Abstract
We examined the relationship between social rank and brain white matter (WM) microstructure, and socioemotional behavior, and its modulation by serotonin (5HT) transporter (5HTT) polymorphisms in prepubertal female macaques. Using diffusion tensor imaging and tract-based spatial statistics, social status differences were found in medial prefrontal cortex (mPFC) WM and cortico-thalamic tracts, with subordinates showing higher WM structural integrity (measured as fractional anisotropy, FA) than dominant animals. 5HTT genotype-related differences were detected in the posterior limb of the internal capsule, where s-variants had higher FA than l/l animals. Status by 5HTT interaction effects were found in (1) external capsule (middle longitudinal fasciculus), (2) parietal WM, and (3) short-range PFC tracts, with opposite effects in dominant and subordinate animals. In most regions showing FA differences, opposite differences were detected in radial diffusivity, but none in axial diffusivity, suggesting that differences in tract integrity likely involve differences in myelin. These findings highlight that differences in social rank are associated with differences in WM structural integrity in juveniles, particularly in tracts connecting prefrontal, sensory processing, motor and association regions, sometimes modulated by 5HTT genotype. Differences in these tracts were associated with increased emotional reactivity in subordinates, particularly with higher submissive and fear behaviors.
Keywords: diffusion tensor imaging, emotional behavior, nonhuman primates, prepuberty, social stress
Introduction
The primate brain has evolved to adapt to complex social environments (Dunbar and Shultz 2007) and, as a consequence, early social experiences have a strong influence on its development. In particular, social stress during childhood is associated with behavioral and cognitive impairments and increased risk for psychopathology and health problems later in life, including anxiety and mood disorders (Teicher et al. 2002, 2003; Gunnar and Quevedo 2007; Weber et al. 2008). These long-term adverse effects of stress are more prevalent in females than in males (Becker et al. 2007) and often emerge during puberty (Seeman 1997; Kessler et al. 2001; Reardon et al. 2009). However, it is likely that adverse social experiences prior to puberty are also critical for neurobehavioral changes.
In addition, genetic factors can affect vulnerability to these stressful experiences. For example, allelic variants in the promoter region of the gene encoding the serotonin (5HT) transporter (5HTT) affect emotional and neuroendocrine stress responses and modulate vulnerability to the deleterious effects of early life stress, both in humans (Caspi et al. 2003; Zalsman et al. 2006; Stein et al. 2008; Caspi et al. 2010) and nonhuman primates (Bennett et al. 2002; Champoux et al. 2002; Barr et al. 2004; McCormack et al. 2009). In both species, the short (s) allele carriers—with less functional 5HTTs—seem more vulnerable to stress (Holmes and Hariri 2003; Suomi 2003). There is also evidence that these gene by environment interactions are more salient in females (Barr et al. 2004; Eley et al. 2004; Sjoberg et al. 2006). Despite the reported role of social stress and genetic variation in the etiology of psychopathology in females, particularly during the peripubertal period, the underlying neurobiological mechanisms are still not clearly understood.
One potential mechanism could be through effects on brain white matter (WM), which is sensitive to adverse experiences (Eluvathingal et al. 2006; Choi et al. 2009; Coplan et al. 2010; Govindan et al. 2010) and 5HT (Whitaker-Azmitia et al. 1996), particularly during development. The important functional role of brain WM has been underscored by the development of neuroimaging methods that visualize fiber tracts and measure their structural integrity in vivo, such as diffusion tensor imaging (DTI; Thomason and Thompson 2011). DTI measures water diffusion in the brain and provides measures of WM fiber tract properties, including fractional anisotropy (FA), a measure of structural integrity of WM tracts. Because increased tract microstructural integrity (via, for example, increased myelin) can affect information transfer by increasing conduction speed along the axon (Lang and Rosenbluth 2003; Paus 2010), brain WM tract integrity is recognized as an important mechanism underlying behavioral control (Fields 2008; Thomason and Thomson 2011). In addition, brain WM integrity is vulnerable to early stress/adversity (Eluvathingal et al. 2006; Katz et al. 2009; Coplan et al. 2010; Govindan et al. 2010; Frodl et al. 2012) and is affected by polymorphisms in the 5HTT gene (Pacheco et al. 2009).
Prospective studies assessing the impact of persistent exposure to social stressors on brain WM in children are difficult. An example of cumulative adverse social experiences is low socioeconomic status (SES), which represents a complex form of chronic early social stress associated with socioemotional and cognitive impairments in children, also involving poor access to material and social resources (Bradley and Corwyn 2002; Hackman and Farah 2009). Low SES during childhood has also been shown to increase the risk for depression in women (Gilman et al. 2002). Although there is some recent evidence of low SES effects on brain gray matter (GM; Hackman and Farah 2009; Jednoróg et al. 2012; Noble et al. 2012), the effects of continual adverse early social experiences such as low SES on brain WM and associated effects on socioemotional deficits in girls are not understood.
To address some of these questions, the present study used a nonhuman primate model of chronic social stress, notably social subordination in rhesus monkeys, to investigate how this adverse experience and 5HTT polymorphisms interact to affect brain WM tracts in prepubertal female macaques using DTI. Subordinate dominance status in rhesus monkeys is enforced through unpredictable and recurring contact and noncontact aggression from more dominant animals in the group. Both subordinate adult (Bernstein and Gordon 1974; Bernstein et al. 1974; Bernstein 1976; Shively and Kaplan 1984) and juvenile females (Bernstein and Ehardt 1985) receive more aggression from higher-ranking group mates and terminate these interactions by emitting submissive behavior, a defining feature of subordination. Because offspring of group living macaques assume the relative rank of their mother (Sade 1967), offspring of subordinate mothers are exposed to high rates of aggression from birth. Consequences of this unpredictable, continual harassment in adult females include stress-related phenotypes, including hypothalamic–pituitary–adrenal axis (HPA) dysregulation, evidenced by hypercortisolemia and reduced glucocorticoid negative feedback (Shively et al. 1997; Jarrell et al. 2008; Kaplan et al. 2010). Thus, social subordination is a well-established nonhuman primate model of chronic psychosocial stress used to study its adverse effects on a broad range of adult behavioral, neuroendocrine, and health outcomes (Gust et al. 1991; Kaplan et al. 1996; Morgan et al. 2002; Michopoulos et al. 2009; Paiardini et al. 2009; Kaplan et al. 2010; Tung et al. 2012). While the subordinate phenotype is less well understood in juveniles, they undergo delayed puberty (Wilson et al. 1986, 2013; Zehr et al. 2005), an effect exacerbated in 5HTT s-allele carriers (Wilson and Kinkead 2008) and associated with increased emotional reactivity (Wilson et al. 2013). However, the neurobiological effects of social subordination on these juvenile females are unknown. In this study, we used DTI and tract-based spatial statistics (TBSS) to examine differences in brain WM tract integrity between dominant and subordinate females prior to puberty, and its modulation by 5HTT polymorphisms. WM tract integrity was measured by FA, in parallel with radial diffusivity (RD) and axial diffusivity (AD) measures to aid with the interpretation of the local microstructural mechanisms involved (Keller and Just 2009; Wheeler-Kingshott and Cercignani 2009; Bennett et al. 2010; Burzynska et al. 2010; Metwalli et al. 2010; Shamy et al. 2010; Hu et al. 2011; Taubert et al. 2011; Shi et al. 2012; Tang et al. 2012). Given recent evidence that social subordination is associated with reduced GM density in the prefrontal cortex (PFC) of adult male macaques as measured by magnetic resonance imaging (MRI; Sallet et al. 2011), we hypothesized that prefrontal tracts would be particularly affected in prepubertal female subordinate monkeys, as well. To assess the functional correlates of social rank-related brain differences, we also examined the associations between brain WM tract integrity, emotional behavior, and measures of stress physiology in these juveniles.
Materials and Methods
Subjects
Subjects were 35 prepubescent female rhesus monkeys (Macaca mulatta) living in 4 social groups consisting of 60–100 adult females and their juvenile offspring, and 4–6 adult males. Animals were housed in outdoor enclosures (three-quarters of an acre area) with access to climate-controlled indoor facilities at the Yerkes National Primate Research Center (YNPRC) Field Station in Lawrenceville, GA. Free access to a standard low-fat, high-fiber diet (Purina Mills Int., Lab Diets, St. Louis, MO, USA) and water was provided. Subjects were studied between 14 and 22 months, prior to menarche, which was reached by these females at 29.92 ± 0.62 months (Wilson et al. 2013), consistent with other reports for outdoor-housed female rhesus monkeys (around 26 months of age; Wilson et al. 1986). All procedures were approved by the Emory University Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act and the US Department of Health and Human Services “Guide for Care and Use of Laboratory Animals.”
Determination of Social Rank
Each subject's relative dominance rank within her natal group was determined based on outcomes of dyadic agonistic interactions in which a subordinate female is one who unequivocally emits a submissive behavior in response to an approach or to an actual aggressive act from a more dominant animal (Bernstein 1976). Thus, dominant rank was not determined by who aggresses whom, but rather who submits to whom. Each animal's relative rank was calculated as the ratio of her rank to the total number of animals in her group, exclusive of animals <12 months old. Thus, a subject ranking 25 out of a group of 100 animals received a relative rank of 0.25. For the present analysis, we compared the most subordinate females in the cohort (animals with a relative rank of >0.60, representing the 40% lowest ranking animals; n = 13) with more dominant females (n = 22). This relative rank value was also used in regression analyses to determine its relation to other behavioral and physiological phenotypes (see below).
5HTT Genotyping
All subjects were genotyped for polymorphisms in the 5HTT promoter gene (SLC6A4), as described previously (Hoffman et al. 2007). Of the 35 females, 17 had both alleles of the long promoter length variant (l/l: 10 dominant and 7 subordinate) and 18 had at least one short allele (s-variant: 12 dominant −9 l/s, 3 s/s− and 6 subordinate −4 l/s, 2 s/s−). Subjects with the l/s or s/s genotype were combined (s-variant or */s) based on the low occurrence of the s/s genotype and previous reports that both genotypes result in similar phenotypes in rhesus monkeys (Champoux et al. 2002).
