Abstract
Cathelicidins are a family of cationic peptides expressed in mammals that possess numerous bactericidal and immunomodulatory properties. In vitro analyses showed that human, mouse, and pig cathelicidins inhibited Bacillus anthracis bacterial growth at micromolar concentrations in the presence or absence of capsule. Combined in vitro analyses of the effects of each peptide on spore germination and vegetative outgrowth by time lapse phase contrast microscopy, transmission electron microscopy, and flow cytometric analysis showed that only the pig cathelicidin was capable of directly arresting vegetative outgrowth and killing the developing bacilli within the confines of the exosporium. C57BL/6 mice were protected from spore-induced death by each cathelicidin in a time- and dose-dependent manner. Protection afforded by the porcine cathelicidin was due to its bactericidal effects, whereas the human and mouse cathelicidins appeared to mediate protection through increased recruitment of neutrophils to the site of infection. These findings suggest that cathelicidins might be utilized to augment the initial innate immune response to B. anthracis spore exposure and prevent the development of anthrax.
Cationic peptides are short (≤100 aa), positively charged, amphiphilic peptides that exert antimicrobial activity at physiological concentrations in their tissues of origin and are involved in host defense (1). In mammals, cationic peptides are categorized into two families: defensins and cathelicidins (2). Defensins are characterized by six highly conserved cysteine residues and are divided into subfamilies based on the arrangement of their disulfide bonds (2). In contrast, cathelicidins are characterized by their N-terminal signal sequence, a conserved cathelin-like domain, and a highly variable antimicrobial C-terminal domain (2). In addition to their direct microbicidal activity against bacteria, viruses, and fungi (3), cationic peptides also induce the expression of chemokines that act as chemoattractants for neutrophils, monocytes, and T cells (4, 5). Although the administration of defensins (6), cathelicidins (7, 8), and engineered cationic peptides (9) have been shown to protect mice in numerous bacterial infection models, through either direct bacterial killing (7, 8) or immunomodulation (6, 9), the therapeutic potential of cationic peptides against spore-forming organisms such as Bacillus anthracis has not been examined.
B. anthracis, the causative agent of anthrax, is a Gram-positive spore-forming soil bacterium classified by the National Institute of Allergy and Infectious Diseases as a category A priority pathogen due to its lethality and potential for misuse (10). Unlike other bacterial pathogens, the endospore is the infectious particle of B. anthracis and is capable of causing infection through three routes: cutaneous, gastrointestinal, and pulmonary (10–12). Regardless of the infectious route, anthrax pathogenesis is thought to occur through the uptake of B. anthracis endospores by resident macrophages and/or dendritic cells, which then transit to the regional lymph nodes (10, 11, 13). While the phagocytes are en route, it is thought that some of the spores not degraded within the phagolysosome germinate, develop into bacilli, escape into the cytoplasm, and kill the cells (12). The subsequent combined expression of lethal toxin (LeTx),4 edema toxin, and an anionic poly-γδ-glutamyl capsule are thought to allow the nascent bacilli to spread rapidly in the host during the early stages of infection without eliciting a detectable immune response (14–16), facilitating the establishment of bacteremia and toxemia (12).
However, recent studies have revealed that intracellular outgrowth is a low-probability event (17, 18). Macrophages (19), neutrophils (20), and dendritic cells (21) have been shown to internalize and kill spores. Time lapse confocal analysis suggested that the ability of macrophages to control intracellular vegetative outgrowth was associated with the number of spores within each cell: macrophages containing ≤4 spores were capable of inhibiting outgrowth, whereas cells that internalized >6 spores were not (18). These in vitro observations were supported by studies that demonstrated that augmenting macrophage numbers at the site of infection increased mouse survival after spore challenge (22). In contrast, systemic and localized depletion of macrophages with chlodronate-loaded liposomes increased the susceptibility of mice to B. anthracis infection (23). These findings suggest that the multiplicity of infection is important for the intracellular survival of spores and the subsequent establishment of infection. As such, we hypothesized that the exogenous administration of cathelicidins would increase the survival rate of mice challenged with B. anthracis endospores by reducing the spore burden on resident phagocytes due to the antimicrobial and chemotactic activities of the peptides.
We chose to concentrate our studies on three peptides: LL-37, the only cathelicidin expressed in humans (4); cathelin-related antimicrobial peptide (CRAMP), the mouse homolog to LL-37 and the only cathelicidin expressed in mice (24); and protegrin-1 (PG-1), a porcine cathelicidin shown to be protective in numerous bacterial infection models (7, 8). Here, we demonstrate in vitro that the vegetative form of B. anthracis is sensitive to the bactericidal activity of each peptide, that this bactericidal activity is not impeded by the presence of capsule, and that PG-1 can kill the developing bacilli within the confines of the exosporium. We further show that the administration of each peptide in vivo causes significant recruitment of neutrophils and protects C57BL/6 mice from s.c. spore challenge. Unique among existing or previously proposed potential therapeutic strategies against B. anthracis infection (11, 25–33), we found that CRAMP- and LL-37-mediated protection occurred independent of direct bacterial killing or toxin neutralization. Instead, our data suggest that the administration of mouse (CRAMP) and human (LL-37) cathelicidins might be utilized to augment the initial innate immune response to B. anthracis spore exposure and prevent the development of anthrax.
Materials and Methods
Mice
Female 7- or 8 wk-old C57BL/6 and A/J mice were purchased from The Jackson Laboratory. Mice were housed under specific pathogen-free conditions and used according to protocols approved by the University of Alabama at Birmingham (UAB). All mice were rested for 1 wk in UAB animal facilities before use, and all experiments were conducted when the mice were between 8 and 10 wk old.
cap-null B. anthracis strain construction
To create the Ames-derived cap-null strain, capBCAD coding sequences were replaced with an Ω-spectinomycin cassette. Briefly, a previously described cap-null mutation was transduced from a UM23C1-1td10 background (34) into the Ames strain using phage CP51 (35). The capsule-negative phenotype of the transductant was evident following growth on nutrient broth yeast agar-CO3 medium in 5% CO2 at 37°C. The mutation was confirmed with a PCR using primers specific for the regions flanking the capBCAD locus. The strain was named UTA8.
Spore preparation and storage
Ames strain
Fully virulent B. anthracis Ames strains were cultured in phage assay medium at 30°C for ~3 days until ~90% of cells appeared phase bright under light microscopy. Cultures were centrifuged at 1780 × g for 20 min. Pellets were resuspended in 10 ml of sterile water, heated for 30 min at 65°C, washed twice in sterile water, and finally resuspended in 3 ml of sterile water. The suspension was filtered through a 3.1 µM GL microfiber syringe filter (National Scientific F2500-20) to remove vegetative cells and cell debris, and the remaining spores were stored at 4°C.
Sterne strain
Attenuated B. anthracis (pX01+pX02−) Sterne 34F2 spores were obtained from Dr. Charles L. Turnbough, Jr. (UAB) and prepared as previously described (36). Briefly, sporulation was induced in bacterial cultures with Difco sporulation medium, and harvested spores were purified from vegetative remnants using a series of thorough washes utilizing distilled (DI) water and a 50% Renografin (Bracco Diagnostics) gradient. Purified spores were suspended in DI water and stored at 4°C, protected from light. Spores and bacilli were quantitated microscopically with a Petroff-Hausser bacterial counting chamber for all experiments unless otherwise noted. All experiments were conducted with the attenuated Sterne strain unless indicated otherwise.
Peptide preparation and storage
Human neutrophil protein-1 (HNP-1) was purchased from Bachem, and CRAMP (37), LL-37 (38), and PG-1 (39) were generated according to their published mature sequences using standard solid-phase synthesis, purified to >95% purity by reversed phase-HPLC, and confirmed by mass spec-troscopic analysis (Alpha Diagnostic Int.). Lyophilized peptides were resuspended in 0.1% BSA in 0.01% acetic acid (peptide diluent; Ref. 40) or PBS (for in vitro and in vivo experiments, respectively) to generate 100 µM working stocks, which were stored at −70°C until time of use.
Radial diffusion assays (RDA)
RDAs were conducted as previously described (40) with minor revisions. Briefly, Sterne strain spore cultures were germinated and grown at 37°C for 3 h in undiluted (21 g/L DI H2O) Mueller-Hinton broth (MHB; BD Bio-sciences) and 2 × 106 CFU were dispersed into 10 ml of a nutrient-deficient underlay composed of 0.3 mg/ml trypticase soy broth (BD Bio-sciences), 10 mM sodium phosphate buffer, and 1% (w/v) Low EEO agarose (Research Products International Corp.). A series of 3.5 mm holes were punched into the solidified agarose and 5 µl of serially diluted peptide was dispensed into each well. Following a 3 h incubation at 37°C, a nutrient-rich overlay (60 mg/ml trypticase soy broth with 1% agarose) was poured over the underlay, and plates were incubated overnight at 37°C to allow for visible growth. Clearance zones were measured under ×5–7 magnification and linear regression analyses were performed to calculate the minimum peptide concentration capable of preventing bacterial growth (minimum effective concentration; MEC).
