syndrome first described in 1944 [7], is actually caused by loss-of-function mutations in MCT8 [8]. Eventually, it took these four publications to convince the field that TH require plasma membrane transport proteins. From then the field grew rapidly and our appreciation of the roles of TH transporters in TH physiology has progressed significantly within only one decade.
Physiological Effects of TH Transporter Deficiency
Patients carrying mutations in MCT8 exhibit high T3, low/normal T4, and normal TSH levels [9,10]. Mice deficient in Mct8 display the same hormone constellation and represent a good model to study the role of Mct8 in the regulation of the TH axis [11,12]. However, these mice exhibit only a mild neurological phenotype very much unlike the corresponding patients [11,12,13]. The expression pattern of Mct8 in neurons, pituitary, thyroid, liver, and kidney may explain why Mct8/MCT8 is important in mice and humans, but did not offer an explanation for the phenotypic differences between mice and humans [13,14,15,16,17]. One possible explanation could be the expression of the L-type amino acid transporter 2 (Lat2, SLC7A8) in developing murine, but not human, neurons [13,18,19]. Another explanation could be that mouse brain microcapillary endothelial cells express the T4-specific transporter Oatp14 (Slco1c1), while human cells do not but depend on MCT8 instead. Accordingly, mice deficient in Mct8 and Slco1c1 suffer from cerebral hypothyroidism and exhibit a neurological phenotype with retarded maturation of a subset of GABAergic interneurons [20]. For a more in-depth discussion of TH transporter physiology, the reader is referred to several recently published reviews [21,22,23]. In this review we highlight the diversity of TH transmembrane transporters and how TH presumably interact with proteins.
Structure and Transport Mechanisms of Transmembrane Transporters
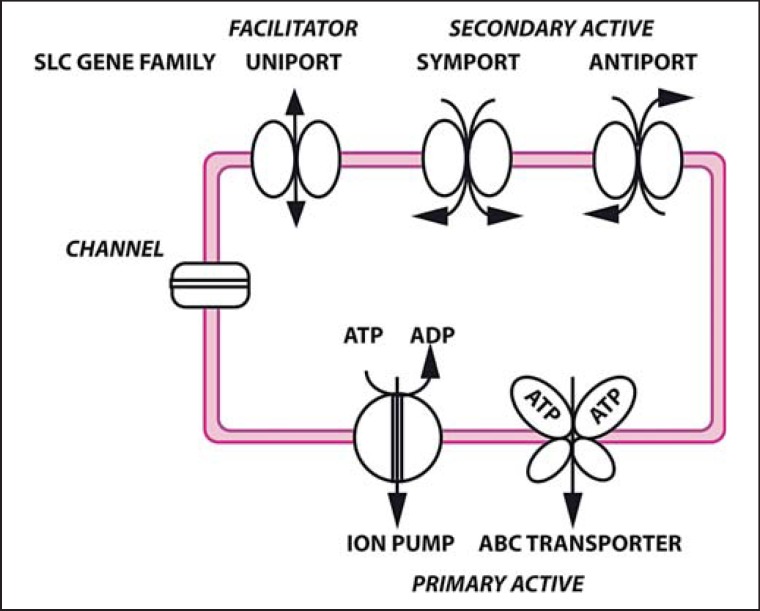
Most plasma membrane transporter proteins in humans are members of the ATP-binding cassette (ABC) and solute carrier (SLC) gene families that together comprise about 400 genes in humans. Transporters are also classified according to their energy source: primary active transporters usually use ATP to take up or extrude solutes from the cells1 and multidrug resistance proteins are probably the best known among them in eukaryotes. Secondary active transporters harness an electrochemical gradient, e.g. Na+, H+, or glutamate, to move solutes across the membrane in a symport or antiport mechanism. Finally, uniporters facilitate diffusion along the concentration gradient of the substrate and are therefore called facilitators (fig. 1). All known thyroid hormone transporter proteins are members of the SLC gene family.
Fig. 1.
Summary of transmembrane transporter classes. Channels are pore-forming proteins which open a gate in a ligand- or electric potential-dependent manner. Transporters are proteins which change their conformation and thereby transport a ligand from one side of the membrane to the other. Human ATP-dependent transporters work against a gradient as exporters (primary active transport). Secondary active transporters harness the electrochemical gradient of one solute to drive the transport of another solute in a symport or antiport mechanism. Uniporters facilitate diffusion of a solute along its gradient, but may exhibit different affinities for the solute depending on the direction of transport.
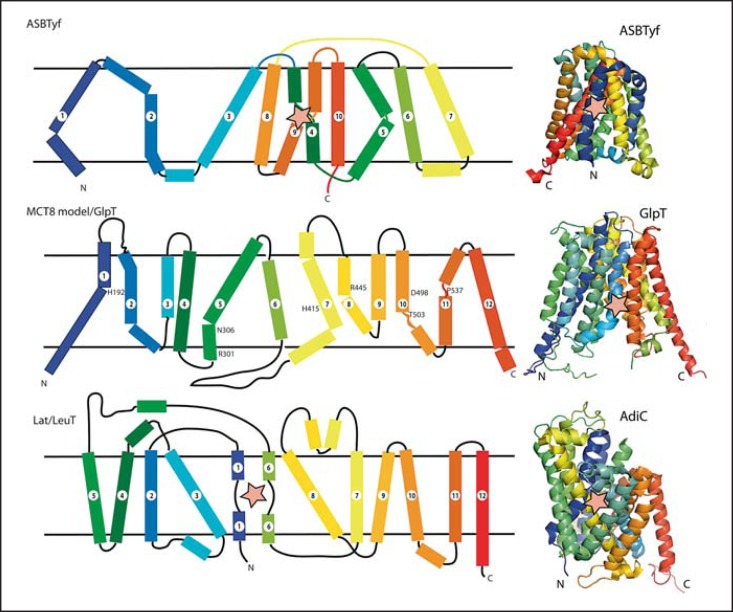
Transporters can also be classified according to their polypeptide chain fold patterns. Interestingly, known TH transporters fall into different classes: the NhaA fold (structure solved for ASBTYf), the major facilitator superfamily (MFS) fold (exemplified by GlpT), and the LeuT fold (exemplified by the AdiC structure). The apical sodium bile acid transporter (ASBT) family folds into two inverted segments of 5 transmembrane helices each. Both segments contain one helix (No. 4 and 9) which is unwound in the center where both interact and form the substrate-binding site (fig. 2). Transporters of the MFS class contain 12 transmembrane helices arranged as two bundles of each 6 helices that can exert a rotatory movement against each other. During this movement, the proteins pass sequentially through conformations that allow alternating access from both sides of the membranes to one central binding site. This transport mechanism is also known as the ‘rocker switch mechanism’. The LeuT fold is well studied, because sodium-dependent neurotransmitter transporters fall into this class. These transporters are arranged as two modules of 5 transmembrane helices, but this time the first helix in each module is discontinuous and participates in substrate binding. Energy coupling in secondary active transporters is a field of active discussion [24], but it appears as if Na+ ions and protons are harnessed to neutralize anionic side chains or substrates to allow closure of transporters around their substrates and initiation of transport (fig. 2).
Fig. 2.