Coefficients of Relatedness
Because this colony is pedigreed, kinship coefficients were determined (Hamilton 1964) per rank and 5HTT genotype. Average coefficients for dominant-l/l, dominant-s-variant, subordinate-l/l, and subordinate s-variant groups were 0.040, 0.045, 0.062, and 0.082, respectively, indicating that any shared genetic factors within each status-genotype category would not explain the phenotypic differences between the groups.
DTI Data
DTI Acquisition
Subjects were transported from their social group to the YNPRC Imaging Center the day before the scans. The scanning age was not different between dominant and subordinate subjects (20.68 ± 0.29 vs. 20.89 ± 0.33 months), nor between l/l and s-allelic variant females (20.68 ± 0.36 vs. 20.90 ± 0.28), as described in Results, and corresponds to a juvenile period before puberty onset (menarche) for these females (29.92 ± 0.62 months) (Wilson et al. 2013). A DTI and a T1-weighted MRI scan were acquired during the same session using a 3-T Siemens Magnetom TRIO system (Siemens Med. Sol., Malvern, PA, USA) and an 8-channel phase array coil. All animals were scanned supine in the same orientation, achieved by placement and immobilization of the head in a custom-made head holder via ear bars and a mouth piece. A vitamin E capsule was taped on the right temple to mark the right side of the brain. Scans were acquired under isoflurane anesthesia (1–1.2% to effect, inhalation), following initial induction with telazol (5 mg/kg, i.m.). Animals were fitted with an oximeter, electrocardiograph, rectal thermistor, and blood pressure monitor for physiological monitoring, an i.v. catheter to administer dextrose/NaCl (0.45%) to maintain normal hydration, and an MRI-compatible heating pad. Upon completion of the scans and full recovery from anesthesia, each female was returned to her social group.
DTI scans were acquired following published protocols by our group for rhesus monkeys (Hecht et al. 2013), using a single-shot dual spin-echo EPI sequence with GeneRalized Auto-calibrating Partially Parallel Acquisitions (GRAPPA) (R = 3), voxel size = 1.3 × 1.3 × 1.3 mm with zero gap, 60 directions, time repetition (TR)/time echo (TE) = 5000/86 ms, field of view (FOV) = 83 mm, b: 0, 1000 s/mm2, and 12 averages. T1-MRI scans were acquired using a 3-dimensional (3D) magnetization-prepared rapid gradient-echo (3D-MPRAGE) parallel imaging sequence with GRAPPA (R = 2), voxel size = 0.5 × 0.5 × 0.5 mm3, time to inversion/TR/TE = 950/3000/3.49 ms, FOV = 96 mm, 8 averages.
DTI Image Analysis
Preprocessing
The FMRIB Software Library (FSL, FMRIB, Oxford, UK; Smith et al. 2004; Woolrich et al. 2009) FDT tool was used to fit a tensor model at each voxel in native diffusion space to calculate the diffusion properties selected for this study (FA and AD; RD was calculated at each voxel by averaging the second and third eigenvalues for each voxel using the fslmaths command in FSL) after correcting for B0 inhomogeneity-induced distortion and eddy current effects, and skull stripping using the BET FSL tool (Smith 2002).
These FSL diffusion analysis tools have been previously applied with success to rhesus brain DTI data by our group (Hecht et al. 2013) and others (Makris et al. 2007; Willette et al. 2010, 2012; Bendlin et al. 2011). The diffusion properties examined (FA, RD, and AD) characterize the local microorganization of brain WM and have been previously used in combination in DTI studies describing developmental changes of brain tracts (Shi et al. 2012) and effects of lesions (Shamy et al. 2010) in this species, as well as effects of experience in humans (e.g. Tang et al. 2012). This is because changes in FA (calculated as the ratio of diffusion parallel to the fibers to the diffusion perpendicular to the fibers) can be either due to changes in perpendicular diffusion along the tract (measured by RD, which decreases with increased axonal myelination; Keller and Just 2009; Zhang et al. 2009; Bennett et al. 2010) or due to changes in parallel diffusivity (measured by AD, which increases with axonal density, caliber, and microtubular packing and organization; Kumar et al. 2010, 2012), evidence also supported by combined DTI and histological studies performed in rodent and nonhuman primate brains (Song et al. 2002, 2003; Choe et al. 2012). Thus, higher FA values in the presence of decreased RD, but no AD changes, are generally interpreted as increased WM tract integrity due to increased myelination, whereas increased FA in parallel to increased AD, without RD changes, indicates increased fiber tract organization.
Tract-Based Spatial Statistics
The FSL TBSS tool (Smith et al. 2006) was used to identify the centers of all major WM tracts present in all subjects. This method uses nonlinear registrations (Rueckert et al. 1999) to align all subjects' FA data to a predetermined common space or template image, from which a group mean FA image is calculated. In our study, the data were registered to the Wisconsin 112RM-SL monkey atlas (McLaren et al. 2009, 2010), resulting in a final resolution of 0.5 × 0.5 × 0.5 mm. The mean FA image was then skeletonized and thresholded (only voxels with FA of >0.2 were included), so that only the centers of major tracts are included in the analysis, excluding small peripheral tracts that may confound findings due to anatomic individual variability and partial volume effects. Each subjects' individual FA values were then projected onto the mean FA skeleton (Smith et al. 2006). Skeletonized FA data significantly minimize the number of voxels included in the voxel-wise statistical analysis (described below), cutting down multiple comparisons, and are less dependent on the accuracy of the initial registrations. A version of the tbss_non_FA script modified to use the Wisconsin 112RM-SL monkey atlas space was applied to generate each subject's skeletonized RD and AD maps.
Statistical Analysis of DTI Measures
A voxel-wise 2-way analysis of variance (ANOVA) was run to examine the effects of social status (high vs. low-ranking) and 5HTT genotype (l/l vs. s-variant) on skeletonized FA data using the Analysis of Functional NeuroImages software package (AFNI; Cox 1996). Results were considered significant at P < 0.05, after cluster correction for clusters >150 significant contiguous voxels (18.75 mm3), a more stringent criteria than previously used for similar rhesus monkey DTI studies (Willette et al. 2010). Results (significant clusters >150 voxels) were displayed in the Wisconsin 112RM-SL rhesus atlas (McLaren et al. 2009, 2010), which is in the brain coordinate space of the Saleem–Logothetis rhesus atlas (Saleem and Logothetis 2007).
Binary masks were created for the regions showing FA differences (i.e. voxel clusters of significant FA differences detected in the voxel-wise ANOVA, status, and/or 5HTT genotype effects). The mean RD and AD were calculated within these regions, following previously published approaches (Smith et al. 2004; Tang et al. 2012). A 2-way ANOVA was run in SPSS to examine the effects of status and 5HTT genotype on RD and AD in those clusters with significant FA differences, with significance level set at P < 0.05. The mean FA was also calculated for each cluster to examine its correlations with behavioral and cortisol data using Pearson correlation (see details below). Finally, a test–retest analysis of stability and replicability of DTI FA, RD, and AD measures was performed in clusters with significant effects in a small group of animals (n = 5) that were scanned twice, a few weeks apart, using a paired t-test.
DTI Probabilistic Tractography
To identify the most likely fiber tract(s) passing through the WM regions with significant status and/or 5HTT genotype effects, we performed probabilistic tractography in each individual macaque brain using the FSL imaging suite and methods published by our group for nonhuman primates (Hecht et al. 2013), rodents (Gutman, Keifer, et al. 2012; Gutman, Magnuson, et al. 2012), and humans (Gutman et al. 2009). A pipeline was constructed using the NiPype framework (Gorgolewski et al. 2011) to perform image registration and to generate tractography maps for each subject. Briefly, the reference volume from the DTI data set (the “nodif” or b0 volume) was registered to the template (Wisconsin 112RM-SL rhesus atlas; McLaren et al. 2009, 2010) using a 12-degrees of freedom linear registration as implemented in the FSL FLIRT module. Regions of interest (ROIs) for probabilistic tractography were derived from the significant cluster masks previously identified in the TBSS analysis. For each ROI, probabilistic tractography was computed in the template space for each voxel within the seed mask using the default parameters in FSL using distance correction, resulting in a probabilistic tractography map for each ROI (based on the 7 significant clusters identified) for each subject. All tractography used a multifiber reconstruction algorithm implemented in FSL, bedpostX, which permits the reconstruction of geometrically complex pathways, including crossing fibers (Behrens et al. 2007). After tractography, each individual raw tract map was thresholded at 1% of the robust mean intensity and binarized, and combined to produce a group probability map; in this composite image (group probability map), the intensity of each voxel represents the number of individual subjects that showed connectivity with that voxel after thresholding. For visualization, the composite images were then thresholded at a group level to highlight only voxels that were common to at least 40% of subjects; of note, we evaluated the effects of both relaxed and more stringent subject- and group-level thresholds which produced qualitatively similar results. Resulting fiber tracts were identified using available rhesus brain and fiber pathway atlases (Schmahmann and Pandya 2006; Saleem and Logothetis 2007; Schmahmann et al. 2007).
Behavioral Data
Group Social Observations
Focal behavioral observations (2 × 30 min) were collected monthly from 14.3 ± 0.24 until 22.5 ± 0.24 months for each subject using an established rhesus ethogram (Altmann 1962) with modifications (Maestripieri et al. 2006). Behavioral categories included affiliative (proximity, grooming, and contact), agonistic (aggression and submission), anxiety-like (yawn, body shake, and scratch; Troisi et al. 1991; Schino et al. 1996), and play behaviors. Data were recorded using netbooks and a custom-designed program that captures initiator, behavior, recipient, and time (Graves and Wallen 2006). Interobserver reliability, calculated as interobserver % agreement on the frequencies and durations of behaviors emitted by a female during formalized observations, exceeded 92%. Durations and frequencies of behaviors were averaged across observation sessions for analysis.