Microbroth dilution assays (MBDA)
MBDAs were conducted as previously described (40), with minor modifications. Peptides were added to flat-bottom 96-well cell culture plates (Corning) containing 5 × 104 mid-log phase Sterne strain bacilli in MHB (cultured and quantitated as described in Radial Diffusion Assays), so that peptide working stocks (100 µM) were diluted 1/10 (v/v) and incubated 4 h at 37°C. Due to the ability of B. anthracis to form chains, bacterial growth was determined by measuring the OD600 with a VERSAmax tunable microplate reader (Molecular Devices) and SOFTmax Proplate reader software.
In vitro bactericidal activity against Ames strains
RDAs and MBDAs were conducted with the Ames strains as described for the Sterne strain with minor modifications. All media used in experiments conducted with the Ames strains contained 0.7% (w/v) NaHCO3, and incubations were done in the presence of 5% CO2 to induce capsule production (35). For both assays, spore cultures were grown for 4 h to allow time for capsule synthesis and were enumerated spectrophotometrically (OD600) using a ThermoSpectronic Genesis 10UV. Capsule synthesis was verified before each experiment with an India ink exclusion assay on a Nikon Eclipse TE2000-U microscope equipped with Metamorph (Imaging Series 6.1) software. For RDAs, 2-mm holes were punched into the underlay with sterile, disposable serological pipets (Falcon 5-ml pipets), whereas the final OD600 values for the MBDAs were determined with a Thermo Electron MultiSkan Spectrum equipped with SkanIt 2.2 (Research Ed.) software.
Time lapse microscopy
Glass coverslips were coated for 30 min with poly-L-lysine (50 µg/ml in DI water), and 1 × 106 spores in DI water were air dried to the coverslips overnight. Coverslips were placed in a Bioptechs Focht Chamber System (FCS2) and incubated with 10 µM peptides in MHB at 37°C and monitored at 10-s intervals for 90 min by phase contrast microscopy using a Leica DM IRBE microscope with a Hamamatsu ORCA-ER digital camera, as previously described (41). Scale bars were generated using a PYSER-Sgi diamond ruled stage micrometer.
Transmission electron microscopy
Thin-section electron microscopy was performed as previously described (36). Briefly, 2 × 107 spores were aliquoted into a 96-well plate (1 × 106 spores/ well) and incubated for 1 h at 37°C in 100 µl of MHB containing peptide diluent, 5 µg/ml gentamicin, or 10 µM PG-1. Samples were then pooled and fixed in a solution of 1.25% formaldehyde, 4% paraformaldehyde, and 2% (v/v) DMSO in PBS, stained with 1% osmium tetroxide and 1% tannic acid, dehydrated in a graded ethanol series and embedded in Spurr’s low-viscosity resin (Electron Microscopy Sciences). Polymerized resin sections (100 nm) were placed on copper grids, poststained with uranyl acetate and lead citrate, and examined with a Hitachi 7000 electron microscope.
Cytotoxicity
The effects of peptide administration on macrophage survival during anthrax LeTx challenge were analyzed as previously described (26), with minor modifications. RAW 264.7 cells (RAW cells; American Type Culture Collection) were dispersed into 96-well, flat-bottom tissue culture plates (3 × 104 cells/well) and incubated overnight at 37°C with 10% CO2 in RPMI 1640 plus 10% FCS. The medium was removed, and 200 µl of fresh RPMI containing PBS or peptide (diluted 1/10, v/v) and recombinant LeTx (List Biological Laboratories) or PBS were added to each well and samples were incubated for 5 h at 37°C. Cell viability was determined by adding 10 µl of alamarBlue (BioSource) to each well. The remaining cells were incubated for an additional 4 h to reduce the alamarBlue reagent, which was quantified by measuring the OD570 and OD600 of each sample. The percentage of surviving cells was calculated according to the manufacturer’s instructions.
Mouse protection experiments
s.c. challenge
Groups of C57BL/6 and A/J mice were briefly anesthetized with Isoflurane (Nova Plus) and injected s.c. with 5 LD50 of Sterne spores (BL/6, 5.0 × 105; A/J, 2.5 × 103; Ref. 42) suspended in 200 µl of PBS or peptides diluted in PBS. To ensure that spores remained dormant, syringes were maintained on ice until injection. Mice were monitored for signs of infection and survival for 10 days after infection.
Cellular depletion
C57BL/6 mice were depleted of Gr-1-binding cells by injecting 50 µg of purified rat anti-mouse Gr-1 Ab (RB6-8C5; BD Bio-sciences) i.p. A purified rat IgG2bk isotype control Ab (BD Biosciences) was administered to control animals. After 24 h, C57BL/6 mice were challenged s.c. as described above.
Intratracheal (i.t.) challenge
Groups of A/J mice were anesthetized with Isoflurane, intubated with SURFLO i.v. 22-gauge × 1-inch catheter tubes (Terumo Medical Corp.), and infected with 5 × 105 spores in 30 µl of PBS or peptides. Mice were monitored for signs of infection and survival for 10 days after infection. The lungs of surviving and uninfected mice were harvested, homogenized, and plated (with and without a 30-min incubation at 65°C) to verify pulmonary spore administration and sterile technique, respectively. Colonies from overnight cultures were confirmed as B. anthracis by staining randomly selected colonies with an Alexa Fluor-488 labeled mAb (EAII) that recognizes the cell wall galactose-N-acetylglucosamine polysac-charide of the bacilli (43) and screening them by flow cytometry (data not shown).
Cellular recruitment
C57BL/6 mice were injected i.p. with 200 µl of PBS or LPS (20 µg; Sigma-Aldrich; L2630) or peptide (50 µM) diluted in PBS. After 4 h, peritoneal cells (PEC) were isolated by lavage with 10 ml of PBS + 2% FCS (FACS buffer). Cells were washed, counted with trypan blue, and stained with Mac-1-PE, CD11c-biotin, Gr-1-biotin, B220-allophycocyanin, CD5-PE, or streptavidin-allophycocyanin (BD Biosciences) and analyzed by flow cytometry with a FACSCalibur. FlowJo software (Treestar) was used to calculate population percentages.
Cytospins and spore clearance
C57BL/6 mice were injected i.p. with 200 µl of PBS or peptides (50 µM) diluted in PBS and infected 4 h later i.p. with 1 × 107 Alexa Fluor 555-labeled spores in 200 µl of PBS. One hour after the infection, PECs were harvested, washed, and enumerated as described in Cellular Recruitment. For cytospin analysis, 4 × 105 cells were removed from each sample, incubated with mAb-93 (44) to block Fc receptors, and subsequently stained with Mac-1-Alexa Fluor 647 (BD Biosciences) and the anti-BclA mAb, EF12 (41) directly conjugated to Alexa Fluor 488. All staining and wash steps were performed on ice in PBS plus 1% BSA (w/v). Cytospins were prepared with a Shandon Elliot Cytospin, fixed, and permeabilized overnight in ethanol at −20°C, and coverslips were mounted with Fluoro-mount G (Southern Biotechnology Associates). Cells were imaged with a Leica DMRB microscope, Hamamatsu digital camera, and Openlab software. Spore clearance was determined by removing 1 × 105 cells from each peritoneal lavage, resuspending the cells in 1 ml of PBS, and lysing them by the addition of 100 µl of 2.5% (v/v) Tween 20. One half of the volume of each sample was directly serially diluted and plated on LB plates, whereas the other half was heat treated at 65°C for 30 min before dilution and plating for colony enumeration.
Statistical analysis
Samples in experiments conducted to determine cellular recruitment, spore burden in Mac-1+ cells, and verification of spore viability during coad-ministration were compared with their respective controls with one-way ANOVAs and Dunnett’s posttests. Two-tailed Student t tests were utilized for comparisons between two groups, whereas one-sample t tests were used to compare groups normalized to their respective controls (which were set to 100). Log rank tests were used to determine statistical significance in mouse survival experiments. For all experiments, statistical significance was defined as p < 0.05, and all graphs and statistical tests were generated/ performed with the Prism software package (version 4.0c; Graphpad software).