Topology diagrams along with experimental structures of transmembrane transporters. The ASBT family has ten transmembrane helices (TMH). Substrate (star) is bound between two interrupted helices, TMH4 and TMH9. MFS proteins are arranged as two bundles of six consecutive TMH each, which form a central substrate-binding site at the interface of the two bundles (amino acids in MCT8 participating in transport are indicated). LeuT fold transporters are arranged as two 5-helix inverted repeats followed by two TMH which do not participate in pseudo-symmetry. The substrate-binding site is formed by two interrupted helices, TMH1 and TMH6. The helices are colored from violet (helix 1) to red (helix 12).
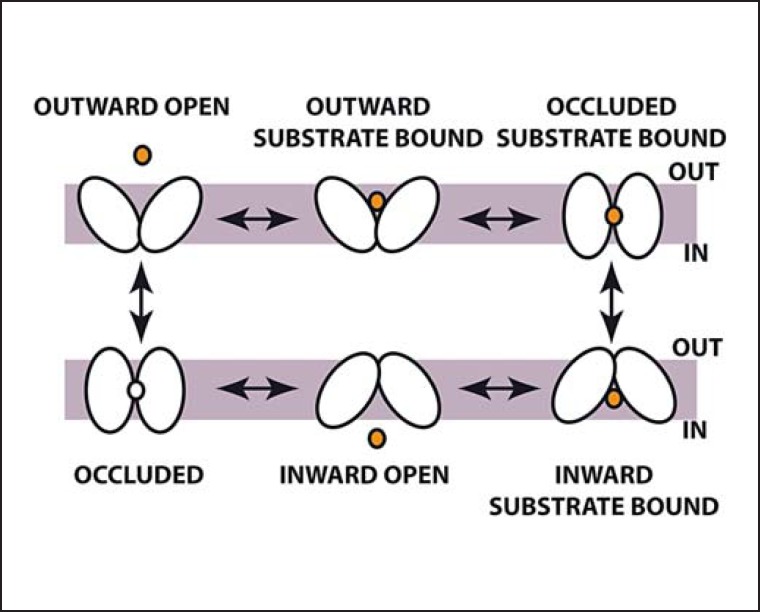
The Rocker Switch Model
The prototypic transmembrane transporter protein of the MFS class is lactose permease (LacY) from E. coli. This protein has been studied for several decades and a plethora of functional data was finally interpreted in light of the crystal structure which was published in 2003 [25]. The structure of the glycerol-phosphate transporter GlpT was published in the same year, and from the first two MFS protein structures published it became evident that these adopt similar structures [26]. In the meantime, six prokaryotic (and recently one eukaryotic [27]) MFS proteins have been crystallized in – luckily – different conformations each. Assuming that MFS proteins in principle function alike, a sequence of six conformations can be assembled through which MFS proteins likely pass while transporting substrate [28]. In those structures in which substrate is present, one centrally located binding site is recognized involving amino acids that are known from biochemical experiments to participate in transport. As mentioned above, two bundles comprising 6 transmembrane helices move against each other during transport (fig. 3).
Fig. 3.
Rocker switch model of transmembrane transport. Transporters pass through a sequence of conformations, which allow solutes alternate access to a central binding site from the two faces of the membrane. Shown is the example of a uniporter, but secondary active transport is easily incorporated in this model.
However, from transporters crystallized in more than one conformation (e.g. FucP), it is also evident that helices may change their lengths during structural transitions or may be kinked in one but straight in another conformation. These joints may often be apparent from the primary structure by prolines and glycines in the middle of helical segments and mislead programs designed to predict transmembrane helices. The lack of a set of structures of the same protein in all different conformations makes it difficult to predict exactly how the transporters perform their movements, what the source of energy for the movements are, and how the substrates or co-substrates help induce the conformational changes [28].
Modes of TH Binding by Proteins
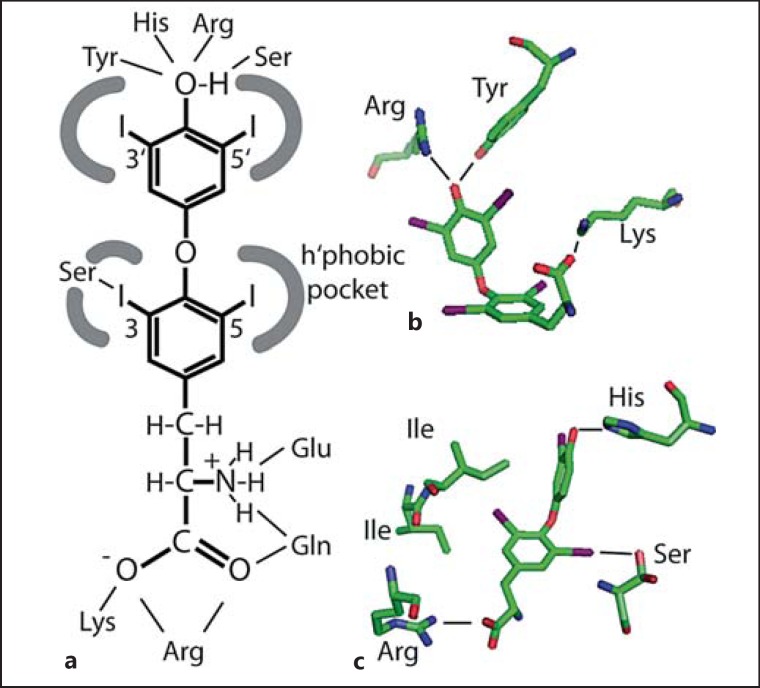
Several different types of protein bind TH, plasma proteins, receptors, enzymes, and transporters. The first studies how TH bind to protein have focused on the plasma transfer protein transthyretin (TTR). Crystal structures for human and rat TTR have been solved [29,30,31] in the presence of several different ligands. In short, two binding sites are present at two ends of a central hydrophobic channel that is formed by the interfaces between two TTR dimers. While the peptide main chains exhibit twofold symmetry, hormones are not bound symmetrically, because T4 adopts an almost skewed conformation which is not symmetrical. Each binding site contains several largely hydrophobic pockets formed by a main chain and side chains of β-sheets which can each accommodate one iodine atom from TH. The innermost binding pocket also allows nucleophiles (e.g. Ser-OH) to form polar interactions specifically with iodine at a similar energetic gain as in a weak H-bond. The polar interactions of the amino and carboxyl groups with Lys and Glu residues at the channel entrance are probably of less importance to hormone binding [31]. The remainder of the binding site is largely a hydrophobic channel that accommodates the two aromatic rings (fig. 4a).
Fig. 4.
Schematic representation how TH interact with proteins. a The iodothyronine molecule is largely hydrophobic, but the 4′- and aminopropionic acid moieties are hydrophilic. Hydrophobic interactions account for most of the TH binding, while interactions with the 4′-hydroxyl and sometimes the aminopropionic acid moiety confer additional specificity. b Polar contacts in the binding pocket for T4 in HSA. The hormone is in a cisoid conformation, i.e. phenolic ring and aminopropionic acid are on the same side of the tyrosyl ring. c Polar and hydrophobic contacts in the binding pocket for T3 in TR-β. The ligand is in the extended conformation. Note the conserved polar contact of Ser with iodine.