Emotionality Testing
The Human Intruder (HI) paradigm (Kalin and Shelton 1989) and the Approach-Avoidance (AA) task (Meunier et al. 1999) were performed before the scans (at 18.37 ± 0.11 months) to examine associations of the subjects' emotional behavior with the neuroimaging data. These tasks evoke strong and distinct behavioral responses to novel, threatening stimuli (Kalin and Shelton 1989; Meunier et al. 1999; Machado et al. 2009) and have been used to examine the effects of early stress (Grand et al. 2005) and 5HTT genotypes (Bethea et al. 2004) in rhesus monkeys. The HI paradigm (Kalin and Shelton 1989) is comprised of 3 consecutive 10-min conditions: (1) An alone (AL) condition that elicits distress vocalizations and locomotion; (2) a profile (PR) condition where an unfamiliar experimenter enters the testing room and presents his/her facial profile toward the monkey, who typically stops locomoting/vocalizing and freezes while scanning the intruder; and a stare (ST) condition, during which the experimenter faces the animal and makes continuous eye contact with it, a threatening behavior for rhesus macaques that induces aggressive and submissive behaviors toward the intruder. Sessions were videotaped and scored for frequencies and durations of behaviors following a previously published ethogram and procedures (Machado and Bachevalier 2006), with an interrater reliability >92%.
The AA task (Meunier et al. 1999) was done a week apart from the HI and consisted of six 5-min sessions, during which a different novel object (3 neutral and 3 fear-evoking) was presented in a tray per session, in parallel to a food reward (jellybean) to drive approach behaviors. For the present analysis, we only report responses to the most fear-evoking object (battery operated toy pig that moved and emitted sounds). AA sessions were also videotaped and scored for frequencies and durations of behaviors (exploration and locomotion; aggressive, submissive, anxiety-like, and fearful behaviors; latency to touch/inspect objects and to eat the jellybean) using a published ethogram (Machado and Bachevalier 2006). Interobserver reliability was >92%.
Statistical Analysis: Behavioral Associations with Rank and Neuroimaging Data
A principal component analysis (PCA) using a rotation method of Oblimin with Kaiser normalization was performed using the SPSS 19.0 software separately for the AA and HI data to group behaviors into similar patterns of responses that represent the most prominent behavioral phenotypes, as previously done in rhesus monkeys (Williamson et al. 2003). The AA PCA included behavioral responses to the motorized pig and the HI PCA included behavioral responses to the most threatening conditions, the PR and ST (Tables 1 and 2). Behaviors with loading scores of ≤0.4 were excluded from the analysis. Composite scores were calculated for each PCA component identified for correlational analysis.
Table 1.
Factor loadings of PCA for the HI test, no eye contact (NEC) condition
| Behaviors | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Fear | Submission | Anxiety | Distress | |
| Freeze duration— NEC | 0.85 | |||
| Turn away frequency—Stare | −0.791 | |||
| Avert gaze frequency—Stare | 0.88 | |||
| Lipsmack frequency—Stare | 0.676 | |||
| Anxiety-like behaviors—Stare | 0.838 | |||
| Toothgrind—Stare | 0.721 | |||
| Coo—Stare | −0.792 | |||
| Threat—Stare | −0.768 |
Table 2.
Factor loadings of PCA for the AA test
| Behaviors | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Anxious aggression | Anxious vigilance | Impulsivity | |
| Threaten object frequency | 0.942 | ||
| Look away from object frequency | 0.763 | ||
| Bite object frequency | −0.439 | ||
| Locomote frequency | 0.823 | ||
| Visually inspect object frequency | −0.805 | ||
| Latency to take reward | −0.761 | ||
| Latency to explore box | −0.664 |
Linear regression assessed the relation between relative social rank and levels of aggression, submission, and affiliation exhibited in the social group. In addition, Pearson product-moment correlations were used to examine the associations between neuroimaging measures (mean FA in clusters with significant status and/or 5HTT genotype effects) and behavioral data (behaviors in the social group and factors derived from the HI and AA PCAs). A Sidak correction for multiple correlations was used to set the significance level for the regression analyses. In addition to the magnitude of the correlation coefficient, Cohen's d values were also reported to provide the effect size of the associations. Using established conventions (Cohen 1992), effect sizes of >0.3 and <0.7 were considered moderate and those of >0.7 large.
Cortisol Data
Serum cortisol levels were measured to examine HPA axis basal activity and stress reactivity a week before the HI and AA tasks. For this, a baseline blood sample was collected from the animal's saphenous vein in the awake state, within 10 min from initial access of the group, following previously published protocols (McCormack et al. 2009; Arce et al. 2010). Immediately after, the subject was transported to a novel behavioral testing room and placed in a testing cage for 30 min, when another blood sample (post-stress) was obtained to measure stress-induced increase in cortisol secretion. This brief social separation is a potent stressor in monkeys that activates the HPA axis (Arce et al. 2010). Cortisol assays were performed at the YNPRC Biomarkers Core Lab using a commercially available RIA kit (Beckman-Coulter/DSL, Webster, TX, USA), with a range from 0.50 to 60 µg/dL, and intra- and interassay coefficient of variabilities of 4.9% and 8.7%, respectively.
Statistical Analysis: Associations with Rank and Neuroimaging Data
Linear regression models assessed the relation between relative social rank and cortisol levels [baseline and stress-induced increases (the latter measured as the delta from baseline to poststress levels)]. Pearson product-moment correlations were used to examine the associations between cortisol (baseline and stress increases) and FA measures. A statistical significance level was set at P < 0.05.
Results
DTI Data
Social Status and 5HTT Genotype Effects on FA Measures
No differences in scanning age were detected by 2-way ANOVA for social status (dominants = 21.05 ± 0.30, subordinates = 20.69 ± 0.33 months; F(1,31) = 0.476, P = 0.495) or 5HTT genotype (l/l = 20.76 ± 0.37, */s = 21.06 ± 0.26 months; F(1,31) = 0.476, P = 0.495).
Two-way ANOVA of the TBSS results identified several regions of the FA skeleton that exceeded the criteria for significance (P < 0.05, cluster-corrected for clusters >150 significant, contiguous, voxels).
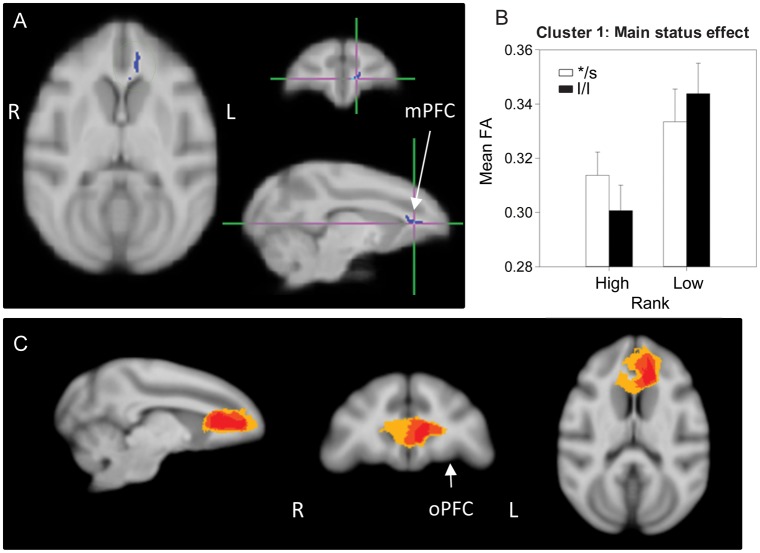
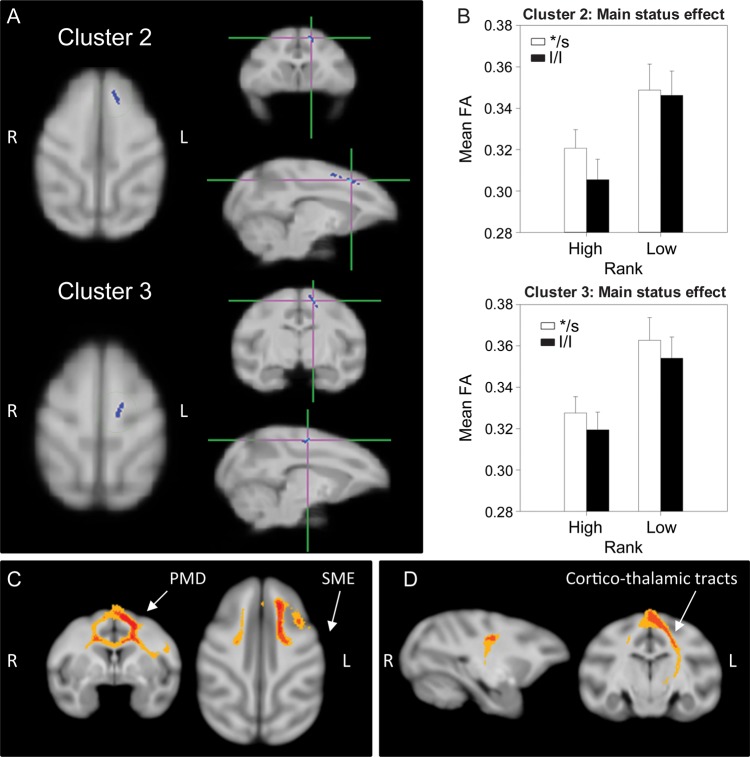
A main effect of status was found in 3 separate clusters (Figs 1 and 2): One in the left medial PFC (mPFC) (cluster 1, 204 voxels), and two in the WM along the left dorsal medial wall (cluster 2, 202 voxels and cluster 3, 196 voxels). In all these clusters, low-ranking animals had higher FA than dominants regardless of the 5HTT genotype. Probabilistic tractography identified cluster 1 as involving local, short-range, intrahemispheric mPFC fibers, some of them cross-hemispheric (crossing through the genu of the corpus callosum (CC)—in 40–60% of the animals; Fig. 1C). Clusters 2 and 3 both involved short-range, cortico-cortical fibers (intra- and interhemispheric) in the dorsomedial wall interconnecting frontal regions corresponding to the supplementary motor, premotor, and primary motor cortex in most animals, and also connecting these frontal motor areas with the somatosensory cortex in 25–50% of subjects (Fig. 2C). In addition, cluster 3 also contained cortico-thalamic tracts connecting somatosensory and primary motor cortices with thalamic regions (Fig. 2D). Based on proximity and overlap in the tractography results, clusters 2 and 3 appear to be part of the same network.