Results
Cathelicidins kill B. anthracis in vitro
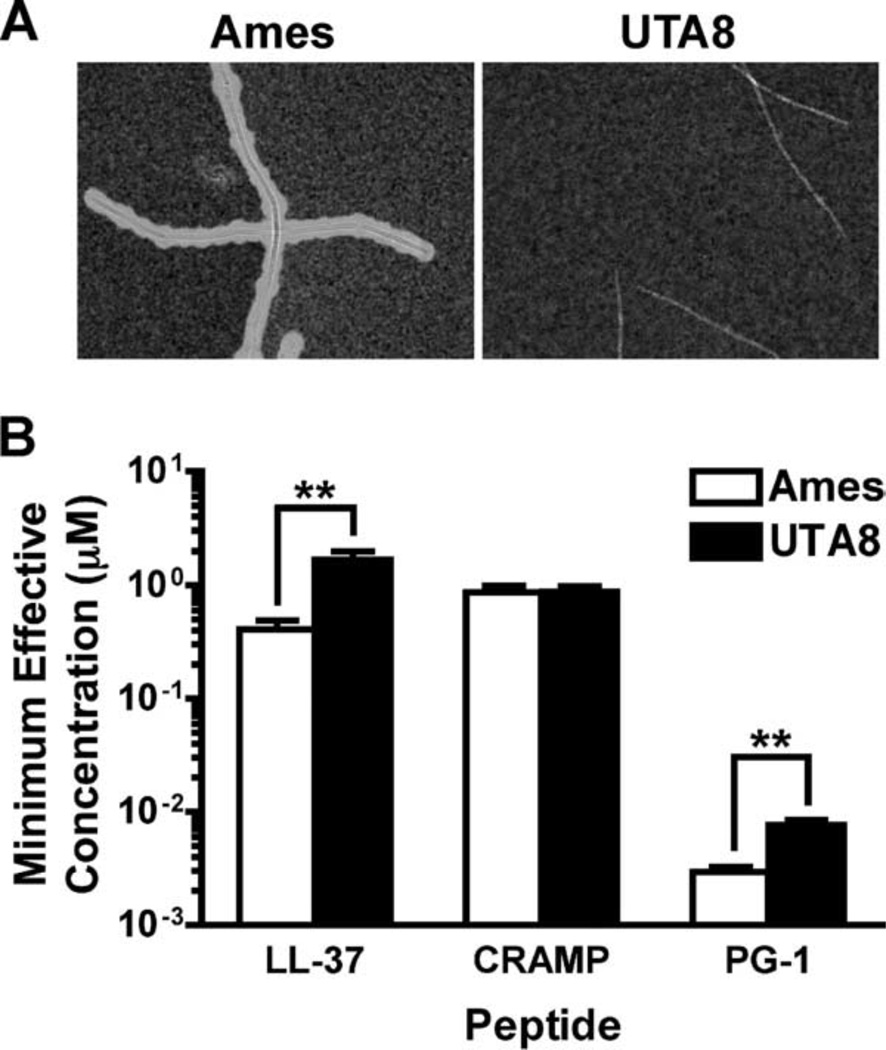
To determine the MEC of peptides capable of inhibiting bacterial growth of the Sterne strain of B. anthracis, each cathelicidin was serially diluted (2-fold) and used in RDAs (Fig. 1A). Consistent with studies conducted with other bacterial species (45, 46), we determined that the MECs of CRAMP (mean ± SEM, 3.2 ± 0.5 µM) and LL-37 (3.4 ± 0.4 µM) were similar, whereas PG-1 (0.8 ± 0.15 µM) was more potent than either of the other two. We also conducted MBDAs (Fig. 1B) to ensure that the MECs calculated in Fig. 1A were not influenced by the ability of the peptides to diffuse through the agarose matrix in the RDAs. In agreement with the MECs determined in Fig. 1A, we observed that CRAMP and LL-37 lost their ability to significantly inhibit bacterial growth between 2.5 and 5 µM, whereas PG-1 remained potent at ≤1.5 µM. Because capsule synthesis plays a pivotal role in anthrax pathogenesis (47, 48), we compared the ability of each cathelicidin to inhibit the growth of an Ames strain of B. anthracis deleted for capsule-biosynthetic genes (UTA8) and its fully virulent isogenic parent strain (Ames) with radial diffusion (Fig. 2) and MBDAs (data not shown). Although the MEC of each peptide was ≤5 µM for both the UTA8 and Ames strains, the presence of capsule reduced the MECs of LL-37 (p < 0.01)- and PG-1 (p < 0.01)-, but not CRAMP-, treated samples (Fig. 2B). These findings show that the in vitro bactericidal activity of these peptides is unaffected by the presence of capsule.
FIGURE 1.
Cathelicidins inhibit B. anthracis growth. A, RDAs were conducted by incubating 2 × 106 ungerminated Sterne strain spores or mid-log phase bacilli with serially diluted peptides (100-0.1 µM). After an overnight incubation to allow for visible colony growth, zones of growth clearance were measured, and the MEC was calculated using linear regression analysis. Graph values represent the mean calculated MEC from at least eight plates ± SEM. Unpaired, two-tailed Student t tests were used to determine statistical significance. *, p < 0.05; **, p < 0.01; ***, p < 0.001. B, MBDAs were performed in which 1 × 106 bacilli/ml were ali-quoted into wells containing serially diluted peptides. Percent growth was calculated by measuring the OD600 of peptide-treated samples normalized to the peptide diluent control samples, which were set to 100% for each experimental repetition. Values represent the mean percentages pooled from three experiments conducted in quintuplicate 6 SEM. Statistical significance was determined by two-tailed, one-sample t tests in which each sample was compared against the peptide diluent control. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FIGURE 2.
Capsule expression does not prevent bactericidal activity of peptides. Spores were grown for 4 h in MHB at 37°C in 5% CO2 and capsule expression was verified by India Ink exclusion (A) before being utilized in RDAs (B) in which the MEC of each peptide was determined against 2 × 106 Ames and UTA8 bacilli. Graph values represent the mean MEC calculated from four plates ± SEM. Unpaired, two-tailed Student’s t tests were used to determine statistical significance. **, p < 0.01.
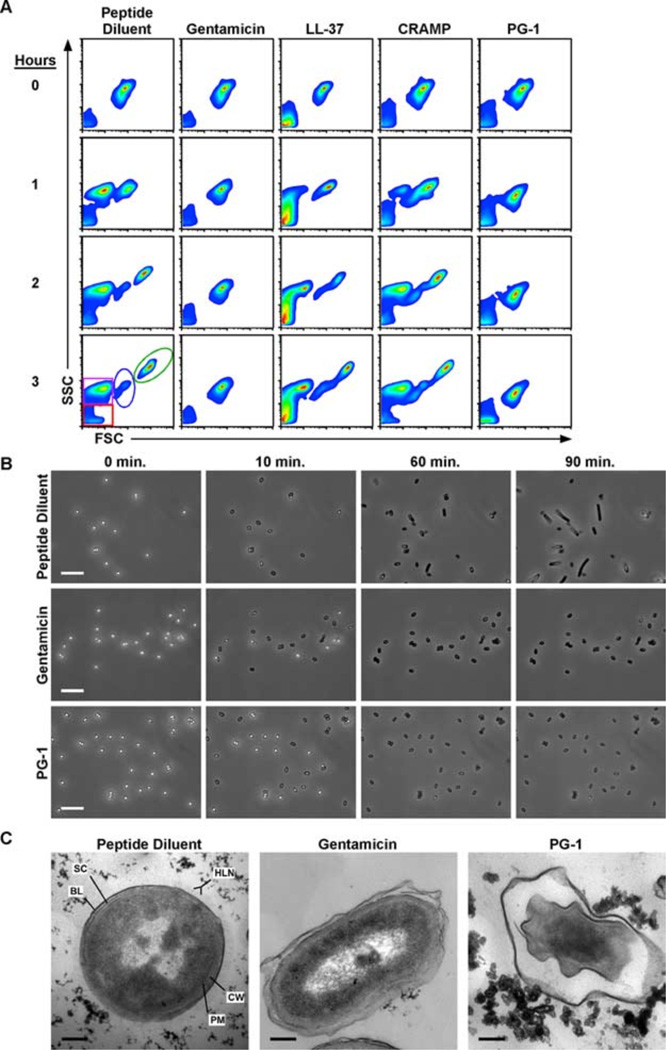
Because the endospore is the infectious form of B. anthracis and is therefore likely to be exposed to cathelicidins in the pleural cavities and the phagolysosomal compartments of professional phagocytic cells, we also examined the sporicidal potential of these cathelicidins. We began by determining the MEC of each peptide against Sterne strain spores by conducting radial diffusion assays in which mid-log phase bacilli were replaced with dormant spores and determined that the MECs of the cathelicidins against spores were similar to those against vegetative bacilli (Fig. 1A), as previously described (33). However, the RDAs did not permit us to distinguish whether the antimicrobial activity observed in Fig. 1A was exerted on the dormant spores, metabolically active germinating spores, or the nascent bacilli. To address this concern, we used a modified MBDA coupled with flow cytometric analysis to observe the germination and outgrowth of spores at hourly intervals in the presence of peptide diluent (negative control), 5 µg/ml gen-tamicin (positive control), or 10 µM concentrations of each peptide. In contrast to our RDA findings, flow cytometric analysis revealed that only gentamicin and PG-1 treatment prevented vegetative outgrowth (Fig. 3A). The inability to detect events representing the exosporium remnants (purple gate) suggested that the growth inhibition of the gentamicin- and PG-1-treated samples occurred at the spore stage. To confirm this observation, germination and outgrowth of individual dormant spores were monitored by time lapse phase contrast microscopy. This analysis revealed that spores treated with gentamicin and PG-1 germinated (as indicated by the phase bright to phase dark transition; Fig. 3B) at the same rate and frequency as PBS-treated spores, but never developed into vegetative bacilli. Because cathelicidins can kill bacteria through membrane disruption or metabolic inhibition (49, 50), we conducted transmission electron microscopy on spores germinated for 1 h in the presence of PG-1 or gentamicin to delineate the effects of compounds on the developing bacilli. We observed that PG-1 treatment caused >99% (357 of 360) of the developing bacilli to display a deflated morphology within the confines of the exospor-ium that did not occur in the gentamicin-treated samples (Fig. 3C). The small number of PG-1-treated spores that did not possess deflated bacilli displayed a phase bright/ungerminated morphology which was also present in the PBS- and gentamicin-treated samples at similar frequencies. Taken together, these in vitro assays indicate that PG-1 is capable of killing metabolically active spores before vegetative outgrowth but that none of the peptides tested was capable of inhibiting the germination process.