Human serum albumin (HSA) was also crystallized in complex with T4[32]. It was found that depending on the presence of fatty acids, 4-5 T4 molecules can bind to one HSA molecule and the binding sites were denoted Tr1-Tr5. Tr1 is surrounded by five α-helices and known to mediate drug or fatty acid binding, too. T4 is wedged into a hydrophobic binding pocket, where a Tyr and an Arg coordinate the 4′-hydroxyl group. Only the aminopropionic acid moiety is exposed to solvent and interacts with K199 and R218 (fig. 4b). Interestingly, the latter interaction appears strained, and mutations in R218 [33,34], which lead to smaller side chains, relax the protein and lead to familial dysalbuminemic hyperthyroxinemia, a condition with abnormally high plasma T4 levels due to increased binding to HSA [35,36]. Notably, iodine atoms make contacts with main chain and side chain nucleophiles as observed in TTR. Tr2 also binds fatty acids in the absence of T4, but is remarkable in that it allows only burial of the phenolic ring in a largely hydrophobic cleft, where a Ser and Tyr coordinate the 4′-phenolic group. The other ring is halfway solvent exposed and the aminopropionic acid moiety completely exposed to solvent. The latter binding site as well as Tr3 and Tr4 may only be available for T4 binding in the absence of fatty acids. Integrating the effects of fatty acids and familial dysalbuminemic hyperthyroxinemia mutations on T4 binding, the authors suggested that only Tr1 represents a physiological T4-binding site [32].
More recently the structure of TH-binding globulin in complex with T4 was reported [37]. The T4-binding site is found on the surface of an antiparallel β-sheet and framed by two α-helices. The TH is mainly held by hydrophobic interactions, including binding pockets for iodine atoms. The 4′-phenolic group is coordinated to a lysine amine group which is further stabilized by a serine. The aminopropionate group is weakly coordinated by two superficial interactions: an H-bond between Asn and the amino group and a salt bridge between the carboxylate and an Arg guanido group.
Crystal structures have also been determined for thyroid hormone receptors (TR)-α and −β in complex with ligands [38]. In both receptors, T3 is almost completely covered in a hydrophobic pocket. The aminopropionic acid moiety is bound in the core of the protein by a conserved Arg residue and the amino group makes a main chain contact with a nitrogen. The 4′-phenolic hydroxyl is interacting with a His from helix 12, which is ordered through this interaction to promote binding of co-activator [38]. With the exception of a conserved Ser which comes close to an inner ring iodine, the His-Arg clamp provides the only polar interactions by side chain residues in TR (fig. 4c). Their relevance is underlined by mutations in patients with resistance to TH, which hamper co-activator recruitment, or in somatic mutations in cancer cell lines. Recently, a second, non-canonical TH-binding site has been observed in TR [39]. The hydrophobic patch the TH binds to appears quite shallow and forms only one pocket to accommodate an inner ring iodine. Interestingly, this is in close contact with the hydroxyl from a Thr. The aminopropionic acid moiety is tightly locked into an H-bond network comprising of an Arg and Glu coordinating the carboxylate and amino groups, respectively, and Gln which coordinates both functional groups from one side [39].
Deiodinases are enzymes that mediate the removal of iodide from TH thereby activating (T4 to T3) or inactivating TH (T4 to rT3, T3 to T2) [40]. In the recently solved crystal structure of murine deiodinase 3 [41], a substrate-binding cleft has been observed that is compatible with substrate pinching between His202 and Arg275, with Glu259 possibly interacting with the amino group. Although no enzyme-substrate complex structure is available at the moment, mutagenesis of the conserved His, Glu, and Arg residues in different deiodinases has reduced their activities consistent with a possible substrate-binding site between His and Arg. More precise understanding of TH binding in deiodinases has to await a high-resolution enzyme-substrate structure.
Taken together, TH are bound in hydrophobic tunnels or grooves where one to three iodine atoms are snugly fit into hydrophobic pockets, which sometimes involve also nucleophilic hydroxyl groups or main chain atoms. The polar 4′-hydroxyl or aminopropionic acid moieties may remain exposed to solvent in some instances, but are in other instances involved in H-bond networks often involving Arg/Lys, Glu, His, Tyr, and Asn or Gln. In TR and possibly in MCT8, TH is pinched between a His and an Arg residue [42]. In none of the presented structures were polar interactions observed with the phenol ether oxygen (fig. 4).
TH Transporters
Early studies by Rao et al. [1] and Krenning et al. [2] have determined a high-affinity/low-capacity and a low-affinity/high-capacity transporter in rat primary hepatocytes with Km values of 52 and 22 nM and 1.4 and 1.8 µM, respectively. In hindsight, these results agree excellently with today's knowledge being compatible with the high-affinity transporter being the sodium-taurocholate transporting polypeptide (NTCP, SLC10A1), a sodium-dependent transporter expressed only in liver, and Mct8, a high-capacity transporter with a Km of 1-5 µM [4,43]. The energy dependence of the high-affinity transporter which was early observed agrees with the Na+ dependence of NTCP [2].
TH Transporters of the Bile Acid Transporter Gene Family
In a screening approach, the group of Theo Visser [44] characterized NTCP expressed in Xenopus oocytes as a TH transporter. NTCP is an integral membrane protein of the ASBT family and takes up taurocholic acid in a symport mechanism with sodium. NTCP is exclusively expressed in hepatocytes where it participates in enterohepatic bile acid circulation. Rat NTCP stimulated the sodium-dependent uptake of T4, T3, rT3, 3,3′-T2, and respective 4′-sulfo-conjugates into oocytes [44]. Recently, the crystal structure of ASBTYf, a bacterial homolog of NCTP, has been solved [45]. In the proposed mechanism, binding of Na+ ions prepares the binding site for the conjugated cholic acid between the two interrupted helices 4 and 9 (fig. 3).
TH Transporters of the Amino Acid Transporter Family
The system L of amino acid transporters was found to be inhibited by TH in astrocytes [46]. This system is composed of a transporter of the MFS family, SLC7A5 (LAT-1) or SLC7A9 (LAT-2) and a heavy chain subunit that serves as an ancillary/escort protein needed for plasma membrane expression, SLC3A1 or SLC3A2. The Visser group also followed up on earlier work from Blondeau's laboratory and others that characterized TH uptake into neurons, astrocytes, and other cell types as sensitive to competition with aromatic amino acids and the LAT inhibitor BCH [47]. Lat1 and to a lesser extent Lat2 mediated T3 and T4 uptake into Xenopus oocytes [47]. Genetic inactivation of Lat2 indeed reduces TH uptake into cultured astrocytes [18]. Thus, Lat2 may play a role in brain TH metabolism. Lat1 is widely expressed, also in brain microvascular endothelial cells, but its expression is apparently essential [48]. To define the physiological role of Lat1, we have to await a cell type-specific Lat1-deficient mouse model in the brain. The LAT transporters belong to the LeuT family of transporters. Recently, a homology model of Lat1 has been presented. The model is based on the crystal structure of the arginine:agmatine antiporter AdiC from E. coli [49]. Based on a virtual ligand screen with the homology model, the authors predicted correctly that Lat1 can transport iodotyrosines [49]. In the AdiC structure, Arg is bound by main chain atoms from the transporter interacting specifically with the L-amino acid moiety and explaining the specificity for amino acids [49]. A hydrophobic pocket close by accommodates the amino acid/substrate side chain and leaves space for TH. Interestingly, in a similar homology model of LAT2, an Asn-to-Ser mutation possibly created a novel polar interaction with an iodine atom within the hydrophobic pocket. This mutation enhanced 3,3′-T2 transport activity [50].