Figure 1.
Results of the DTI analysis: Main effect of status. (A) TBSS 2 × 2 ANOVA results showing the main effect of status (high/dominant vs. low/subordinate), cluster 1: Left medial prefrontal cortex (mPFC) WM. (B) Results from the 2-way ANOVA showing that low-ranking animals have significantly higher FA than high-ranking ones. (C) Representation of the affected tracts using probabilistic tractography: Group probability map (subject-level threshold at 1% of the robust mean intensity with distance correction applied) showing that the cluster of significant voxels in (A) includes local, short-range, intrahemispheric mPFC fibers, with some cross-hemispheric fibers (crossing through the genu of the CC), in 40–60% of the animals (yellow). Left-to-right images represent sagittal, coronal, and axial planes. The composite images (group probability maps) show voxels that were common to at least 15 (40%) animals. Colors represent the percentage of subjects that showed connectivity with that voxel in the single-subject analysis: 40–60% animals in yellow, 60–80% in orange, and 80–100% in red. oPFC: orbital prefrontal cortex.
Figure 2.
Results of the DTI analysis: Main effect of status. (A) TBSS 2 × 2 ANOVA results showing the main effect of status in 2 clusters, 2 and 3: WM along the left dorsal medial wall. (B) Results from the 2-way ANOVA showing that low-ranking animals have significantly higher FA than high-ranking ones. (C and D) Probabilistic tractography: Group probability map (subject-level threshold at 1% of the robust mean intensity with distance correction applied) showing that (C) both clusters of significant voxels in (A) involved short-range, cortico-cortical fibers (intra- and interhemispheric) in the dorsomedial wall, interconnecting frontal regions corresponding to the primary and supplementary motor area (SMA) and premotor cortex in most of the animals, as well as connections of these frontal motor areas with somatosensory cortex (SSC) in about 25–50% of subjects. Left-to-right images represent coronal and axial planes. The composite images (group probability maps) show voxels that were common to at least 15 (40%) animals. Colors represent the percentage of subjects that showed connectivity with that voxel in the single-subject analysis: 40–60% animals in yellow, 60–80% in orange, and 80–100% in red. PMD: dorsal premotor cortex; SME: somatosensory cortex (D) represents the group probability map of additional caudal tractography in cluster 3, suggesting the involvement of cortico-thalamic tracts connecting somatosensory and primary motor cortices with thalamic regions. Left-to-right images represent sagittal and coronal planes. Thresholds and color codes as in C.
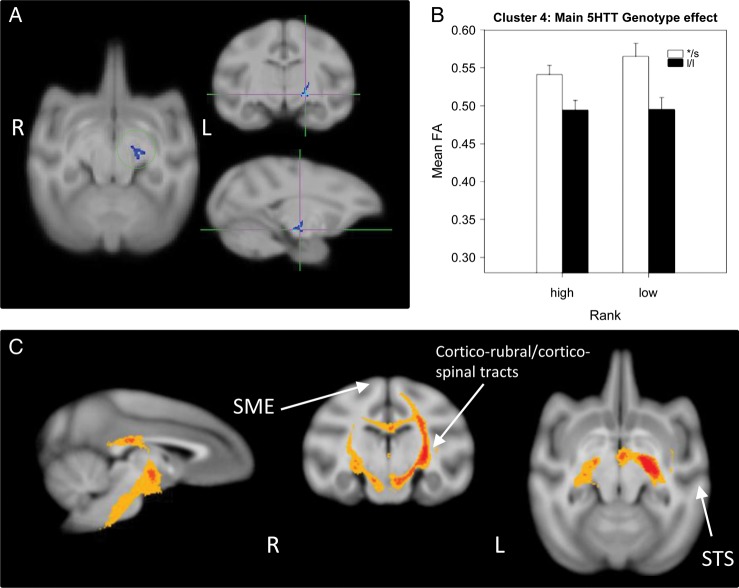
A main 5HTT genotype effect was detected in a single cluster (cluster 4, 153 voxels), in the left posterior limb of the internal capsule (PLIC, Fig. 3), with s-variants showing higher FA than l/l animals regardless of social status. Probabilistic tractography identified this cluster as involving mostly cortical descending pathways, including corticorubral and corticospinal tracts with bilateral connectivity (via the corpus callosum) in about 50% of the animals (Fig. 3A).
Figure 3.
Results of the DTI analysis: Main effect of the 5HTT genotype. (A) TBSS 2 × 2 ANOVA results showing the main effect of 5HTT genotype (l/l vs */s –or short-variant-) in a cluster (cluster 4) in the left PLIC. (B) Results from the 2-way ANOVA showing that l/l animals have significantly lower FA than */s ones. (C) Probabilistic tractography: Group probability map (subject-level threshold at 1% of the robust mean intensity with distance correction applied) showing that the cluster of significant voxels in (A) involves mostly cortical descending pathways, including corticorubral and corticospinal tracts with bilateral connectivity via the corpus callosum in about 50% of the animals. Left-to-right images represent sagittal, coronal, and axial planes. The composite images (group probability maps) show voxels that were common to at least 9 (25%) animals. Colors represent the percentage of subjects that showed connectivity with that voxel in the single-subject analysis: 25–50% animals in yellow, 50–75% in orange, and 75–100% in red. The slightly different group threshold applied to the composite image of this cluster was chosen for display purposes, but more stringent group-level threshold results showed qualitatively similar results (just slightly narrower bands). STS: superior temporal sulcus; SME: somatosensory cortex.
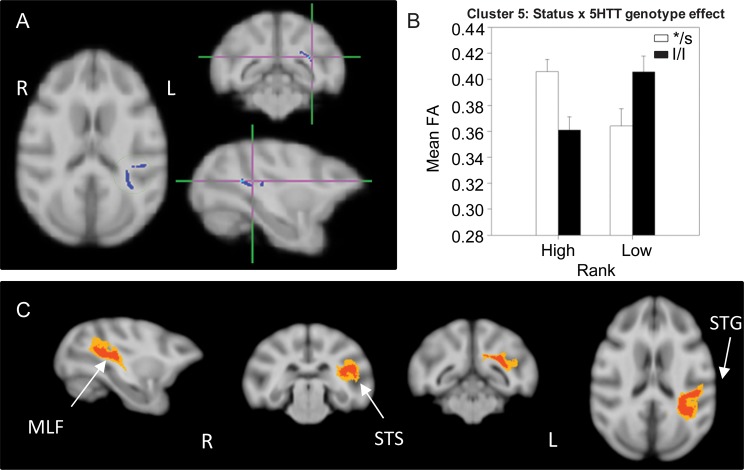
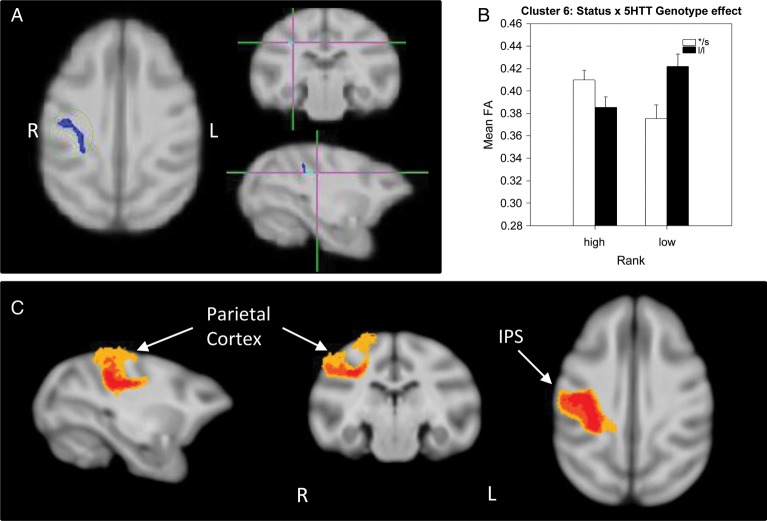
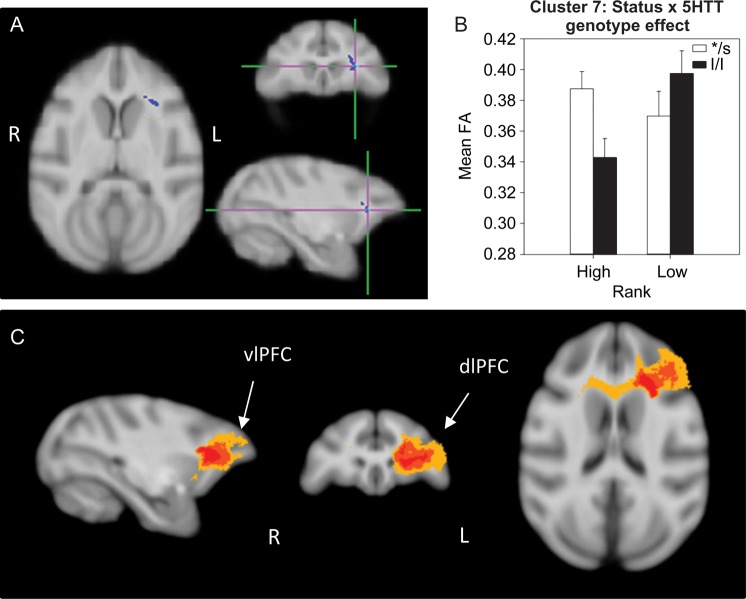
A significant status × 5HTT genotype interaction effect was detected in 3 different clusters: One in the left external capsule (EC; cluster 5, 242 voxels; Fig. 4), one in the right parietal cortex WM (cluster 6, 167 voxels; Fig. 5), and a third in the left PFC WM (cluster 7, 157 voxels; Fig. 6). In all 3 of these clusters, dominant l/l animals had lower FA than s-variant animals, while the opposite was true for low-ranking animals (l/l had higher FA than s-variants). Probabilistic tractography identified cluster 5 (EC) as involving the left middle longitudinal fasciculus (MLF; Fig. 4C), a long association parieto-temporal tract that reciprocally connects the inferoparietal cortex with cortical areas in the superior temporal sulcus (STS) and gyrus (STG) as identified in macaques by tract-tracing (Schmahmann and Pandya 2006) and diffusion spectrum imaging (Schmahmann et al. 2007). Cluster 6 (parietal cortex WM) involved short-range, parietal “U” fibers (Fig. 5C), short cortico-cortical association fiber tracts. Cluster 7 involved short-range prefrontal tracts connecting the dorsolateral, ventrolateral, and dorsomedial PFC in the left hemisphere and across the 2 hemispheres in 40–60% of subjects (Fig. 6C).