FIGURE 3.
PG-1 treatment prevents vegetative outgrowth by disrupting the plasma membrane of the developing bacilli. In all experimental conditions, 1 × 106 spores were incubated in peptide diluent control, genta-micin (5 µg/ml), or peptides (10 µM) diluted in MHB at 37°C for the indicated lengths of time. A, Flow cytom-etry was used to monitor the effects of each peptide on germination and outgrowth. Density plots depict the forward scatter (FSC-H) and side scatter (SSC-H) profiles of germinating spores in which debris (red), exosporium remnants (purple), spores (blue), and bacilli (green) can all be monitored as distinct populations. Scatter profiles are representative of three experiments conducted in duplicate. B, Representative photomicrograph excerpts taken from time lapse phase contrast microscopic analyses in which spores were incubated at 37°C and imaged every 10 s for 90 min. Photomicrographs are representative of three experiments. Bars = 10 µm. C, Representative transmission electron photomicrographs of spores treated with peptide diluent, gentamicin, or PG-1 for 1 h at 37°C. The plasma membrane (PM) and cell wall (CW) of the developing bacilli are shown in relation to the spore coat (SC) and basal layer (BL) and hair-like nap (HLN) of the exosporium. Bars = 0.2 µm.
Cathelicidin administration protects C57BL/6 mice from spore challenge
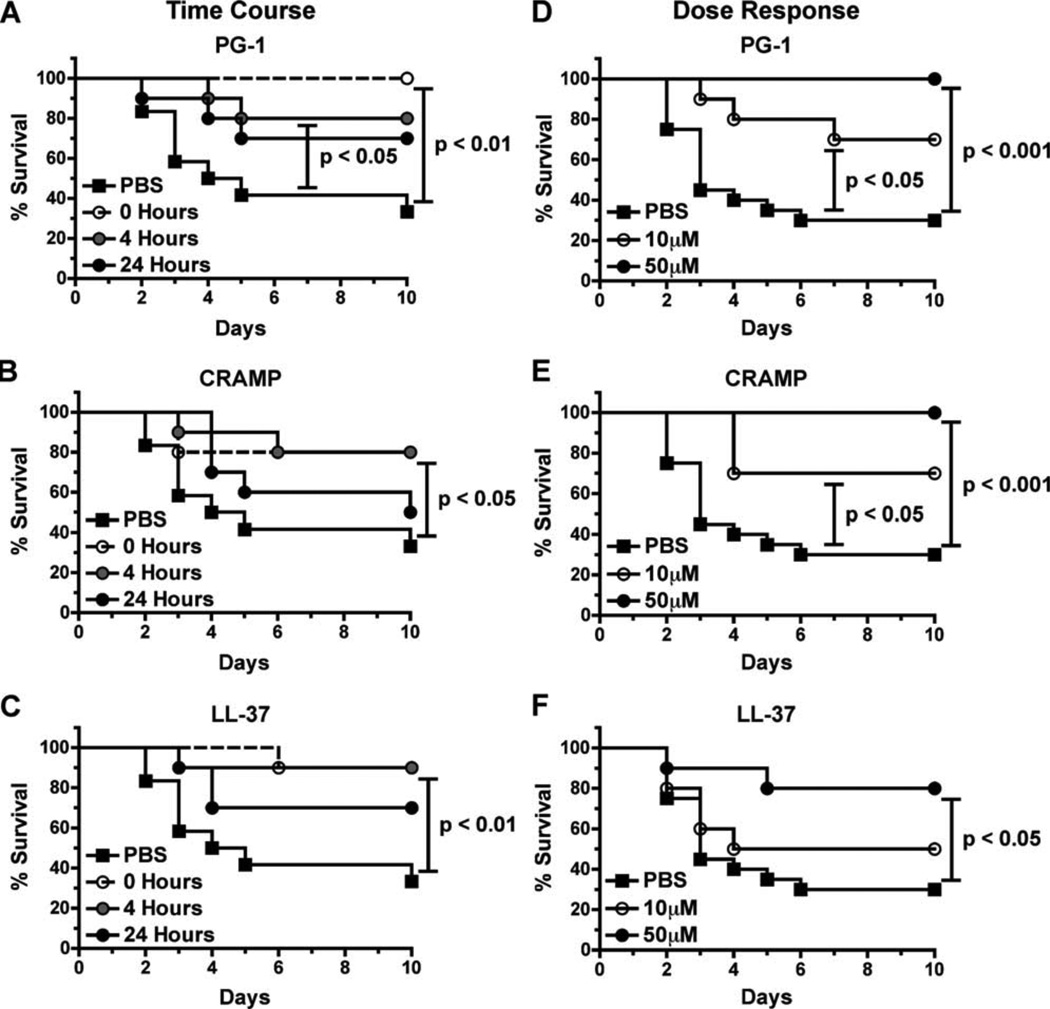
Although the exogenous administration of cathelicidins, defensins, and engineered cationic peptides have been shown to reduce bacterial burden (6, 8) and protect mice (7, 9) from Gram-negative and Gram-positive bacterial pathogens, we are unaware of studies that examined the protective potential of cationic peptide administration against spore-forming bacterial species in vivo. To determine the protective potential of cathelicidins against B. anthracis spore challenge, C57BL/6 mice were injected s.c. with 5 LD50 of Sterne strain spores in 1) PBS alone (PBS), 2) PBS followed by the delayed administration of 50 µM peptide 4 or 24 h postinfection, or 3) spores coadministered with peptides (0 h). The mice were then monitored for the onset of disease symptoms (i.e., edema) and survival for 10 days after infection. Only 33% of PBS-treated mice survived the challenge (Fig. 4, A–C). In contrast, we observed that a single administration of each peptide (up to 4 h postinfection) significantly improved the survival rate (80–100% survival) of infected mice. Although PG-1 (p = 0.0950) and LL-37 ( p = 0.0822) treatment 24 h after infection increased mouse survival rates to 70%, these differences did not achieve statistical significance.
FIGURE 4.
Cathelicidin administration protects C57BL/6 mice from s.c. spore challenge. A–C, Survival of mice inoculated s.c. with 5 LD50 (5 × 105) of spores in 200 µl of PBS (n = 12 mice) or 50 µM solutions of PG-1 (A), CRAMP (B), or LL-37 (C) simultaneously (0 h) or 4 or 24 h after infection (n = 10 mice per group). D–F, Survival of mice inoculated s.c. with 5 LD50 (5 × 105) of spores in 200 µl of PBS (n = 20 mice) or 50 µM or 10 µM solutions of PG-1 (D), CRAMP (E), or LL-37 (F; = 5 10 mice per group). In both experiments, mice were monitored for 10 days, and log rank tests were utilized to determine whether differences in survival were statistically significant.
Because mouse survival was similar between mice that received the cathelicidins 0 or 4 h postinfection, we chose to further characterize the nature of this peptide-mediated protection utilizing peptide coadministration (0 h) to minimize experimental variables. Because the ability of cationic peptides to disrupt bacterial membranes is thought to be threshold dependent (49, 50), we next determined whether the peptide-mediated increase in mouse survival was dose dependent by challenging C57BL/6 mice s.c. with 5LD50 of Sterne strain spores suspended in PBS or peptides (10 or 50 µM). Consistent with our previous findings (Fig. 4, A–C), only 35% of PBS-treated mice survived the challenge (Fig. 4, D–F), whereas 80–100% of the mice that received the 50 µM dose of each cathelicidin survived. Statistically significant improvements in mouse survival rates also occurred in mice that were treated with the 10 µM dose of CRAMP and PG-1 (Fig. 4, D–E). Although no peptide possessed antimicrobial activity against dormant spores in vitro (Fig. 3), we ensured that we were administering viable spores by dilution-plating extra inocula of spores suspended in PBS or peptides (10 or 50 µM). We observed no reduction in viable CFUs derived from peptide-treated spores compared with PBS-treated controls (data not shown), indicating that the spores in the challenge experiment were viable at the time of infection. Taken together, these findings provide the first in vivo evidence that cationic peptide administration protects against spore challenge and that this protection is both time and dose dependent.