TH Transporters of the OATP/SLCO Gene Family
Organic anion-transporting polypeptides are a large gene family involved in the transport of many metabolites and xenobiotics. These proteins usually have a broad substrate specificity. Oatp1 was cloned from rat as an organic anion transporter [51] and was later shown to mediate cellular uptake of TH [44]. Oatp1/Slco1a1 transported T4, T3, rT3, 3,3′-T2, and respective 4′-sulfo-conjugates into Xenopus oocytes [44]. However, this gene has no ortholog in the human genome, and the close homolog, OATP-A/SLCO1A2 is a bile acid, but not TH, transporter. Oatp2 and Oatp3 have been cloned from rat and shown to transport TH, but are not found in the human genome [52]. OATP-E/SLCCO4A1 was cloned from human brain and transports T3 at Km of 6.5 µM and T4 at Km of 8 µM[53]. This transporter is also present in rodents [53]. SLCO4C1 was cloned from human kidney and transports T3 at a Km of 1.9 µM[54].
Oatp14/Slco1c1 was identified as a T4-specific TH transporter predominantly expressed in brain with a Km of 90 nM [55,56]. It is expressed in brain microvascular endothelium and is involved in brain T4 uptake [57]. SLCO1C1 belongs to the MFS family of 12-transmembrane helix transporters. Accordingly, a homology model of rat Oatp14 based on the crystal structures of three MFS proteins (LacY, GlpT, EmrD) has been presented [58]. While the transporter likely resembles one of the homology models, significant uncertainty remained, and no specific interactions between protein and its substrate T4 have been identified [58]. It thus remains unclear how the protein achieves its remarkable substrate specificity of accepting T4 and rT3, but not T3.
TH Transporters of the MCT Family
MCT have been named according to the substrates of MCT-1-4, pyruvate and lactate [59]. MCT1 was initially cloned as a point mutant that was able to transport mevalonate and later found to transport pyruvate [60]. Several MCT require escort proteins of the immunoglobulin superfamily. The two MCT able to transport TH, MCT8/SLC16A2 and MCT10/SLC16A10, do not need ancillary proteins to reach the plasma membrane [61], but are known to form multimers [62]. MCT10 had been previously characterized as the T-type amino acid transporter, TAT1, which transports aromatic amino acids. Interestingly, MCT10 transports T3, but not T4[63]. MCT8 is highly homologous to MCT10 and able to transport T4, but inactive as an amino acid transporter [64]. As mentioned above, human and rat MCT8 is a specific TH transporter accepting T4, T3, rT3 and 3,3′-T2 [4,43]. A detailed study of possible substrates revealed that MCT8, is specific for L-enantiomers of TH, requires both the amino and the carboxy groups, and at least one iodine atom in each iodothyronine ring [43]. Several compounds that compete with TH binding in serum proteins or deiodinases do not affect MCT8, underlining its remarkable specificity [43]. Desipramine, an inhibitor of LeuT, also inhibits MCT8 and MCT10, despite the fact that MCT belong to the MFS, while LeuT belongs to the LeuT family of transporters [65]. Moreover, several tyrosine kinase inhibitors interfere with the TH axis by non-competitive inhibition of MCT8 [66,67].
We hypothesized that MCT8 may interact with its amino acid substrates via charged amino acids which should be located within transmembrane regions to interact with the predicted central substrate-binding site in MFS transporters. Hydropathy plots are of limited accuracy to predict transmembrane helices in transporters which change helix lengths upon transport movements or introduce sharp kinks in helices within the lipid phase. We therefore generated a homology model of MCT8 using E. coli GlpT as a template [43]. Two amino acids, Arg445 and Asp498, caught our attention owing to their predicted location within the membrane plane. Mutation of each amino acid to Ala completely inactivated the enzyme towards TH and homologs without carboxy or amino groups, respectively. Rather, the homology model predicted a salt bridge between both amino acids in the inward-open conformation [43]. The relevance of this pair of charged amino acids was recently supported by independent experiments which also showed that a charge reversal mutant, Arg445Asp/Asp498Arg, regained activity [68]. A salt bridge also stabilizes a conformation in MCT1, but in this protein the interaction occurs between amino acids within one helix, while in MCT8 the interaction is between two transmembrane helices [43,69]. Based on the finding of a TH-binding His-Arg pair in TR and Dio3 [38,41], we tested whether His-Arg pairs spaced by about 16 Å could be found in the MCT8 homology model. His192 occurs in transmembrane helix 2 at the same position as the substrate-binding Asp45 in GlpT [42]. Targeted mutations probing position 192 in MCT8 revealed effects on transport activity [64]. The location of His192, however, suggests substrate interaction in the outward-open conformation, which was not modeled. His415 and Arg301 constitute another His-Arg clamp, which, according to our model, satisfies the geometrical constraints in the modeled inward-open conformation as seen in TR [64]. Effects of mutations on TH transport are compatible with a role of both residues in MCT8-mediated substrate transport. Interestingly, His415 occupies the same position in MCT8 as Phe in MCT1, which, when mutated to Cys, makes MCT1 a mevalonate transporter [60]. In a completely independent approach, chemical modification of MCT8 histidines with diethylpyrocarbamate (DEPC) supported a role of His192 in TH transport [70]. His415 may be buried too deeply to be accessible for extracellular DEPC. Similarly, chemical modification of cysteines suggested Cys481 and Cys497 close to the transport channel [71]. Again, since we only have a homology model of the inward-open conformation of MCT8, these findings with a non-cell permeant reagent are hard to interpret. A model in the outward-open conformation is therefore needed.
Based on the high sequence identity between MCT8 and MCT10, we hypothesized that those sequence differences that are located along the substrate translocation channel should be responsible for the different substrate specificity between MCT8 and MCT10, namely the transport of T4 by MCT8, but not MCT10. We showed recently that several amino acid exchanges suffice to turn MCT10 into a T4 transporter [72]. Our homology models of MCT8 and 10 may thus be of suitable accuracy to further explore possible protein-substrate interactions.