Figure 4.
Results of the DTI analysis: Status × 5HTT genotype interaction effect. (A) TBSS 2 × 2 ANOVA results showing significant status × 5HTT genotype interactions in a cluster (cluster 5) in the left EC. (B) Results from the 2-way ANOVA showing that dominant l/l animals had lower FA than s-variant ones, whereas l/l subordinates had higher FA than */s. (C) Probabilistic tractography: Group probability map (subject-level threshold at 1% of the robust mean intensity with distance correction applied) showing that the cluster of significant voxels in (A) corresponds to the left middle longitudinal fasciculus, which reciprocally connects the inferoparietal area with cortical regions in the STS and STG. Left-to-right images represent sagittal, coronal (rostral vs. caudal), and axial planes. The composite images (group probability maps) show voxels that were common to at least 15 (40%) animals. Colors represent the percentage of subjects that showed connectivity with that voxel in the single-subject analysis: 40–60% animals in yellow, 60–80% in orange and 80–100% in red. STG: superior temporal gyrus; STS: superior temporal sulcus.
Figure 5.
Results of the DTI analysis: Status × 5HTT genotype interaction effect. (A) TBSS 2 × 2 ANOVA results showing a status × genotype interaction in a cluster (cluster 6) in the right parietal WM. (B) Results from the 2-way ANOVA showing that dominant l/l animals had lower FA than s-variant ones, and l/l subordinates had higher FA than low */s. (C) Probabilistic tractography: Group probability map (subject-level threshold at 1% of the robust mean intensity with distance correction applied) showing that the cluster of significant voxels in (A) corresponds to short-range, parietal cortico-cortical “U” fibers in the right hemisphere. Left-to-right images represent sagittal, coronal, and axial planes. The composite images (group probability maps) show voxels that were common to at least 15 (40%) animals. Colors represent the percentage of subjects that showed connectivity with that voxel in the single-subject analysis: 40–60% animals in yellow, 60–80% in orange, and 80–100% in red. ips: intraparietal sulcus.
Figure 6.
Results of the DTI analysis: status × 5HTT genotype interaction effect. (A) TBSS 2 × 2 ANOVA results showing a status × genotype interaction effect in a cluster (cluster 7) in the left prefrontal WM. (B) Results from the 2-way ANOVA showing that dominant l/l animals had lower FA than s-variant ones, and l/l subordinates had higher FA than low */s. (C) Probabilistic tractography: group probability map (subject-level threshold at 1% of the robust mean intensity with distance correction applied) showing that the cluster of significant voxels in (A) corresponds to the left, short-range, prefrontal WM tracts connecting dorsolateral,ventrolateral, and dorsomedial PFC in the left hemisphere and bilaterally via the genu of the corpus callosum in 40–60% of subjects. Left-to-right images represent sagittal, coronal, and axial planes. The composite images (group probability maps) show voxels that were common to at least 15 (40%) animals. Colors represent the percentage of subjects that showed connectivity with that voxel in the single-subject analysis: 40–60% animals in yellow, 60–80% in orange, and 80–100% in red. dlPFC: dorsolateral PFC; vlPFC: ventrolateral PFC.
Status and 5HTT Genotype Effects on RD and AD Measures
We used a 2-way ANOVA to analyze status and 5HTT genotype effects on RD and AD in those clusters with significant FA differences and found 2 different patterns of results: (1) In most of the clusters, the significant FA effects (status, 5HTT genotype, or status × genotype) were accompanied by opposite effects on RD, but not on AD; thus, in clusters 1–3, where a main status effect of FA was detected (with subordinates showing increased FA), an opposite effect was found on RD, so that subordinate animals showed lower RD than dominants (cluster 1, F(1,31) = 4.445, P = 0.043; cluster 2, F(1,31) = 10.025, P = 0.003; cluster 3, F(1,31) = 8.993, P = 0.005), without differences in AD; similarly, in cluster 4, where a main 5HTT genotype effect was detected on FA (with s-variants showing higher FA than l/l animals), the opposite genotype effect was detected on RD, so that s-allele carriers showed lower RD than l/l animals (F(1,31) = 18.272, P = 0.0000169), without differences in AD; furthermore, in clusters 6 and 7, which showed status × genotype effects on FA (with dominant l/l animals showing lower FA than s-variant animals, while the opposite was true for low-ranking animals), an opposite interaction effect was detected on RD, so that dominant l/l subjects showed higher RD than s/* and the opposite was true for low-ranking animals (cluster 6: F(1,31) = 8.369, P = 0.007; cluster 7: F(1,31) = 4.535, P = 0.041), with no effects detected on AD Only cluster 5 showed a different pattern of effects, where the status × genotype interaction effect on FA (with dominant l/l animals showing lower FA than s-variant animals, while the opposite was true for low-ranking animals) was accompanied by similar effects on AD, but no effects at all on RD, so that high-ranking, l/l animals had lower AD than high s/*, and the opposite was true for low-ranking animals (F(1,31) = 8.305, P = 0.007).
Test–Retest Analysis of Stability and Replicability of DTI Measures
No significant test–retest differences were detected for DTI measures in any of the 7 identified clusters described above. This analysis suggests that the measures are stable and reproducible, and that the experimental group differences reported are not spurious.
Behavioral Data
Group Social Observations: Status and 5HTT Genotype Associations
Regression analyses demonstrated that higher-ranking juveniles, indeed, direct higher rates of aggressive behaviors toward others (r = −0.46, P < 0.01), whereas rates of aggression received (r = −0.35, P < 0.03) and submissive behaviors emitted increased significantly with more subordinate status (r = 0.34, P = 0.04). The degree by which social rank predicted these behaviors was not affected by adding the 5HTT genotype to the regression model.
Emotionality Testing: PCA
Four components were identified in the HI paradigm PCA (Table 1): Fear, submission, anxiety, and distress. The first component accounted for 22.1% of the variance and was labeled as “fear” based on the positive loading of freezing during the PR condition, behavior reflecting fearfulness/anxiety in this task (Kalin and Shelton 1989). In addition, turn away during the ST condition loaded negatively. The second component accounted for 19.1% of the variance and included lipsmack and gaze avert during the ST condition, both of which loaded positively and indicate “submissive” or pacificatory behaviors used to neutralize a potentially threatening interaction (Altmann 1962). The third component (labeled “anxiety”) accounted for 17.5% of the variance and included anxiety-like behaviors (yawn, scratch; Schino et al. 1996) and toothgrinding (also reflecting distress and anxiety; Williamson et al. 2003; Machado and Bachevalier 2006) during the ST condition, all with positive loadings. The fourth component (labeled as “distress”) accounted for 13.6% of the variance and included coo vocalizations and threats during ST (both with negative loadings), a positive score in this component indicating low frequencies of these behaviors and thus lower reactivity and distress based on previous reports of PCA loadings for rhesus monkeys (Williamson et al. 2003).
Three components were identified in the AA task PCA for behaviors exhibited in response to the most fear-evoking object (motorized pig) (Table 2): Anxious aggression, vigilance/anxiety, and impulsivity. The first component accounted for 30.2% of the variance and included threat object (positive loading), look away from object (functioning to avoid interaction with the object; positive loading), and bite object (negative loading). While threat is considered an aggressive behavior for this species (Altmann 1962), in the context of the AA and other behaviors it may reflect more defensive aggression (Meunier et al. 1999) and we considered positive scores on this component as indicating “anxious aggression.” The second component accounted for 20.5% of the variance and included locomotion (positive loading) and visually inspecting the object (negative loading), thus a high score on this component indicates high exploration and low “vigilance/anxiety.” The third component accounted for 15.3% of the variance and included latency to take the jellybean and latency to explore the testing box, both with negative loadings, thus a high score on this component indicates shorter latencies indicating increased “impulsivity.”
Behavioral Correlations with FA Measures
Mean FA in significant clusters was associated with specific behaviors from the social group and from component scores of the HI and AA PCAs, all showing moderate-to-large effect sizes (Table 3). Mean FA in cluster 1 (left mPFC tracts) positively correlated with HI-component-1 (fear) and with submissive behavior in the social group, so that animals with higher FA were more fearful and submissive. Mean FA in cluster 2 (left cortico-thalamic tracts) was also positively correlated with social submission. Mean FA in cluster 4 (left PLIC) was positively correlated with group observations of submissive behavior and negatively with play behavior. FA in cluster 5 (left MLF) positively correlated with HI-component-2 (submission toward HI). FA in cluster 6 (right parietal “U” fibers) negatively correlated with HI-component-3 (anxiety) and AA-component-1 (anxious aggression), so that animals with higher FA showed less anxiety-related behaviors. FA in cluster 7 (left short-range prefrontal fibers) negatively correlated with AA-component-1 (anxious aggression), and positively with HI-component-2 (submissive behaviors toward HI), with animals with higher FA exhibiting less anxious aggression and more submission toward HI.
Table 3.