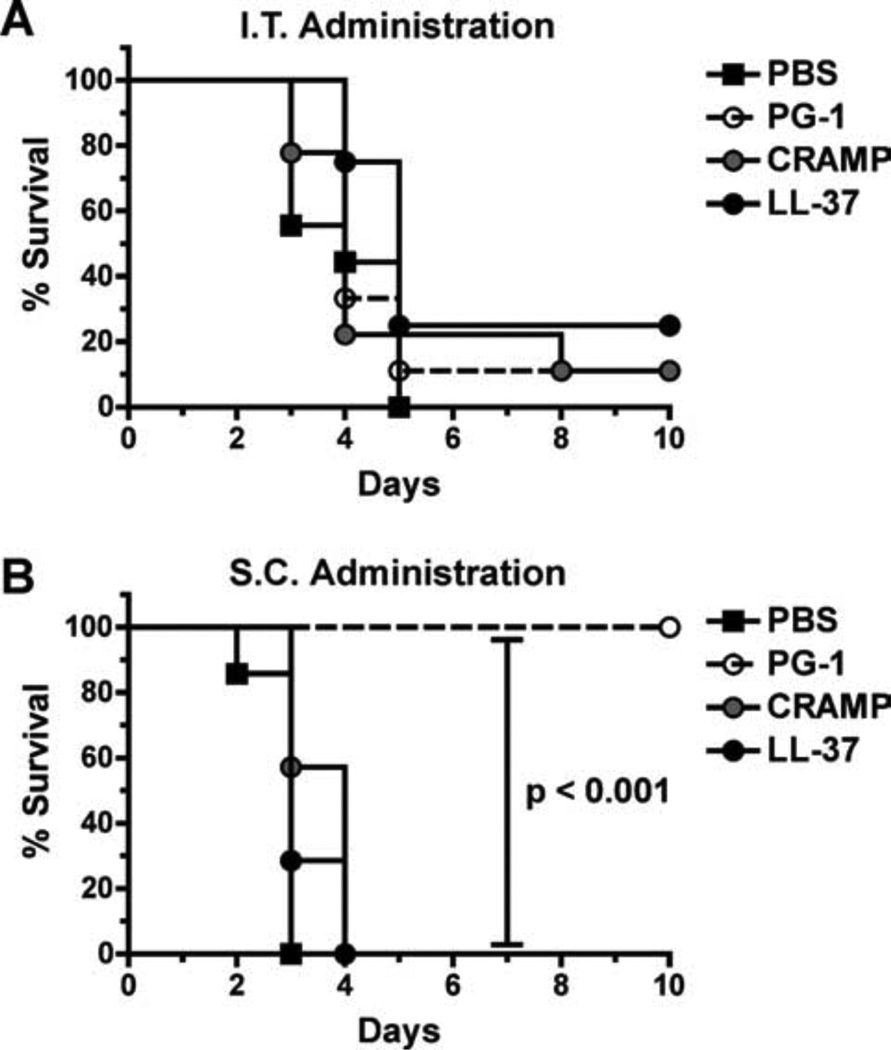
To determine the protective potential of cathelicidin administration against inhalational anthrax, we utilized an i.t. infection model in A/J mice (51, 52), because C57BL/6 mice are highly resistant to pulmonary spore challenge with the Sterne strain (51). We coadministered 5 LD50 of spores with PBS or peptides (50 µM) i.t. to A/J mice, which were subsequently monitored for 10 days after infection for morbidity and survival. Unlike the C57BL/6 s.c. infection model, statistically significant protection was not observed between mice that received spores suspended in PBS or peptides (Fig. 5A), suggesting that peptide-mediated protection does not occur in a pulmonary model of infection. However, the necessity to change mouse strains to determine the protective potential of peptide administration against inhalational anthrax could have influenced this result. Unlike C57BL/6 mice, mouse strains that are susceptible to pulmonary infection from Sterne strain spores (i.e., A/J and DBA2/J) are all C5 deficient and are immunocompromised as a result (53).
FIGURE 5.
PG-1 but not CRAMP or LL-37 protects A/J mice against s.c. spore challenge, whereas none protect against i.t. administration. A, Survival of mice challenged i.t. with 5 LD50 (5 × 105) of spores suspended in 30 µl of PBS or 50 µM peptides (diluted in PBS) and monitored for 10 days. n = 8–9 mice per group. B, Survival of mice inoculated with 5 LD50 (2.5 × 103) of spores suspended in 200µl of PBS or 50µM peptides (diluted in PBS) s.c. and monitored for 10 days. n = 7 mice per group. Log rank tests were utilized in both experiments to determine whether differences in survival were statistically significant.
To determine whether the disparity of protection observed in our A/J i.t. and C57BL/6 s.c. infection models was due to differences in the administrative routes or mouse strains, we re-examined the protective potential of peptide administration in A/J mice which were challenged s.c. with 5 LD50 of spores suspended in PBS or peptides (50 µM). All of the PG-1-treated A/J mice survived the s.c. spore challenge, whereas the CRAMP, LL-37, and PBS control groups rapidly succumbed to the infection (Fig. 5B). These results suggested that the inability of CRAMP and LL-37 to protect A/J mice from inhalation anthrax was mouse strain specific, whereas the lack of PG-1-mediated protection was infectious route specific. It also suggested that the CRAMP- and LL-37-mediated protection in the C57BL/6 model was not due to the direct antimicrobial activities of these peptides (which are thought (49, 50) to be threshold dependent), given that the infectious dose for the C57BL/6 mice (5 × 105 spores) was 200-fold greater than that of the A/J mice (2.5 × 103 spores), whereas the amount of peptide administered was the same in both infection models.
Cathelicidins protect mice through direct and indirect mechanisms
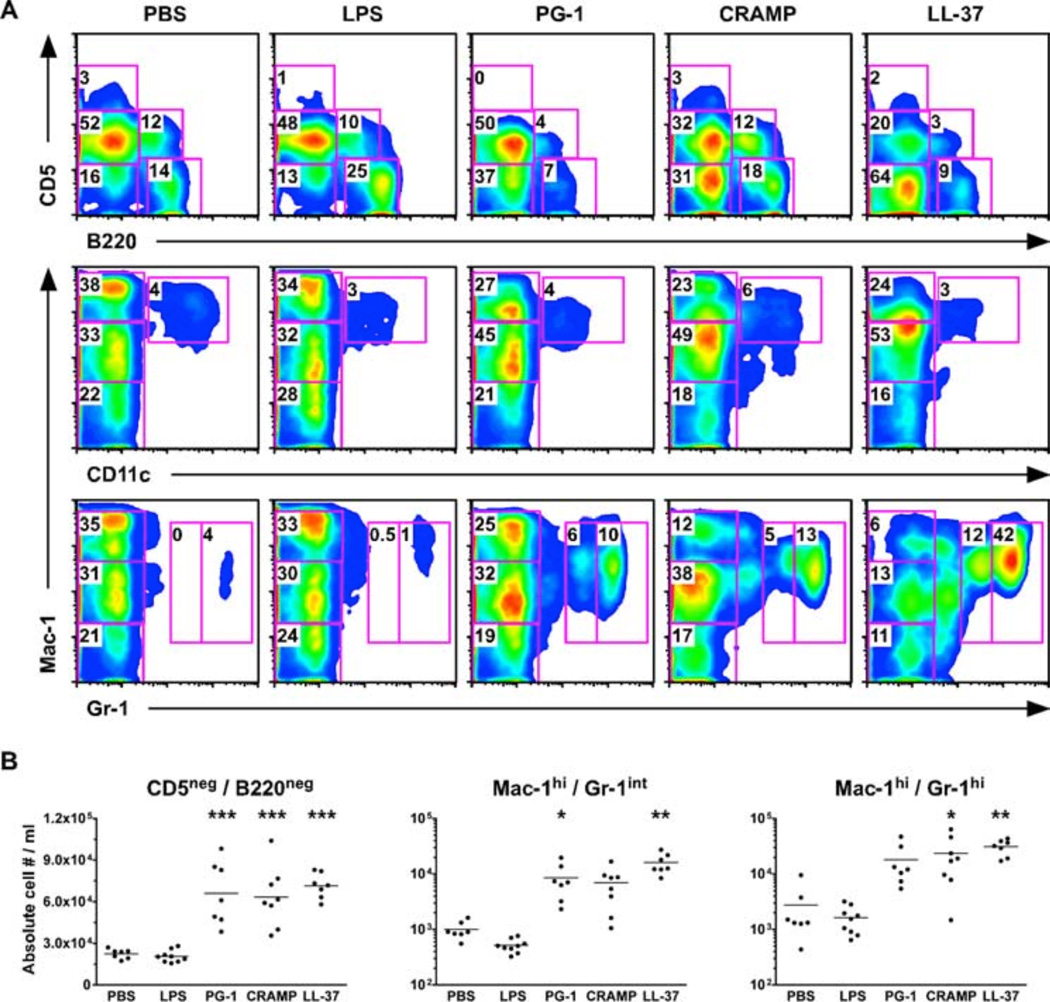
We next examined the mechanism of protection provided by each peptide. Because cellular recruitment has been implicated as an important mechanism of cationic peptide-mediated protection in multiple bacterial infection models (6, 9, 54) and C5-deficient mice suffer from delayed neutrophil and reduced macrophage recruitment following i.p. spore administration (55), we next determined whether any of the cathelicidins enhanced cellular recruitment in vivo. PBS or peptides (50 µM) were injected into the peritoneal cavities of C57BL/6 mice, and 4 h later the mice were euthanized, the peritoneal cavity was lavaged, and peritoneal cellular populations were differentiated by flow cytometric analysis. LPS (20 µg) was also administered to a cohort of mice to ensure that any alterations in cellular recruitment were not due to endotoxin contamination of our sample preparations. In contrast to LPS treatment, which resulted in the migration of myeloid and lymphocyte populations out of the peritoneum, we found that each peptide caused statistically significant ( p < 0.001) recruitment of myeloid cells (CD5−B220− population) into the peritoneal cavity (Fig. 6). Further characterization of the peritoneal fluids for Mac-1, CD11c, and Gr-1 expression revealed that each peptide significantly increased the proportion (Fig. 6A) and absolute cell numbers (Fig. 6B) of immature (Mac-1highGr-1intermediate) and mature (Mac-1highGr-1high) neutrophils. Identification of cellular subsets by cell surface marker expression was augmented by forward-side scatter analysis, as well as differential cell counts in which cell size, granularity, nuclear profile, and Mac-1 expression were determined. Although we are not aware of previous studies that examined the ability of PG-1 treatment to influence cellular recruitment in vivo, our findings that LL-37 and CRAMP increased neutrophil recruitment is consistent with other in vivo models (56). Only CRAMP (1.9 × 105 cells/ml) administration resulted in a significant ( p < 0.05) increase in the concentration of recovered peritoneal cells compared with PBS (1.2 × 105 cells/ml) controls (data not shown).