Molecular Pathology of Pathogenic MCT8 Mutations
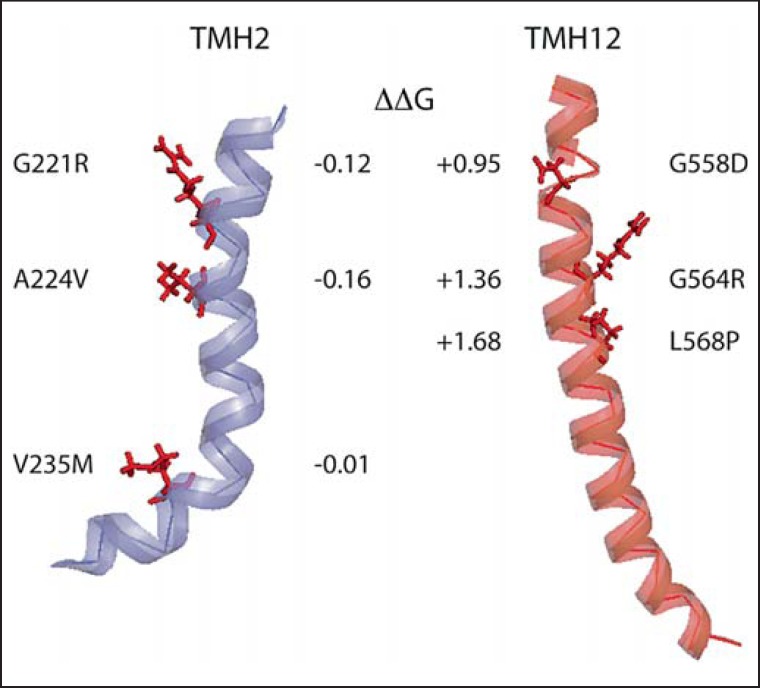
Genotype-phenotype studies on MCT8 mutations have been performed several times [73,74,75,76,77]. With few exceptions, all described mutations in MCT8 severely impaired TH transport and led to severe phenotypes. To understand the molecular pathology, we aimed to categorize pathogenic mutations in MCT8 [76]. Mutations that delete parts of the gene, affect promoter or splice regions, or induce frameshifts are not informative for our purpose to understand the membrane protein. Likewise, effects by premature termination are likely trivial. This leaves us with missense mutations and deletions and insertions of single amino acids, on which we have focused our study. As a polytopic membrane protein with 12 transmembrane domains, MCT8 is an inherently complicated protein to synthesize, and mutations that change the vital interactions with the Sec61 translocon or impair membrane insertion likely lead to misfolding and target the protein for degradation. Indeed, several missense mutants of MCT8 were found unstable or not translocated to the plasma membrane [76,78]. To illustrate this approach, we have calculated the free energy change of water-to-membrane transitions against wild-type MCT8 for three mutants affecting transmembrane helix 2 (which is close to the substrate-binding site) and compared these to three mutants in transmembrane helix 12 (fig. 5). Calculations were based on the MPEx Translocon TM Analysis tool [79] based on quantitative analysis of the contribution of amino acids to membrane insertion [80]. The free energy change of these mutations in TMH2 was very small, likely not affecting membrane insertion. In contrast, three mutations in TMH12 appreciably increase the free energy of the protein, likely affecting membrane insertion (fig. 5).
Fig. 5.
Pathological mutations may change transmembrane helix (TMH) insertion into the membrane. TMH2 and TMH12 from the MCT8 homology model are depicted with each three missense mutations which inactivate the protein. Mutations in TMH2 affect TH transport, but are translocated to the plasma membrane, indicative of near-normal folding. Mutations in TMH12 disrupt plasma membrane translocation. Compared to wild-type MCT8, all three mutations lead to unfavorable free energy changes reducing the driving force for correct membrane insertion.
Mutations which introduce or delete Gly and Pro residues [42] likely impair protein folding, because Gly allow and Pro restrict flexibility. Transmembrane helices also often have Gly residues at positions where they approach each other closely. Clearly, insertion of any side chain will act like a wedge pushing the helices apart. Other mutants may lack vital intramolecular interactions and therefore be unstable. Such proteins are targets for pharmacological chaperones.
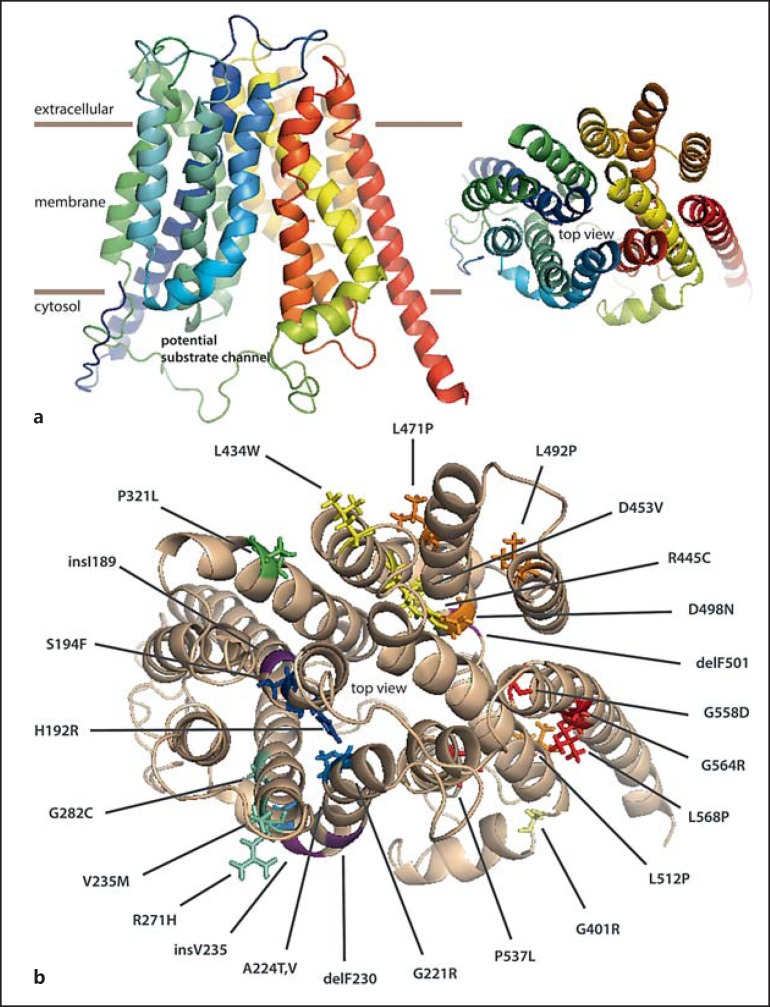
Finally, missense mutations that affect interactions with substrate may be particularly revealing [43]. For example, His192Arg, Arg445Cys, and Asp498Asn mutations likely affect substrate interactions, if we consider above results by targeted mutations and chemical modification (fig. 6). Ala224Val, Ser194Phe, and Val235Met are also assumed to be located towards the substrate-binding site. Moreover, insV235 and delPhe230 change the helix register of helix 2, which on one side forms the substrate channel and at the same time interacts with helix 11 from the other 6-helix bundle.
Fig. 6.
Homology model of MCT8. a The model is based on the structure of the bacterial glycerol-3-phosphate transporter GlpT. TMH1 is violet and TMH12 is red. In the top view, i.e. from the extracellular side, a central channel is apparent which is closed on the extracellular side. b Missense and small indel mutations mapped on the homology model of MCT8. P321L is located at the interface of the two 6-helix bundles and G401R marks a sharp kink between the horizontal helix and TMH7.
Dynamics of the protein may also be affected by pathogenic mutations. According to our model, delPhe501 (and Asp498) is located exactly in the part of helix 10 which in other transporters undergoes a major rearrangement [28], i.e. a sharp kink is formed in another conformation which helps close the cytoplasmic side of the pore.