Pearson correlations of mean FA in regions where significant effects of status and/or 5HTT genotype with behavioral data collected in the social group and factors derived from the PCA analysis of emotionality tests (HI and AA)
| Cluster of significant FA voxels | Behavior/components |
R df = 33 |
P-value | Cohen's d |
|---|---|---|---|---|
| Cluster 1, main status effect | HI-component-1, fear | 0.364 | 0.044 | 0.782 |
| Submissive behaviors in the group | 0.389 | 0.023 | 0.845 | |
| Cluster 2, main status effect | Submissive behaviors in the group | 0.346 | 0.045 | 0.738 |
| Cluster 4, main 5HTT genotype effect | Submissive behaviors in the group | 0.438 | 0.01 | 0.974 |
| Play in the group | −0.349 | 0.043 | −0.745 | |
| Cluster 5, status by genotype effect | HI-component-2, submissive | 0.422 | 0.018 | 0.931 |
| Cluster 6, status by genotype effect | AA-component-1, anxious aggression | −0.525 | 0.002 | −1.234 |
| HI-component-3, anxiety | −0.616 | 0.0002 | −1.564 | |
| Cluster 7, status by genotype effect | AA-component-1, anxious aggression | −0.41 | 0.018 | −0.899 |
| HI-component-2, submissive | 0.439 | 0.013 | 0.977 |
Note: Shown are P-values associated with these correlations. Applying a Sidak correction yields a critical P-value of 0.005. However, all effects sizes, reflected in the magnitude of the correlation coefficient as well as the associated Cohen's d value, indicate medium-to-large effects.
Additional correlational analyses were performed to better understand how behaviors in different contexts related to each other as well as to relative dominance ranks within the social groups. HI-component-1 (fear) was negatively correlated with social aggression (r = −0.445, P = 0.014, Cohen's d = −0.994), suggesting that animals that were more fearful during the HI displayed less social aggression in their groups (i.e. subordinate animals). Indeed, more subordinate status predicted higher scores on this fear-related component (r = 0.343, P = 0.059, Cohen's d = 0.730). In addition, HI-component-3 (anxiety) correlated positively with AA-component-1 (anxious aggression; r = 0.613, P = 0.0003, Cohen's d = 1.552), but negatively with submissive behaviors in the social group (r = −0.400, P = 0.029, Cohen's d = 0.873), so that less anxiety during the HI task was associated with less anxious aggression during the AA, and with more submission in the group. Finally, more subordinate status predicted higher scores on the HI-submissive component (r = 0.390, P = 0.030, Cohen's d = 0.847), consistent with higher rates of lip-smacking during the ST condition of the HI (r = 0.459, P = 0.005, Cohen's d = 1.033). However, subordinate status also predicted a higher frequency of threats (r = 0.326, P = 0.053, Cohen's d = 0.690), whereas more dominant status predicted longer duration of freezing during the ST condition of the HI (r = −0.358, P = 0.032, Cohen's d = −0.767), reflecting two different strategies for dealing with a threatening situation, depending on social rank.
Cortisol Data: Associations with Status, 5HTT Genotype, and FA Measures
Relative rank was also included in regression analyses to determine its relation to HPA axis activity (baseline and stress-induced cortisol). More subordinate status significantly predicted higher baseline cortisol concentrations (r = 0.37, P = 0.04, Cohen's d = 0.797), but a more blunted cortisol stress response to the social separation test (r = −0.44, P < 0.01, Cohen's d = −0.980). The degree by which social rank predicted these phenotypes was not affected by adding the 5HTT genotype to the regression model.
When examining the associations between mean FA in each significant cluster and cortisol measures (baseline and stress-induced increases), no significant correlations were found in any brain regions.
Discussion
To the best of our knowledge, this is the first DTI study to examine the relationship between social subordination and brain WM tract integrity in association with socioemotional behavior, and its modulation by 5HTT polymorphisms in a prepubertal female macaque model. At this juvenile stage of development, social rank-related differences were found in tracts connecting the prefrontal, motor, association, and sensory processing brain regions, with subordinate females showing higher WM integrity than dominant animals, sometimes modulated by the 5HTT genotype. Microstructural differences in these tracts could affect emotional and sensory processing, leading to increased emotional reactivity in subordinates, as supported by the associations detected between FA and submissive and fear behaviors, and consistent with reports in human conditions (Kim and Whalen 2009; Noriuchi et al. 2010). In most of the brain regions showing differences in FA, a measure of WM tract integrity, opposite differences were detected in RD, but not in AD, suggesting that differences in tract integrity likely involve differences in myelin (Song et al. 2002, 2003; Keller and Just 2009; Zhang et al. 2009; Bennett et al. 2010; Hu et al. 2011; Choe et al. 2012). The one exception was the EC (MLF), where FA differences were accompanied by differences in AD, but not in RD, indicating different mechanisms involved (e.g. differences in axon density/caliber, cytoskeletal organization; Song et al. 2002; Kumar et al. 2010, 2012; Choe et al. 2012). While our study design cannot disentangle genetic or prenatal effects from the postnatal experience of social subordination, these findings provide important evidence that differences in social status up to the prepubertal period are associated with differences in brain WM structure linked to behavioral function.
At the behavioral level, subordinate juveniles received more aggression from higher-ranking animals and exhibited more submission than dominants, consistent with the behavioral phenotype reported in the literature for preadult low-ranking rhesus females (Bernstein and Ehardt 1985). In adults, the unpredictable, continual harassment experienced by subordinate females results in stress-related phenotypes, including hypercortisolemia and HPA dysregulation (Shively et al. 1997; Jarrell et al. 2008; Kaplan et al. 2010; Michopoulos et al. 2012). Consistent with those reports, the juvenile subordinates in this study also showed elevated basal cortisol, suggesting that subordination during the prepubertal period is, indeed, a psychosocial stress that results in neuroendocrine signs of chronic stress already at this young age.
Neuroimaging analyses showed that subordinate females had increased WM tract structural integrity (increased FA) compared with higher-ranking animals in 3 regions. These included mPFC WM involving local, intra-, and interhemispheric tracts, as well as WM along the dorsomedial wall which involved: (1) Fibers interconnecting frontal regions in the supplementary motor, premotor, and primary motor cortex, and these frontal motor areas with the somatosensory cortex and (2) cortico-thalamic tracts connecting somatosensory and primary motor cortices with the thalamus, which transform sensory information into action and are also potentially part of the mirror neuron or motor representation systems (Rizzolatti and Fadiga 1998). The fact that the effects of social subordination were limited to limbic prefrontal and frontal tracts suggests specificity of rank-related differences, that is, these were not widespread effects. This observation is important given that PFC and frontal regions are particularly vulnerable to early life stress in primates (Sanchez et al. 1998, 2001; Kaufman and Charney 2001; De Bellis et al. 2002; Teicher et al. 2003; Spinelli et al. 2009), and WM alterations have been reported in these regions in children and nonhuman primates exposed to other forms of chronic stress during development (Eluvathingal et al. 2006; Paul et al. 2008; Coplan et al. 2010; Govindan et al. 2010). A caveat of our study design, though, is that it cannot separate the contribution of postnatal social stress experience from potential genetic or maternal experiential prenatal effects on the structural differences observed in subordinates. However, consistent with the possibility that subordination effects on brain WM could be a consequence of chronic stress (as supported by the elevated cortisol levels in subordinates), region-specific effects of chronic stress have been reported specifically on mPFC neuronal structure, including retraction of dendritic fields and synaptic loss, in primates and rodents (Joels et al. 2007; Arnsten 2009). These smaller dendritic fields with fewer synapses can actually result in electrically more compact neurons with enhanced neuronal output due to reduced integration of input (Joels et al. 2007; Thomason and Thompson 2011) and, consequently, in less flexible/versatile functional and behavioral responses. Our findings of increased FA in subordinates' local mPFC tracts potentially involved in emotional regulation (Drevets 2001; Arnsten 2009), as well as in frontal and cortico-thalamic tracts that transform sensory information into action (Rizzolatti and Fadiga 1998), and the association of this increased tract integrity with higher fear and submission—behaviors associated with lower rank—would be consistent with this notion of decreased behavioral flexibility. That is, these brain structural differences in subordinates (increased tract integrity potentially due to increased myelination) could lead to increased conduction speed along mPFC and frontal tracts (Paus 2010), which would support prepotent behavioral responses potentially adaptive for survival of subordinates in their social groups (i.e. higher submission and fear behaviors). Although these interpretations are speculative because our design does not allow us to establish causality between brain and behavioral differences, it is possible that the brain structural phenotypes in subordinates function to facilitate attending and reacting to cues to successfully navigate the social environment and to minimize the risk of aggression from more dominant animals (Silk 2002). These speculations are consistent with reports of increased FA in the ventromedial PFC of squirrel monkeys exposed to early life stress, an observation that was interpreted as “adaptive,” preparing the individual to cope with challenges in their environment (Katz et al. 2009). Altogether these findings suggest that different positions in the social hierarchy likely lead to different female strategies to deal with threatening or uncertain situations, potentially related to brain structural differences.
Structural effects of social subordination have been recently reported in prefrontal GM of adult male rhesus monkeys by MRI, as well as in the STS and amygdala (Sallet et al. 2011), and there is evidence that low SES, a complex mix of chronic social stress and limited access to resources, results in reduced prefrontal and frontal volumes and gyrification, associated with socioemotional and cognitive deficits in children (Hackman and Farah 2009; Jednoróg et al. 2012). Our findings provide further evidence that structural differences associated with social status in primates extend to brain WM as well, specifically affecting the integrity of medial prefrontal and frontal tracts in developing females. Consistent with our findings of a positive correlation between FA in these tracts and fear, increased FA in prefrontal regions involved in emotional control has also been linked to increased emotional reactivity, in particular anxiety, in human studies (Han et al. 2008; Phan et al. 2009; Zhang et al. 2012). Thus, dispelling the common misconception that higher FA is better functionally, altogether this evidence supports the recent view (e.g., Thomason and Thompson 2011) that higher FA in developing individuals may reflect less cognitive flexibility and could potentially impact neurocircuit organization during learning, resulting in prepotent responses potentially adaptive to that specific environment, as suggested for subordinates in our study.