FIGURE 6.
Administration of pep-tides i.p. to C57BL/6 mice causes recruitment of neutrophils. Mice were injected i.p. with 200 µl of PBS, LPS (20 µg), or peptides (50 µM), and the peritoneal cavity was lavaged 4 h later. A, Cell populations were determined based on the relative expression levels of the cell surface markers B220, CD5, Mac-1, CD11c, and Gr-1. B, The absolute numbers of myeloid cells (CD5−B220−) and immature (Mac-1highGr-1intermediate) and mature (Mac-1highGr-1high) neutrophils were significantly larger in mice that received peptides than those injected with PBS as determined by a one-way ANOVA with a Dunnett posttest. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
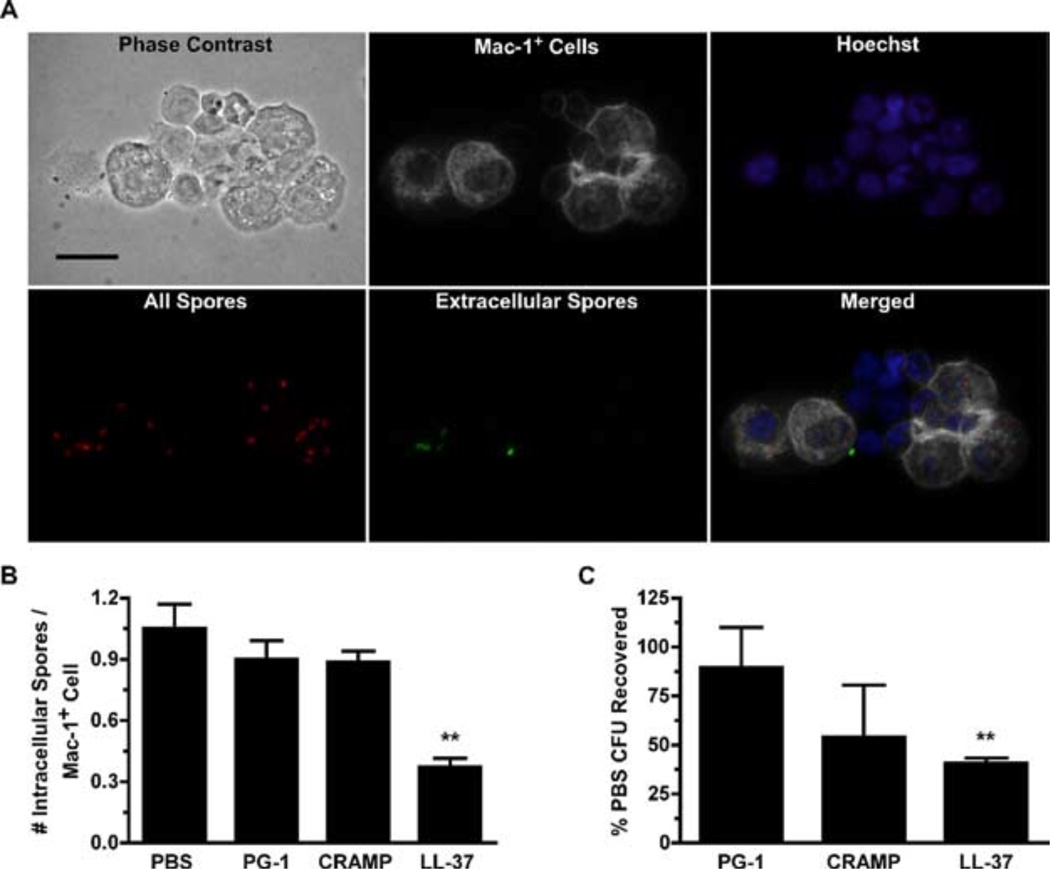
To determine whether the recruited neutrophils affected spore clearance in vivo, 200 µl of PBS or peptides (50 µM) were injected into the peritoneal cavities of C57BL/6 mice, followed 4 h later by 1 × 107 directly Alexa Fluor 555-labeled spores. Mice were sacrificed, and the peritoneal contents were lavaged 1 h after spore administration. Microscopic analysis of the peritoneal exudates (representative field from PBS-treated mouse shown in Fig. 7A) revealed that neutrophil recruitment induced by LL-37 administration was associated with a significant decrease ( p < 0.05) in the spore burden of Mac-1+ cells (Fig. 7B). Plating assays revealed that there was a direct correlation between Mac-1+ cell spore burden and the number of recovered CFU (Fig. 7C). These results suggest that cellular recruitment following peptide administration can promote spore clearance by decreasing the spore burden at the individual cell level, consistent with the mounting evidence that the multiplicity of infection is important in the establishment of an anthrax infection (18, 22, 23).
FIGURE 7.
Recruitment of neutrophils i.p. following LL-37 administration reduces spore burden in Mac-1+ cells and increases spore clearance. Mice were injected i.p. with PBS or peptides as described in Fig. 6 and after a 4-h incubation, 1 × 107 Alexa Fluor 555-labeled spores were injected i.p. Peritoneal lavages were performed 1 h later, and cells were subsequently stained for cytospin analysis or plated to determine spore survival. Graph values are pooled from three experiments. A, Representative photomicrographs of cytospins prepared from the PECs collected from PBS-treated mice illustrating differential staining that allows intracellular spores (red only) to be distinguished from extracellular spores (green and red) within Mac-1+ cells (white) and the total cell population (phase and Hoechst). Bars, 20 µm. B, Intracellular spore burden was determined from 300 randomly selected cells from cytospins shown in A by counting the number of Alexa Fluor 555-labeled spores (red) that did not costain with the Alexa Fluor 488-labeled anti-BclA mAb (EF12; green). Values represent the mean number of intracellular spores per Mac-1+ peritoneal cell ± SEM. Statistical significance was determined with a one-way ANOVA and Dunnett posttest. n = 5 mice per group. C, Aliquots of 1 × 105 cells from each mouse were lysed, serially diluted, and plated overnight. The percent recovered was calculated by normalizing the number of CFU quantitated from PBS-pretreated mice to 100% for each experimental repetition. Values represent the mean percentages ± SEM. n = 9 mice per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Because it was previously reported that defensins are capable of protecting macrophages (26, 33) and mice from anthrax LeTx challenge (26), we also tested the ability of each cathelicidin to inhibit anthrax LeTx induced cytolysis of RAW 264.7 cells. In agreement with previous studies using LL-37 (26, 33), none of the cathelicidins were capable of protecting the RAW 264.7 cells from exposure to high or low concentrations of LeTx (Fig. 8), suggesting that the peptide-mediated protection of the C57BL/6 mice (Fig. 4) was not associated with activity against toxin.
FIGURE 8.
Cathelicidins do not protect macrophages from anthrax LeTx-induced cytolysis. RAW 264.7 cells were incubated for 5 h with PBS or serially diluted HNP-1 (A), PG-1 (B), CRAMP (C), or LL-37 (D) with low or high levels of LeTx (400 ng/ml lethal factor and 200 ng/ml or 1600 ng/ml protective Ag, respectively). Cell viability was determined by alamarBlue reduction. Points represent the mean values pooled from three experiments conducted in duplicate ± SEM.
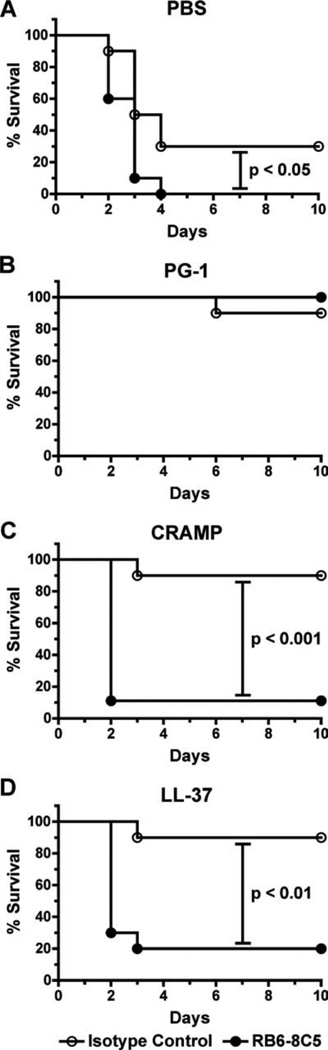
Given that the i.p. administration of LL-37 and CRAMP increased neutrophil recruitment (Fig. 6), which was associated with a reduction in the spore burden of Mac-1+ cells and decreased spore survival in vivo (Fig. 7), we hypothesized that the protection C57BL/6 mice received from these peptides during s.c. spore challenge was mediated by increased neutrophil recruitment to the site of infection. To test this hypothesis, mice were injected i.p. with 50 µg of an anti-mouse Gr-1 mAb to deplete Gr-1+ cells and subsequently challenged s.c. with 5 LD50 of spores suspended in either PBS or peptides (50 µM) the following day (Fig. 9). We observed 90% mouse survival in each peptide-treated group that received the control mAb and 100% survival in the Gr-1-depleted, PG-1-treated mice, suggesting that PG-1-mediated protection can occur independent of cellular recruitment. In contrast, only 10–20% of the Gr-1-depleted mice that received LL-37 or CRAMP survived. This finding confirmed the importance of Gr-1+ cells (i.e., neu-trophils) in mediating the protection induced by these peptides against spores in the C57BL/6 s.c. infection model.