Clearly, we do not have an experimental structure of MCT8, and only recently the first eukaryotic MFS transporter, GLUT1, has been crystallized [27]. Therefore, we can interpret biochemical data only on the basis of (homology) models. Available data suggest that the homology model based on GlpT is of some utility for the interpretation of biochemical experiments involving MCT8.
Conclusion
Nature has been creative in designing TH interaction sites in proteins. An analysis of plasma TH-binding proteins and TH receptors (for which experimental TH:protein complex structures are available), deiodinase (for which an experimental structure and biochemical data point to a substrate-binding site), and TH transmembrane transporters (for which only homology models and biochemical data on possible substrate interaction sites exist) reveals that proteins interact with all features of TH except the ether bond (i.e. hydrophobic rings, iodine atoms, L-aminopropionic acid moiety). However, no single ‘TH-binding domain’ exists. Evolution converged on TH binding from different starting points in fundamentally different protein families. While binding proteins, receptors, and enzymes may be optimized for tight substrate/ligand binding, transporters have to achieve specific interactions, while avoiding tight binding of the transport substrate, as binding would stall the transport process. Finding out how this is achieved is an import goal in MCT8 research. Such data will be of importance for the rational design of specific activators or inactivators of MCT8 which may be useful for the treatment of thyroid disease.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft for their generous support (DFG Schw914/3-1; Sch914/4-1) as well as the Graduate College GK1208. Dorothea Bayer is supported by the University of Technology, Sydney, and the Sherman Foundation.
References
- 1.Rao GS, Eckel J, Rao ML, Breuer H. Uptake of thyroid hormone by isolated rat liver cells. Biochem Biophys Res Commun. 1976;73:98–104. doi: 10.1016/0006-291x(76)90502-7. [DOI] [PubMed] [Google Scholar]
- 2.Krenning EP, Docter R, Bernard HF, Visser TJ, Hennemann G. Active transport of triiodothyronine (T3) into isolated rat liver cells. FEBS Lett. 1978;91:113–116. doi: 10.1016/0014-5793(78)80029-5. [DOI] [PubMed] [Google Scholar]
- 3.Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451–476. doi: 10.1210/edrv.22.4.0435. [DOI] [PubMed] [Google Scholar]
- 4.Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 5.Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 6.Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allan W, Herndon CN, Dudley FC. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic. 1944;48:325–334. [Google Scholar]
- 8.Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 gene. Am J Hum Genet. 2005;77:41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu J, Refetoff S, Dumitrescu AM. Inherited defects of thyroid hormone-cell-membrane transport: review of recent findings. Curr Opin Endocrinol Diabetes Obes. 2013;20:434–440. doi: 10.1097/01.med.0000432531.03233.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friesema EC, Jansen J, Heuer H, Trajkovic M, Bauer K, Visser TJ. Mechanisms of disease: psychomotor retardation and high T3 levels caused by mutations in monocarboxylate transporter 8. Nat Clin Pract Endocrinol Metab. 2006;2:512–523. doi: 10.1038/ncpendmet0262. [DOI] [PubMed] [Google Scholar]
- 11.Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (MCT) 8-deficient mice. Endocrinology. 2006;147:4036–4043. doi: 10.1210/en.2006-0390. [DOI] [PubMed] [Google Scholar]
- 12.Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirth EK, Roth S, Blechschmidt C, Hölter SM, Becker L, Racz I, Zimmer A, Klopstock T, Gailus-Durner V, Fuchs H, Wurst W, Naumann T, Bräuer A, de Angelis MH, Köhrle J, Grüters A, Schweizer U. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in MCT8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci. 2009;29:9439–9449. doi: 10.1523/JNEUROSCI.6055-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trajkovic-Arsic M, Visser TJ, Darras VM, Friesema EC, Schlott B, Mittag J, Bauer K, Heuer H. Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology. 2010;151:802–809. doi: 10.1210/en.2009-1053. [DOI] [PubMed] [Google Scholar]
- 15.Trajkovic-Arsic M, Muller J, Darras VM, Groba C, Lee S, Weih D, Bauer K, Visser TJ, Heuer H. Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology. 2010;151:5053–5062. doi: 10.1210/en.2010-0593. [DOI] [PubMed] [Google Scholar]
- 16.Heuer H, Maier MK, Iden S, Mittag J, Friesema EC, Visser TJ, Bauer K. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005;146:1701–1706. doi: 10.1210/en.2004-1179. [DOI] [PubMed] [Google Scholar]
- 17.Wirth EK, Sheu SY, Chiu-Ugalde J, Sapin R, Klein MO, Mossbrugger I, Quintanilla-Martinez L, de Angelis MH, Krude H, Riebel T, Rothe K, Köhrle J, Schmid KW, Schweizer U, Grüters A. Monocarboxylate transporter 8 deficiency: altered thyroid morphology and persistent high triiodothyronine/thyroxine ratio after thyroidectomy. Eur J Endocrinol. 2011;165:555–561. doi: 10.1530/EJE-11-0369. [DOI] [PubMed] [Google Scholar]
- 18.Braun D, Kinne A, Brauer AU, Sapin R, Klein MO, Köhrle J, Wirth EK, Schweizer U. Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia. 2011;59:463–471. doi: 10.1002/glia.21116. [DOI] [PubMed] [Google Scholar]
- 19.Braun D, Wirth EK, Wohlgemuth F, Reix N, Klein MO, Grüters A, Köhrle J, Schweizer U. Aminoaciduria, but normal thyroid hormone levels and signalling, in mice lacking the amino acid and thyroid hormone transporter Slc7a8. Biochem J. 2011;439:249–255. doi: 10.1042/BJ20110759. [DOI] [PubMed] [Google Scholar]
- 20.Mayerl S, Muller J, Bauer R, Richert S, Kassmann CM, Darras VM, Buder K, Boelen A, Visser TJ, Heuer H. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124:1987–1999. doi: 10.1172/JCI70324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuer H, Visser TJ. The pathophysiological consequences of thyroid hormone transporter deficiencies: insights from mouse models. Biochim Biophys Acta. 2013;1830:3974–3978. doi: 10.1016/j.bbagen.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta. 2013;1830:3987–4003. doi: 10.1016/j.bbagen.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweizer U, Köhrle J. Function of thyroid hormone transporters in the central nervous system. Biochim Biophys Acta. 2013;1830:3965–3973. doi: 10.1016/j.bbagen.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y. Common folds and transport mechanisms of secondary active transporters. Annu Rev Biophys. 2013;42:51–72. doi: 10.1146/annurev-biophys-083012-130429. [DOI] [PubMed] [Google Scholar]