In contrast to the correlations detected between status-related differences in tract integrity and behavior, no associations were found with the levels of the stress hormone cortisol, despite being elevated in subordinate juveniles. This suggests that rank-related FA differences are not mediated by the concurrently high basal cortisol concentrations. Future studies will need to elucidate whether subordination-related brain structural effects are a consequence of chronic exposure to high cortisol prior to this prepubertal period, or whether they are mediated by other biological or genetic factors.
We also examined how 5HTT polymorphisms modulated social rank effects. The effects of 5HTT allelic variation on emotional reactivity are thought to reflect brain structural differences caused by exposure to different levels of 5HT during development (Whitaker-Azmitia et al. 1996), since adult levels of brain transporters are similar (Willeit et al. 2001; Shioe et al. 2003; Jedema et al. 2010; Murthy et al. 2010). In fact, there is evidence that s-allele carriers have reduced GM volume in mPFC, frontal, temporal, and parietal cortices and amygdala (Canli et al. 2005; Pezawas et al. 2005; Jedema et al. 2010), and reduced FA in the uncinate fasciculus (UF, connecting the ventromedial PFC with temporal regions) (Pacheco et al. 2009), which could underlie activity differences in these limbic circuits (Hariri et al. 2002; Rao et al. 2007). In this study, we did not detect genotype effects on UF, but rather on the PLIC, with s-variant animals showing higher FA than l/l subjects, and higher FA being associated with increased social submission and less play, suggesting a potential effect on social interactions. Tractography identified the tracts affected as cortical descending pathways, including the corticospinal and corticorubral tracts, involved in the voluntary motor control of upper and lower limbs for locomotion and manipulation of objects, as well as of posture and muscle tone control (Humphrey et al. 1984). Reduced FA in the PLIC has been associated with motor impairments (Sach et al. 2004; Puig et al. 2011) and hypotonia (Yamada et al. 2006) in clinical conditions. Based on this evidence, the higher FA in s-variants could result in higher muscle tone and readiness to locomote associated with their higher submission, but the association with reduced play is difficult to explain.
We also found social status by 5HTT genotype interaction effects in several regions, with opposite genotype effects in subordinates and higher-ranking animals. These included the left EC, identified by tractography as the left MLF, a long association fiber pathway connecting the inferoparietal area with cortical regions in the STS and STG in macaques (Schmahmann and Pandya 2006; Schmahmann et al. 2007). In this cluster, higher FA was associated with higher gaze-averting/submission rates toward the HI. The MLF is involved in the processing of auditory spatial information in macaques (Schmahmann and Pandya 2006; Schmahmann et al. 2007), with left STG dominance reported for processing of conspecific vocalizations in fMRI studies (Joly et al. 2012). Increased FA in left parieto-temporal tracts including the MLF, involved in auditory processing, has been associated with increased language comprehension in humans (Wong et al. 2011). Based on all this evidence, the higher MLF FA shown by s-variant than l/l females, at least in higher-ranking animals, could potentially result in faster, more efficient, cortical auditory processing.
Additional interaction effects were found in “U” parietal tracts (cortico-cortical association fibers connecting adjacent gyri and involved in sensory information integration) and short-range tracts connecting PFC regions within and between hemispheres. Although the opposite effects of genotype on subordinate and dominant animals are difficult to interpret, the negative correlation between FA in these tracts and anxiety during the HI task is interesting given that similar associations have been reported between microstructural WM abnormalities (low FA) in frontolimbic and parietal regions and psychopathology in some clinical populations, also modulated by the 5HTT genotype (Alexopoulos et al. 2009).
It is important to note the limitations of this study. First of all, our research design does not allow to demonstrate causality between social rank, brain WM microstructure, and behavioral differences, because it cannot disentangle whether the WM structural differences in subordinates are the result of postnatal social experience, maternal prenatal experience (e.g., prenatal stress; Wadhwa 2005), or transgenerational transmission of brain and behavioral phenotypes via genetic or epigenetic mechanisms (Nelson and Nadeau 2010). Because rhesus monkey offspring assume their mother's social rank, effects of postnatal social experience are potentially confounded by their mother's prenatal experience. Thus, if the mothers experienced social stress during gestation, this could alter brain maturation and lead to behavioral deficits later in life (Seckl 2008; Harris and Seckl 2011). Furthermore, our recent report that social status differences in adult females' peripheral gene expression are associated with differences in DNA methylation patterns (Tung et al. 2012) supports the possibility that some of the social rank effects on brain and behavioral/neuroendocrine phenotypes are mediated via epigenetic modifications, which could also be transmitted from mothers to daughters. An additional limitation is that, because we only studied the animals at one age point, we cannot determine, either, when the social rank- or 5HTTgenotype-related differences emerged or whether they reflect differences in developmental trajectories. Given all these caveats, the findings of this study should be interpreted with caution. While future studies need to determine the unique contributions of genetic/epigenetic factors versus pre- and postnatal experience on social rank-related brain differences, evidence from our group and others support that at least some of the systems studied are very plastic and sensitive to social experience, including social rank changes during adulthood. For example, rank rearrangement results in distinct behavioral and physiological phenotypes (Shively et al. 1997) and genome-wide expression patterns (Tung et al. 2012) that match a female's current, but not former, rank. The central question for future studies is whether brain structure exhibits similar social experience-induced plasticity.
In addition to the study design limitations described above, technical limitations of the DTI neuroimaging procedures also need to be noted. We performed a combined analysis of FA, RD, and AD, because these DTI properties provide good overall indices of changes in tract structural integrity and the underlying mechanisms due to their strong relationships with histological measures of WM microstructure (i.e. myelin thickness, axon density/caliber/diameter, fiber spread, etc.). However, some of those relationships remain controversial (Wheeler-Kingshott and Cercignani 2009) and our level of analysis cannot truly demonstrate the microstructural/cellular processes involved in the social status and/or 5HTT genotype differences detected, or their actual biological/physiological meaning besides the behavioral correlations reported here. These questions are particularly complex in developing primates, where it is not clearly understood whether ongoing WM maturation processes such as myelination and axon pruning may contribute to DTI properties in a different way than in adults (Paus 2010). Finally, the FA thresholding used for the TBSS method excluded small fiber tracts from the analysis that could also be affected, which is a methodological limitation due to the generation of potential false negatives.
Taking into account all these potential mitigating factors, our data nonetheless suggest the existence of differences in brain structural connectivity between subordinate and higher-ranking prepubertal females, in part modulated by 5HTT polymorphisms. Structural differences in the main tracts affected, which connect the prefrontal, motor, association, and sensory processing brain regions, were associated with specific behavioral phenotypes, particularly submissive, fear, and anxious behaviors that vary as a function of social status. Ongoing longitudinal analyses will determine if these rank-related differences persist into adolescence (a period of important brain circuitry reorganization; Lebel et al. 2008; Giedd and Rapoport 2010; Knickmeyer et al. 2010; Shi et al. 2012) and adulthood, while planned studies will begin to examine whether prenatal and genetic/epigenetic factors synergize with postnatal social experiences to influence neurobehavioral development.
Funding
This work was supported by the National Institutes of Health (National Institute of Mental Health: grant numbers MH079100, P50 MH078105, and MH078105-S1, MH091645, and F31 MH086203 to B.R.H., F31 MH085445 to V.M.; National Institute of Child Health & Human Development: HD055255) and the National Center for Research Resources (grant number P51RR165—YNPRC base grant—currently supported by the Office of Research Infrastructure Programs/OD—grant number OD P51OD11132). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institute of Child Health & Human Development or the National Institutes of Health.
Notes
We would like to thank Jennifer Whitley, Marta Checchi, Shannon Bounar, Erin Olmoguez, and the entire staff of the Yerkes National Primate Research Center Field Station for their invaluable help in collecting the data presented. We would also like to thank Dr Todd Preuss for his help in identifying the affected tracts. Conflict of Interest: None declared.