FIGURE 9.
Gr-1 depletion abrogates CRAMP- and LL-37-, but not PG-1-, induced protection of C57BL/6 mice challenged s.c. with spores. Survival curves of mice injected i.p. with 50 µg of an anti-GR-1 mAb to deplete neutrophils or an isotype-matched control Ab. The following day, 5 LD50 (5 × 105) of spores were coadministered s.c. in 200 µl of PBS (A) or 50 µM solutions of PG-1 (B), CRAMP (C), or LL-37 (D), and survival was monitored for 10 days. Log rank tests were conducted to determine whether differences in survival were statistically relevant. n = 10 mice per group.
Discussion
Most of the previous work on the susceptibility of B. anthracis to cationic peptides and the use of these peptides as potential anthrax therapeutics have centered on defensins (20, 26, 33, 57). The first study to examine the bactericidal activity of cationic peptides against B. anthracis demonstrated that the peptides expressed by human pulmonary epithelial cells (i.e., LL-37 and human β-defen-sin 3) are capable of killing vegetative bacilli in vitro (58). Subsequent studies demonstrated that human neutrophil peptides (α-defensins) and synthetically derived θ-defensins (retrocyclins) are capable of killing the vegetative form of B. anthracis and neutralizing the cytotoxic effects of lethal toxin on RAW 264 macrophages and protecting BALB/c mice from toxemia (26, 33). Some retrocyclins even appeared to possess sporicidal abilities in vitro (33). In this paper, we characterized the sensitivity of B. anthracis to three cathelicidins: LL-37 (human), CRAMP (mouse), and PG-1 (pig). We determined that the vegetative form of B. anthracis was sensitive to the direct antimicrobial activity of all three peptides at micromolar concentrations and that sensitivity was not affected by the presence of capsule. We also examined the previously reported sporicidal potential of these peptides (33, 59) and established that only PG-1 was capable of altering the vegetative outgrowth process. Expanding on these in vitro findings, we ascertained that cationic peptide administration protected mice from spore challenge and that each cathelicidin protected C57BL/6 mice in a time-and dose-dependent manner. We determined that this protection was mediated by the direct killing of germinated spores or bacilli following inoculation (PG-1) or through enhanced cellular recruitment to the site of infection (CRAMP and LL-37), but not by neutralizing the effects of lethal toxin.
Previous studies indicate that unencapsulated strains of B. anthracis are highly sensitive to the bactericidal activities of LL-37 and PG-1 (33, 58). Although capsule synthesis is important in anthrax pathogenesis, our studies using in vitro assays with fully virulent Ames and an isogenic capsule-negative mutant showed that the presence of the B. anthracis poly-D-glutamic acid capsule did not impede the bactericidal activity of these peptides. Although the polysaccharide capsule of Kleb-siella pneumoniae contributes to antimicrobial resistance (60), our findings are consistent with studies of group A Streptococcus (61) and Streptococcus pneumoniae (62) which demonstrated that D-alanylation of lipotechoic acids by DltA contributed more to antimicrobial peptide resistance than capsule expression. B. anthracis was recently reported to possess a homologous dltABCD operon and that its deletion increased the sensitivity of unencapulated bacilli to defensin-mediated killing 3- to 5-fold (57). However, because our study included only three cationic peptides and is the first to examine the protective potential of capsule to peptides in B. anthracis, it remains to be seen whether capsule synthesis contributes to protection from less potent cationic peptides (i.e., human β-defensins 1 and 2; see Ref. 58).
The sporicidal potential of cationic peptides was previously examined in vitro with RDAs (33) and kinetic MBDAs that correlated colony counts with spore and bacterial levels spec-trophotometrically (59). Both studies suggested that cationic peptides might possess sporicidal activities, but neither assay was capable of distinguishing the stage during germination and outgrowth or the mechanism involved in the killing. To better understand the sporicidal properties of each peptide, we monitored the effects of the cathelicidins on germination and outgrowth by direct single-spore observation using flow cytometric analysis and time lapse phase contrast microscopy. Only PG-1 prevented vegetative outgrowth, and transmission electron microscopy revealed ultra structural damage to the plasma membranes of the developing bacilli within the exosporium. We are not aware of previous studies that used transmission electron microscopy to examine the effects of cationic peptides on Gram-positive bacilli. However, the membrane disruption and cytoplasmic content release that we observed were similar to those shown following SMAP29 and CAP18 treatment of Pseudomonas aeruginosa PA01 (63) as well as LL-37 and cecropin B treatment of Escherichia coli D21 (64). In agreement with previous studies, no peptide was observed to kill dormant spores (33).
Size, sequence, charge, hydrophobicity, amphipathicity, conformation, and structure are all characteristics that affect the antimicrobial activity and specificity of cationic peptides (49). As such, it is difficult to speculate what characteristic causes PG-1 to be more potent than CRAMP or LL-37 against both the encapsulated and unencapsulated strains of B. anthracis as well as germinated spores, but this finding is consistent with previous studies utilizing multiple bacterial species (46). However, it is not surprising that our results with LL-37 and CRAMP so closely parallel each other. Both peptides are similarly sized (30–40 aa depending on the extent of proteolytic cleavage), α-helical peptides with charges of ~6 at pH 7 (50, 65, 66). However, PG-1 is significantly different from either CRAMP or LL-37 in that it is an 18-aa β-sheet peptide containing 2 disulfide bonds and a charge of 7 at neutral pH (50, 67). These structural and charge dissimilarities could account for the differences in the intensity of cellular recruitment observed following i.p. administration of the peptides. Although we are unaware of previous studies that examined the chemoattractant properties of PG-1, both LL-37 and CRAMP were previously reported to attract leukocytes using formyl peptide receptor-like (FPRL) 1 and mouse FPRL-2 (56). Considering the structural dissimilarities between the α-helical (LL-37 and CRAMP) and β-sheet (PG-1) peptides, it is unlikely that PG-1 binds FPRL-1 or mouse FPRL-2.
Based on numerous studies indicating that cationic peptide administration increases bacterial clearance (6, 8, 9, 49, 68) and host survival (7, 9, 49, 68) following bacterial infections and the growing demand for alternative therapeutic strategies against anthrax, we examined whether cathelicidin administration would protect mice from spore challenge. Consistent with the previous bacterial infection models (7, 9, 49, 68), our s.c. infection studies with C57BL/6 mice revealed that the administration of each cathelicidin 4 h after spore administration significantly improved mouse survival rates (and trended toward increased survival 24 h postinfection) in a dose-dependent manner. Cellular recruitment and depletion studies suggested that this cathelicidin-mediated protection resulted not only from the direct killing of germinated spores but also through the increased recruitment of neutrophils, which reduced the spore burden on resident phagocytes and facilitated increased spore clearance. These findings are in agreement with previous studies, in which: 1) the in vivo administration of CRAMP and LL-37 resulted in a 6-fold increase in neutrophil recruitment 4 h after injection (56); 2) the enhanced bacterial clearance observed following cathelicidin (LL-37; Ref. 54), defensin (HNP-1; Ref. 6), or engineered cationic peptide (innate defense regulator-1; Ref. 9) administration in various infectious models was dependent on cellular recruitment; 3) PG-1 administration protected mice from i.p., i.v., and intradermal bacterial infections by bacterial killing (7, 8); and 4) the importance of spore burden on resident macrophages for preventing intracellular vegetative outgrowth was demonstrated with time lapse confocal microscopy (18), as well as in vivo macrophage depletion and augmentation infection models (22, 23).
In contrast to the C57BL/6 s.c. infection models, we did not observe any peptide-mediated protection in pulmonary spore challenge models conducted in A/J mice. To discern whether this protection discrepancy was mouse strain or infectious route specific, we also conducted a s.c. infection with the A/J mice and only observed increased rates of survival in mice treated with PG-1. Since the Gr-1 depletion studies suggested that CRAMP and LL-37 protected C57BL/6 mice through cellular recruitment, it is not surprising that both peptides failed to protect A/J mice from s.c. or i.t. spore challenge, given that A/J mice were previously reported to have delayed neutrophil recruitment (55) and reduced accumulation of macrophages following thioglycollate (69), Listeria monocytogenes (70), and B. anthracis spore administration (55). Similarly, it is not startling that PG-1 failed to protect A/J mice from pulmonary spore challenge, because it has been shown in multiple animal models that spore germination does not occur in the lung (52, 71), thus negating the bactericidal activities of PG-1. Because we could not demonstrate pulmonary protection in A/J mice following peptide administration and the fact that C57BL/6 mice are highly resistant to inhalation anthrax (51), the efficacy of cathelicidin administration against pulmonary anthrax will need to be re-examined in follow-up studies with a fully virulent strain of B. anthracis. The use of a fully virulent strain of B. anthracis would facilitate the use of s.c. and i.t. studies within the same mouse strain (72). Regardless, the fact that only PG-1 protected A/J mice from s.c. spore challenge is in agreement with our findings that PG-1 was the only peptide capable of killing germinated spores in vitro and for which in vivo killing activity did not require the presence of Gr-1+ cells (i.e., neutrophils) to protect C57BL/6 mice. This is especially notable given a recent report indicating that spores became metabolically active within 2 h of s.c. injection (73).