- 25.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 27.Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N. Crystal structure of the human glucose transporter GLUT1. Nature. 2014;510:121–125. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 28.Yan N. Structural advances for the major facilitator superfamily transporters. Trends Biochem Sci. 2013;38:151–159. doi: 10.1016/j.tibs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Blake CC, Oatley SJ. Protein-DNA and protein-hormone interactions in prealbumin: a model of the thyroid hormone nuclear receptor? Nature. 1977;268:115–120. doi: 10.1038/268115a0. [DOI] [PubMed] [Google Scholar]
- 30.Wojtczak A, Cody V, Luft JR, Pangborn W. Structures of human transthyretin complexed with thyroxine at 2.0 a resolution and 3′,5′-dinitro-N-acetyl-L-thyronine at 2.2 Å resolution. Acta Crystallogr D Biol Crystallogr. 1996;52:758–765. doi: 10.1107/S0907444996003046. [DOI] [PubMed] [Google Scholar]
- 31.Wojtczak A, Cody V, Luft JR, Pangborn W. Structure of rat transthyretin (RTTR) complex with thyroxine at 2.5 Å resolution: first non-biased insight into thyroxine binding reveals different hormone orientation in two binding sites. Acta Crystallogr D Biol Crystallogr. 2001;57:1061–1070. doi: 10.1107/s0907444901007235. [DOI] [PubMed] [Google Scholar]
- 32.Petitpas I, Petersen CE, Ha CE, Bhattacharya AA, Zunszain PA, Ghuman J, Bhagavan NV, Curry S. Structural basis of albumin-thyroxine interactions and familial dysalbuminemic hyperthyroxinemia. Proc Natl Acad Sci USA. 2003;100:6440–6445. doi: 10.1073/pnas.1137188100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sunthornthepvarakul T, Angkeow P, Weiss RE, Hayashi Y, Refetoff S. An identical missense mutation in the albumin gene results in familial dysalbuminemic hyperthyroxinemia in 8 unrelated families. Biochem Biophys Res Commun. 1994;202:781–787. doi: 10.1006/bbrc.1994.1998. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg SM, Ferrara AM, Nicholas ES, Dumitrescu AM, Cody V, Weiss RE, Refetoff S. A novel mutation in the albumin gene (R218S) causing familial dysalbuminemic hyperthyroxinemia in a family of Bangladeshi extraction. Thyroid. 2014;24:945–950. doi: 10.1089/thy.2013.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz M, Rajatanavin R, Young RA, Taylor C, Brown R, Braverman LE, Ingbar SH. Familial dysalbuminemic hyperthyroxinemia: a syndrome that can be confused with thyrotoxicosis. N Engl J Med. 1982;306:635–639. doi: 10.1056/NEJM198203183061103. [DOI] [PubMed] [Google Scholar]
- 36.Hennemann G, Docter R, Krenning EP, Bos G, Otten M, Visser TJ. Raised total thyroxine and free thyroxine index but normal free thyroxine. A serum abnormality due to inherited increased affinity of iodothyronines for serum binding protein. Lancet. 1979;1:639–642. doi: 10.1016/s0140-6736(79)91080-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhou A, Wei Z, Read RJ, Carrell RW. Structural mechanism for the carriage and release of thyroxine in the blood. Proc Natl Acad Sci USA. 2006;103:13321–13326. doi: 10.1073/pnas.0604080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nascimento AS, Dias SM, Nunes FM, Aparicio R, Ambrosio AL, Bleicher L, Figueira AC, Santos MA, de Oliveira NM, Fischer H, Togashi M, Craievich AF, Garratt RC, Baxter JD, Webb P, Polikarpov I. Structural rearrangements in the thyroid hormone receptor hinge domain and their putative role in the receptor function. J Mol Biol. 2006;360:586–598. doi: 10.1016/j.jmb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Souza PC, Puhl AC, Martinez L, Aparicio R, Nascimento AS, Figueira AC, Nguyen P, Webb P, Skaf MS, Polikarpov I. Identification of a new hormone-binding site on the surface of thyroid hormone receptor. Mol Endocrinol. 2014;28:534–545. doi: 10.1210/me.2013-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianco AC, Kim BW. Deiodinases: Implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweizer U, Schlicker C, Braun D, Köhrle J, Steegborn C. The crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism. Proc Natl Acad Sci USA. 2014;111:10526–10531. doi: 10.1073/pnas.1323873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleinau G, Schweizer U, Kinne A, Kohrle J, Gruters A, Krude H, Biebermann H. Insights into molecular properties of the human monocarboxylate transporter 8 by combining functional with structural information. Thyroid Res. 2011;4(suppl 1):S4. doi: 10.1186/1756-6614-4-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinne A, Kleinau G, Hoefig CS, Grüters A, Köhrle J, Krause G, Schweizer U. Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J Biol Chem. 2010;285:28054–28063. doi: 10.1074/jbc.M110.129577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friesema EC, Docter R, Moerings EP, Stieger B, Hagenbuch B, Meier PJ, Krenning EP, Hennemann G, Visser TJ. Identification of thyroid hormone transporters. Biochem Biophys Res Commun. 1999;254:497–501. doi: 10.1006/bbrc.1998.9974. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Levin EJ, Pan Y, McCoy JG, Sharma R, Kloss B, Bruni R, Quick M, Zhou M. Structural basis of the alternating-access mechanism in a bile acid transporter. Nature. 2014;505:569–573. doi: 10.1038/nature12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blondeau JP, Beslin A, Chantoux F, Francon J. Triiodothyronine is a high-affinity inhibitor of amino acid transport system L1 in cultured astrocytes. J Neurochem. 1993;60:1407–1413. doi: 10.1111/j.1471-4159.1993.tb03302.x. [DOI] [PubMed] [Google Scholar]
- 47.Friesema EC, Docter R, Moerings EP, Verrey F, Krenning EP, Hennemann G, Visser TJ. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology. 2001;142:4339–4348. doi: 10.1210/endo.142.10.8418. [DOI] [PubMed] [Google Scholar]
- 48.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T-cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geier EG, Schlessinger A, Fan H, Gable JE, Irwin JJ, Sali A, Giacomini KM. Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc Natl Acad Sci USA. 2013;110:5480–5485. doi: 10.1073/pnas.1218165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinne A, Hinz K, Wittner M, Krause G. Involvement of LAT2 in the transport of 3,30-T2 across the plasma membrane and first structural insights into transport mechanisms by homology model generation. 16th European Congress of Endocrinology, Wroclaw. Endocr Abstr. 2014;35:P1081. [Google Scholar]
- 51.Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na+-independent organic anion transporter. Proc Natl Acad Sci USA. 1994;91:133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, Nomura H, Hebert SC, Matsuno S, Kondo H, Yawo H. Molecular characterization and tissue distribution of a new organic anion transporter subtype (OATP3) that transports thyroid hormones and taurocholate and comparison with OATP2. J Biol Chem. 1998;273:22395–22401. doi: 10.1074/jbc.273.35.22395. [DOI] [PubMed] [Google Scholar]
- 53.Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, Onogawa T, Suzuki T, Asano N, Tanemoto M, Seki M, Shiiba K, Suzuki M, Kondo Y, Nunoki K, Shimosegawa T, Iinuma K, Ito S, Matsuno S, Abe T. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology. 2001;142:2005–2012. doi: 10.1210/endo.142.5.8115. [DOI] [PubMed] [Google Scholar]
