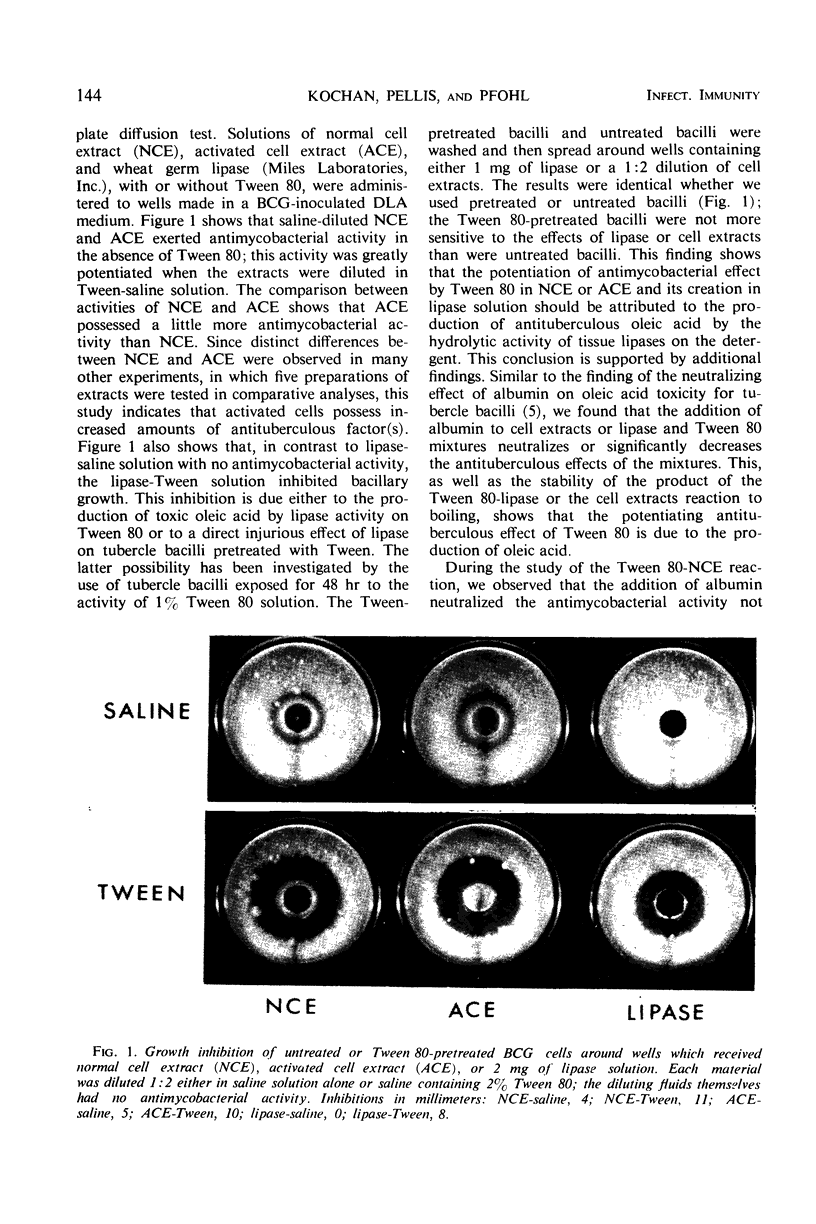
Abstract
Fractions prepared from normal and activated liver cells were tested for the antimycobacterial activity by the agar plate diffusion test. Results showed that lysosome extracts of normal and activated cells exerted no antibacterial activity, cell extracts and lysosome membranes exerted some activity, and cell membranes exerted the strongest activity. The active materials of activated cells exerted stronger antituberculous activity than the corresponding materials of normal cells. Degrees of the antimycobacterial activity of various cell fractions showed a close correlation with the amounts of nonesterified fatty acids. This correlation, as well as other data, suggested that the antimycobacterial activity of cell fractions was caused by toxic fatty acids which were produced during the hydrolysis of lipoporteins or phospholipids by the activity of tissue lipases. The relationship of these findings to the mechanism of cellular immunity is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C. A., Draper P., Hart P. D. Mycobacteria and lysosomes: a paradox. Nature. 1969 Feb 15;221(5181):658–660. doi: 10.1038/221658a0. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai K., Kondo E. A suggested role of the lysosomal membrane as a part of the defence mechanism against tuberculous infection. Jpn J Med Sci Biol. 1970 Oct;23(5):295–302. doi: 10.7883/yoken1952.23.295. [DOI] [PubMed] [Google Scholar]
- Kanai K., Kondo E. Separation and properties of "in vivo grown tubercle bacilli" associated with the lysosomal membrane. Jpn J Med Sci Biol. 1970 Oct;23(5):303–314. [PubMed] [Google Scholar]
- Kochan I., Cahall D. L., Golden C. A. Employment of tuberculostasis in serum-agar medium for the study of production and activity of Mycobactin. Infect Immun. 1971 Aug;4(2):130–137. doi: 10.1128/iai.4.2.130-137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Golden C. A., Bukovic J. A. Mechanism of tuberculostasis in mammalian serum. II. Induction of serum tuberculostasis in guinea pigs. J Bacteriol. 1969 Oct;100(1):64–70. doi: 10.1128/jb.100.1.64-70.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I. Mecahnism of tuberculostasis in mammalian serum. I. Role of transferrin in human serum tuberculostasis. J Infect Dis. 1969 Jan;119(1):11–18. doi: 10.1093/infdis/119.1.11. [DOI] [PubMed] [Google Scholar]
- Kochan I., Pellis N. R., Golden C. A. Mechanism of Tuberculostasis in Mammalian Serum III. Neutralization of Serum Tuberculostasis by Mycobactin. Infect Immun. 1971 Apr;3(4):553–558. doi: 10.1128/iai.3.4.553-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz P. E., Di Luzio N. R. Biochemical characterization of Kupffer and parenchymal cells isolated from rat liver. Exp Cell Res. 1971 Jul;67(1):17–26. doi: 10.1016/0014-4827(71)90616-1. [DOI] [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969 Aug 1;130(2):315–326. doi: 10.1084/jem.130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. I. The effects of endotoxin, endotoxin tolerance, and cortisone on the release of acid hydrolases from a granular fraction of rabbit liver. J Exp Med. 1962 Oct 1;116:433–450. doi: 10.1084/jem.116.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]