References
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Glatt CE, Latoussakis V, Kelly RE, Jr, Kanellopoulos D, Klimstra S, Lim KO, Young RC, et al. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord. 2009;119:132–141. doi: 10.1016/j.jad.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann N Y Acad Sci. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol Behav. 2010;101:446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–5. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Canu E, Willette A, Kastman EK, McLaren DG, Kosmatka KJ, Xu G, Field AS, Colman RJ, Coe CL, et al. Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol Aging. 2011;32:2319. doi: 10.1016/j.neurobiolaging.2010.04.008. e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bennett I, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Ehardt CL. Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. J Comp Psychol. 1985;99:115–132. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974;62:304–311. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behav Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci USA. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Choe AS, Stepniewska I, Colvin DC, Ding Z, Anderson AW. Validation of diffusion tensor MRI in the central nervous system using light microscopy: quantitative comparison of fiber properties. NMR Biomed. 2012;25:900–908. doi: 10.1002/nbm.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Abdallah CG, Tang CY, Mathew SJ, Martinez J, Hof PR, Smith EL, Dwork AJ, Perera TD, Pantol G, et al. The role of early life stress in development of the anterior limb of the internal capsule in nonhuman primates. Neurosci Lett. 2010;480:93–96. doi: 10.1016/j.neulet.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Chugani DC, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. J Psychiatry Neurosci. 2012;37:37–45. doi: 10.1503/jpn.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31:359–367. [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cereb Cortex. 2010;20:561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand A, McCormack KM, Maestripieri D, Sanchez MM. Effects of infant maltreatment on emotional and HPA axis reactivity in juvenile rhesus macaques. 35th Annual Meeting of the Society for Neuroscience; Washington (DC). 2005. [Google Scholar]
- Graves FC, Wallen K. Androgen-induced yawning in rhesus monkey females is reversed with a nonsteroidal anti-androgen. Horm Behav. 2006;49:233–236. doi: 10.1016/j.yhbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Keifer OP, Jr, Magnuson ME, Choi DC, Majeed W, Keilholz S, Ressler KJ. A DTI tractography analysis of infralimbic and prelimbic connectivity in the mouse using high-throughput MRI. Neuroimage. 2012;63:800–811. doi: 10.1016/j.neuroimage.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Magnuson M, Majeed W, Keifer OP, Jr, Davis M, Ressler KJ, Keilholz S. Mapping of the mouse olfactory system with manganese-enhanced magnetic resonance imaging and diffusion tensor imaging. Brain Struct Funct. 2012;218(2):527–537. doi: 10.1007/s00429-012-0413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I . J Theor Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Han DH, Renshaw PF, Dager SR, Chung A, Hwang J, Daniels MA, Lee YS, Lyoo IK. Altered cingulate white matter connectivity in panic disorder patients. J Psychiatr Res. 2008;42:399–407. doi: 10.1016/j.jpsychires.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Hecht EE, Gutman DA, Preuss TM, Sanchez MM, Parr LA, Rilling JK. Process versus product in social learning: comparative diffusion tensor imaging of neural systems for action execution-observation matching in macaques, chimpanzees, and humans. Cereb Cortex. 2013;23(5):1014–1024. doi: 10.1093/cercor/bhs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin transporter promoter polymorphism (5-HTTLPR) in adult female rhesus monkeys (Macaca mulatta) Endocrine. 2007;31:202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes Brain Behav. 2003;2:332–335. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Geng F, Tao L, Hu N, Du F, Fu K, Chen F. Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp. 2011;32:10–21. doi: 10.1002/hbm.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey DR, Gold R, Reed DJ. Sizes, laminar and topographic origins of cortical projections to the major divisions of the red nucleus in the monkey. J Comp Neurol. 1984;225:75–94. doi: 10.1002/cne.902250109. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, et al. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15:512–522, 446. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Dehaene-Lambertz G, Ramus F. The influence of socioeconomic status on children's brain structure. PLoS One. 2012;7(8):e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Joly O, Ramus F, Pressnitzer D, Vanduffel W, Orban GA. Interhemispheric differences in auditory processing revealed by fMRI in awake rhesus monkeys. Cereb Cortex. 2012;22:838–853. doi: 10.1093/cercor/bhr150. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson ME, Manuck SB, Clarkson TB. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25:3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Liu C, Schaer M, Parker KJ, Ottet MC, Epps A, Buckmaster CL, Bammer R, Moseley ME, Schatzberg AF, et al. Prefrontal plasticity and stress inoculation-induced resilience. Dev Neurosci. 2009;31:293–299. doi: 10.1159/000216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, Gilmore JH. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20:1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Harper RM. Rostral brain axonal injury in congenital central hypoventilation syndrome. J Neurosci Res. 2010;88:2146–2154. doi: 10.1002/jnr.22385. [DOI] [PubMed] [Google Scholar]
- Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Regional brain axial and radial diffusivity changes during development. J Neurosci Res. 2012;90:346–355. doi: 10.1002/jnr.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Rosenbluth J. Role of myelination in the development of a uniform olivocerebellar conduction time. J Neurophysiol. 2003;89:2259–2270. doi: 10.1152/jn.00922.2002. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring's behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res. 2006;175:90–95. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, van der Kouwe A, Kennedy DN, Hodge SM, Dale AM, Benner T, Wald LL, Wu O, Tuch DS, et al. Frontal connections and cognitive changes in normal aging rhesus monkeys: a DTI study. Neurobiol Aging. 2007;28:1556–1567. doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Kastman EK, Bendlin BB, Johnson SC. Rhesus macaque brain morphometry: a methodological comparison of voxel-wise approaches. Methods. 2010;50:157–165. doi: 10.1016/j.ymeth.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. Neuroimage. 2009;45:52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res. 2010;1348:156–164. doi: 10.1016/j.brainres.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009;81:1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012;37:1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Murthy NV, Selvaraj S, Cowen PJ, Bhagwagar Z, Riedel WJ, Peers P, Kennedy JL, Sahakian BJ, Laruelle MA, Rabiner EA, et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. Neuroimage. 2010;52:50–54. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Nelson VR, Nadeau JH. Transgenerational genetic effects. Epigenomics. 2010;2:797–806. doi: 10.2217/epi.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sovell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, Kamio Y. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 2010;1362:141–149. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J Neurosci. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav Immun. 2009;23:286–293. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, Bruce S, Williams LM, Richard CC, Cohen RA, et al. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat. 2008;4:193. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Blasco G, Daunis IEJ, Prados F, Remollo S, Prats-Galino A, Soria G, Boada I, Castellanos M, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR. Am J Neuroradiol. 2011;32:857–863. doi: 10.3174/ajnr.A2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GM, Kaercher KA, Brodkin ES, Detre JA, Farah MJ. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62:600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Reardon LE, Leen-Feldner EW, Hayward C. A critical review of the empirical literature on the relation between anxiety and puberty. Clin Psychol Rev. 2009;29:1–23. doi: 10.1016/j.cpr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L. Grasping objects and grasping action meanings: the dual role of monkey rostroventral premotor cortex (area F5) Novartis Found Symp. 1998;218:81–95. doi: 10.1002/9780470515563.ch6. discussion 95–103. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, Buchel C, Weiller C. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain. 2004;127:340–350. doi: 10.1093/brain/awh041. [DOI] [PubMed] [Google Scholar]
- Sade D. Determinants of dominance in a group of free ranging rhesus monkeys. In: Altmann SA, editor. Social communication amoung primates. Chicago (IL): University of Chicago Press; 1967. pp. 99–114. [Google Scholar]
- Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. London (UK): Academic Press; 2007. [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O'Reilly JX, Jbabdi S, Croxson PL, Jenkinson M, Miller KL, Rushworth MF. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford (UK): Oxford University Press; 2006. [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Carpenter DM, Fong SG, Murray EA, Tang CY, Hof PR, Rapp PR. Alterations of white matter tracts following neurotoxic hippocampal lesions in macaque monkeys: a diffusion tensor imaging study. Hippocampus. 2010;20:906–910. doi: 10.1002/hipo.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Short SJ, Knickmeyer RC, Wang J, Coe CL, Niethammer M, Gilmore JH, Zhu H, Styner MA. Diffusion tensor imaging-based characterization of brain neurodevelopment in primates. Cereb Cortex. 2012;23(1):36–48. doi: 10.1093/cercor/bhr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, Hirano M, Shinohara M, Kagami M, Okubo Y, et al. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Silk JB. Practice random acts of aggression and senseless acts of intimidation: the logic of status contests in social groups. Evol Anthropol. 2002;11:221–225. [Google Scholar]
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33:312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Gene-environment interactions and the neurobiology of social conflict. Ann N Y Acad Sci. 2003;1008:132–139. doi: 10.1196/annals.1301.014. [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci USA. 2012;109:10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Villringer A, Ragert P. Learning-related gray and white matter changes in humans: an update. Neuroscientist. 2011;18(4):320–325. doi: 10.1177/1073858411419048. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Troisi A, Schino G, D'Antoni M, Pandolfi N, Aureli F, D'Amato FR. Scratching as a behavioral index of anxiety in macaque mothers. Behav Neural Biol. 1991;56:307–313. doi: 10.1016/0163-1047(91)90469-7. [DOI] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci USA. 2012;109(17):6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Weber K, Rockstroh B, Borgelt J, Awiszus B, Popov T, Hoffmann K, Schonauer K, Watzl H, Propster K. Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry. 2008;8:63. doi: 10.1186/1471-244X-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About "axial" and "radial" diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A, Asenbaum S, Tauscher J, Fuchs K, Sieghart W, Hornik K, et al. No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Biol Psychiatry. 2001;50:8–12. doi: 10.1016/s0006-3223(00)01123-9. [DOI] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, McLaren DG, Canu E, Kastman EK, Kosmatka KJ, Xu G, Field AS, Alexander AL, Colman RJ, et al. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. Neuroimage. 2010;51:987–994. doi: 10.1016/j.neuroimage.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Coe CL, Colman RJ, Bendlin BB, Kastman EK, Field AS, Alexander AL, Allison DB, Weindruch RH, Johnson SC. Calorie restriction reduces psychological stress reactivity and its association with brain volume and microstructure in aged rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):903–916. doi: 10.1016/j.psyneuen.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DE, Coleman K, Bacanu SA, Devlin BJ, Rogers J, Ryan ND, Cameron JL. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: a preliminary report. Biol Psychiatry. 2003;53:284–291. doi: 10.1016/s0006-3223(02)01601-3. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Bounar S, Godfrey J, Michopoulos V, Higgins M, Sanchez M. Social and emotional predictors of the tempo of puberty in female rhesus monkeys. Psychoneuroendocrinology. 2013;38(1):67–83. doi: 10.1016/j.psyneuen.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Collins DC. Ontogeny of luteinizing hormone secretion and first ovulation in seasonal breeding rhesus monkeys. Endocrinology. 1986;118:293–301. doi: 10.1210/endo-118-1-293. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Kinkead B. Gene-environment interactions, not neonatal growth hormone deficiency, time puberty in female rhesus monkeys. Biol Reprod. 2008;78:736–743. doi: 10.1095/biolreprod.107.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FC, Chandrasekaran B, Garibaldi K, Wong PC. White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. J Neurosci. 2011;31:8780–8785. doi: 10.1523/JNEUROSCI.0999-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yamada K, Matsuzawa H, Uchiyama M, Kwee IL, Nakada T. Brain developmental abnormalities in Prader-Willi syndrome detected by diffusion tensor imaging. Pediatrics. 2006;118:e442–e448. doi: 10.1542/peds.2006-0637. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biol Reprod. 2005;72:1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jones M, DeBoy CA, Reich DS, Farrell JA, Hoffman PN, Griffin JW, Sheikh KA, Miller MI, Mori S, et al. Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J Neurosci. 2009;29:3160–3171. doi: 10.1523/JNEUROSCI.3941-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li WH, Shu N, Zheng HR, Zhang ZJ, Zhang Y, He Z, Hou CL, Li ZX, Liu J, et al. Increased white matter integrity of posterior cingulate gyrus in the evolution of post-traumatic stress disorder. Acta Neuropsychiatr. 2012;24:34–42. doi: 10.1111/j.1601-5215.2011.00580.x. [DOI] [PubMed] [Google Scholar]