- 54.Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, Abe M, Satoh F, Unno M, Hishinuma T, Inui K, Ito S, Goto J, Abe T. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci USA. 2004;101:3569–3574. doi: 10.1073/pnas.0304987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion-transporting polypeptide as a high-affinity thyroxine transporter. Mol Endocrinol. 2002;16:2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- 56.Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y. Functional characterization of rat brain-specific organic anion transporter (OATP14) at the blood-brain barrier: high-affinity transporter for thyroxine. J Biol Chem. 2003;278:43489–43495. doi: 10.1074/jbc.M306933200. [DOI] [PubMed] [Google Scholar]
- 57.Tohyama K, Kusuhara H, Sugiyama Y. Involvement of multispecific organic anion transporter, OATP14 (SLC21A14), in the transport of thyroxine across the blood-brain barrier. Endocrinology. 2004;145:4384–4391. doi: 10.1210/en.2004-0058. [DOI] [PubMed] [Google Scholar]
- 58.Westholm DE, Marold JD, Viken KJ, Duerst AH, Anderson GW, Rumbley JN. Evidence of evolutionary conservation of function between the thyroxine transporter OATP1C1 and major facilitator superfamily members. Endocrinology. 2010;151:5941–5951. doi: 10.1210/en.2010-0640. [DOI] [PubMed] [Google Scholar]
- 59.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 60.Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 61.Visser WE, Philp NJ, van Dijk TB, Klootwijk W, Friesema EC, Jansen J, Beesley PW, Ianculescu AG, Visser TJ. Evidence for a homodimeric structure of human monocarboxylate transporter 8. Endocrinology. 2009;150:5163–5170. doi: 10.1210/en.2009-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biebermann H, Ambrugger P, Tarnow P, von Moers A, Schweizer U, Grueters A. Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone-specific transporter MCT8. Eur J Endocrinol. 2005;153:359–366. doi: 10.1530/eje.1.01980. [DOI] [PubMed] [Google Scholar]
- 63.Friesema EC, Jansen J, Jachtenberg JW, Visser WE, Kester MH, Visser TJ. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol. 2008;22:1357–1369. doi: 10.1210/me.2007-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun D, Lelios I, Krause G, Schweizer U. Histidines in potential substrate recognition sites affect thyroid hormone transport by monocarboxylate transporter 8. Endocrinology. 2013;154:2553–2561. doi: 10.1210/en.2012-2197. [DOI] [PubMed] [Google Scholar]
- 65.Roth S, Kinne A, Schweizer U. The tricyclic antidepressant desipramine inhibits T3 import into primary neurons. Neurosci Lett. 2010;478:5–8. doi: 10.1016/j.neulet.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 66.Braun D, Kim TD, Le Coutre P, Kohrle J, Hershman JM, Schweizer U. Tyrosine kinase inhibitors noncompetitively inhibit MCT8-mediated iodothyronine transport. J Clin Endocrinol Metab. 2012;97:E100–E105. doi: 10.1210/jc.2011-1837. [DOI] [PubMed] [Google Scholar]
- 67.Braun D, Schweizer U. Authentic bosutinib inhibits triiodothyronine transport by monocarboxylate transporter 8. Thyroid. 2014;24:926–927. doi: 10.1089/thy.2013.0660. [DOI] [PubMed] [Google Scholar]
- 68.Groeneweg S, Friesema EC, Kersseboom S, Klootwijk W, Visser WE, Peeters RP, Visser TJ. The role of Arg445 and Asp498 in the human thyroid hormone transporter MCT8. Endocrinology. 2014;155:618–626. doi: 10.1210/en.2013-1521. [DOI] [PubMed] [Google Scholar]
- 69.Manoharan C, Wilson MC, Sessions RB, Halestrap AP. The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Mol Membr Biol. 2006;23:486–498. doi: 10.1080/09687860600841967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groeneweg S, Lima de Souza EC, Visser WE, Peeters RP, Visser TJ. Importance of His192 in the human thyroid hormone transporter MCT8 for substrate recognition. Endocrinology. 2013;154:2525–2532. doi: 10.1210/en.2012-2225. [DOI] [PubMed] [Google Scholar]
- 71.Lima de Souza EC, Groeneweg S, Visser WE, Peeters RP, Visser TJ. Importance of cysteine residues in the thyroid hormone transporter MCT8. Endocrinology. 2013;154:1948–1955. doi: 10.1210/en.2012-2101. [DOI] [PubMed] [Google Scholar]
- 72.Schweizer U, Braun D, Johannes J. Training MCT10 to transport thyroxine: structure-based targeted mutations in MCT10. 2014;35:OC7. 16th European Congress of Endocrinology, Wroclaw. Endocr Abstr 4. [Google Scholar]
- 73.Jansen J, Friesema EC, Kester MH, Milici C, Reeser M, Gruters A, Barrett TG, Mancilla EE, Svensson J, Wemeau JL, Busi da Silva Canalli MH, Lundgren J, McEntagart ME, Hopper N, Arts WF, Visser TJ. Functional analysis of monocarboxylate transporter 8 mutations identified in patients with X-linked psychomotor retardation and elevated serum triiodothyronine. J Clin Endocrinol Metab. 2007;92:2378–2381. doi: 10.1210/jc.2006-2570. [DOI] [PubMed] [Google Scholar]
- 74.Jansen J, Friesema EC, Kester MH, Schwartz CE, Visser TJ. Genotype-phenotype relationship in patients with mutations in thyroid hormone transporter MCT8. Endocrinology. 2008;149:2184–2190. doi: 10.1210/en.2007-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Visser WE, Jansen J, Friesema EC, Kester MH, Mancilla E, Lundgren J, van der Knaap MS, Lunsing RJ, Brouwer OF, Visser TJ. Novel pathogenic mechanism suggested by ex vivo analysis of MCT8 (SLC16A2) mutations. Hum Mutat. 2009;30:29–38. doi: 10.1002/humu.20808. [DOI] [PubMed] [Google Scholar]
- 76.Kinne A, Roth S, Biebermann H, Köhrle J, Grüters A, Schweizer U. Surface translocation and tri-iodothyronine uptake of mutant MCT8 proteins are cell type-dependent. J Mol Endocrinol. 2009;43:263–271. doi: 10.1677/JME-09-0043. [DOI] [PubMed] [Google Scholar]
- 77.Capri Y, Friesema EC, Kersseboom S, Touraine R, Monnier A, Eymard-Pierre E, Des Portes V, De Michele G, Brady AF, Boespflug-Tanguy O, Visser TJ, Vaurs-Barriere C. Relevance of different cellular models in determining the effects of mutations on SLC16A2/MCT8 thyroid hormone transporter function and genotype-phenotype correlation. Hum Mutat. 2013;34:1018–1025. doi: 10.1002/humu.22331. [DOI] [PubMed] [Google Scholar]
- 78.Kersseboom S, Kremers GJ, Friesema EC, Visser WE, Klootwijk W, Peeters RP, Visser TJ. Mutations in MCT8 in patients with Allan-Herndon-Dudley syndrome affecting its cellular distribution. Mol Endocrinol. 2013;27:801–813. doi: 10.1210/me.2012-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snider C, Jayasinghe S, Hristova K, White SH. Mpex: a tool for exploring membrane proteins. Protein Sci. 2009;18:2624–2628. doi: 10.1002/pro.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hessa T, Meindl-Beinker NM, Bernsel A, Kim H, Sato Y, Lerch-Bader M, Nilsson I, White SH, von Heijne G. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature. 2007;450:1026–1030. doi: 10.1038/nature06387. [DOI] [PubMed] [Google Scholar]