The role of neutrophils in anthrax pathogenesis has only recently been examined. Historically, the levels of resident and recruited macrophages were thought to dictate the outcome of infection. We recently found that the C-terminal domain of BclA, a collagen-like protein on the outermost surface of the exosporium, directs cellular uptake to Mac-1-expressing cells (42), suggesting that neutrophils are also capable of binding and internalizing spores. This was demonstrated by Mayer-Scholl et al. (20), who found that human neutrophils were capable of internalizing and killing B. anthracis spores and bacilli. However, these in vitro findings were not supported by the only mouse infection study to examine the role of neutrophils in anthrax pathogenesis (22). In that study, mice were injected with the RB6-8C5 Ab to induce neutropenia 2 days before i.p. or aerosol spore challenge. Statistically significant reductions in survival rates (compared with saline controls) were observed only in mice that received a high dose of inhaled spores. Neutrophil recruitment, induced by the injection of 1 ml of starch into the peritoneal cavity of mice 4 h before i.p. spore challenge did not increase survival rates or mean times to death. Because we used different mouse and spore strains, infectious routes, and elicitants, it is difficult to directly compare these previous findings with our own.
The treatment strategy for an anthrax infection is the same now as it has been for decades: vaccination with an attenuated spore strain or purified bacterial supernatant to generate antitoxin Abs, and a timely and sustained antibiotic regiment (11). A common concern is that an antibiotic-resistant strain of B. anthracis could be used in an attack to undermine one-half of the current treatment protocol. As a result, following the anthrax attacks in 2001, numerous attempts have been made to develop novel therapeutic agents that directly lyse germinated spores or bacilli (i.e., human group IIa phospholipase A2 (29), retrocyclins (33), or recombinant γ phage (30) lysin) or counter the antiphagocytic properties of capsule synthesis through degradation (CapD; Ref. 31) or mAb binding (27). Major efforts have also been made to develop reagents that abrogate the effects of toxin expression (i.e., small peptides (32), defensins (26, 33), or mAbs (25) and Ab fragments (28) to LeTx components). We chose to determine whether cathelicidin administration would protect mice from B. anthracis infection because it allowed us to examine the protective potential of a natively expressed peptide (CRAMP) and its human homolog (LL-37). We believed that this was important considering previous concerns that the protective potential of exogenous human neutrophil defensin mediated against bacterial infection in mice may be exaggerated because mouse neutrophils do not express defensins (6). We found that CRAMP and LL-37 significantly improved mouse survival following spore challenge at previously used infectious doses (29, 74) and that this protection was mediated through increased cellular recruitment to the site of infection. Unlike previous therapeutic strategies, LL-37 and CRAMP were not capable of directly killing germinated spores or bacteria or of negating the effects of lethal toxin. Instead, our data suggest that these peptides exploited a naturally occurring bottleneck in anthrax pathogenesis in which the spores must survive passage to the regional lymph nodes within APCs to establish an infection. As such, these peptides could potentially be the first preventative therapeutic agents against an anthrax infection. This is significant because although the current treatment regimen of immunization and antibiotic treatment is sufficient to prevent death from inhalational anthrax (74), the pathogen is still capable of imparting on its hosts lasting and debilitating neurological disorders (75). The development of therapeutic strategies that could prevent the establishment of infection, used in conjunction with the current treatment regiment, might help to alleviate the downstream effects of anthrax exposure.
Acknowledgments
We thank Dr. Charles L. Turnbough, Jr. (UAB) for providing Sterne strain spores for use in these experiments; Albert Tousson, Shawn Williams, Leigh Millican, and Melissa Chimento (UAB imaging core facility) for their assistance with the confocal and electron microscopy; and Dr. Nicholas Kin (UAB) and Dr. Claudia Oliva (UAB) for critically reading the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants AI057699-03 (to J.F.K.) and AI057156 and AI33537 (to T.M.K.). M.W.L. and M.K.S. were supported by Grant T32AI55438, B.L.P.D. was supported by Grant 5T32GM008361-16, and K.J.P. was supported by T32AI055449. This research is part of the dissertation research conducted by M.W.L. a predoctoral student in the Department of Microbiology, University of Alabama at Birmingham.
Abbreviations used in this paper: LeTx, lethal toxin; CRAMP, cathelin-related antimicrobial peptide; PG-1, protegrin-1; UAB, University of Alabama at Birmingham; DI, distilled; RDA, radial diffusion assay; MHB, Mueller-Hinton broth; MBDA, microbroth dilution assay; i.t., intratracheal; PEC, peritoneal cell; MEC, minimum effective concentration; FPRL-1, formyl peptide receptor-like 1; HNP-1, human neutrophil protein-1.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 2.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J. Leukocyte Biol. 2001;69:691–697. [PubMed] [Google Scholar]
- 3.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finlay BB, Hancock RE. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- 5.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defenses. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 6.Welling MM, Hiemstra PS, van den Barselaar MT, Paulusma-Annema A, Nibbering PH, Pauwels EK, Calame W. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J. Clin. Invest. 1998;102:1583–1590. doi: 10.1172/JCI3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg DA, Hurst MA, Fujii CA, Kung AH, Ho JF, Cheng FC, Loury DJ, Fiddes JC. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinstraesser L, Klein RD, Aminlari A, Fan MH, Khilanani V, Remick DG, Su GL, Wang SC. Protegrin-1 enhances bacterial killing in thermally injured skin. Crit. Care Med. 2001;29:1431–1437. doi: 10.1097/00003246-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, Wang A, Lee K, Doria S, Hamill P, et al. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 2007;25:465–472. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- 10.Mock M, Fouet A. Annu. Rev. Microbiol. 55: 2001. Anthrax; pp. 647–671. [DOI] [PubMed] [Google Scholar]
- 11.Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N. Engl. J. Med. 1999;341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 12.Guidi-Rontani C. The alveolar macrophage: the Trojan horse of Bacillus anthracis . Trends Microbiol. 2002;10:405–409. doi: 10.1016/s0966-842x(02)02422-8. [DOI] [PubMed] [Google Scholar]
- 13.Cleret A, Quesnel-Hellmann A, Vallon-Eberhard A, Verrier B, Jung S, Vidal D, Mathieu J, Tournier JN. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 2007;178:7994–8001. doi: 10.4049/jimmunol.178.12.7994. [DOI] [PubMed] [Google Scholar]
- 14.Baldari CT, Tonello F, Paccani SR, Montecucco C. Anthrax toxins: a paradigm of bacterial immune suppression. Trends Immunol. 2006;27:434–440. doi: 10.1016/j.it.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Fukao T. Immune system paralysis by anthrax lethal toxin: the roles of innate and adaptive immunity. Lancet Infect Dis. 2004;4:166–170. doi: 10.1016/S1473-3099(04)00940-5. [DOI] [PubMed] [Google Scholar]
- 16.Raymond B, Leduc D, Ravaux L, Goffic RL, Candela T, Raymondjean M, Goossens PL, Touqui L. Edema toxin impairs anthracidal phospho-lipase A2 expression by alveolar macrophages. PLoS Pathog. 2007;3:e187. doi: 10.1371/journal.ppat.0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu H, Sa Q, Koehler TM, Aronson AI, Zhou D. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell Microbiol. 2006;8:1634–1642. doi: 10.1111/j.1462-5822.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 18.Ruthel G, Ribot WJ, Bavari S, Hoover TA. Time-lapse confocal imaging of development of Bacillus anthracis in macrophages. J. Infect. Dis. 2004;189:1313–1316. doi: 10.1086/382656. [DOI] [PubMed] [Google Scholar]
- 19.Kang TJ, Fenton MJ, Weiner MA, Hibbs S, Basu S, Baillie L, Cross AS. Murine macrophages kill the vegetative form of Bacillus anthracis . Infect. Immun. 2005;73:7495–7501. doi: 10.1128/IAI.73.11.7495-7501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer-Scholl A, Hurwitz R, Brinkmann V, Schmid M, Jungblut P, Weinrauch Y, Zychlinsky A. Human neutrophils kill Bacillus an-thracis . PLoS Pathog. 2005;1:e23. doi: 10.1371/journal.ppat.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickering AK, Osorio M, Lee GM, Grippe VK, Bray M, Merkel TJ. Cytokine response to infection with Bacillus anthracis spores. Infect. Im-mun. 2004;72:6382–6389. doi: 10.1128/IAI.72.11.6382-6389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote CK, Van Rooijen N, Welkos SL. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect. Immun. 2006;74:469–480. doi: 10.1128/IAI.74.1.469-480.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cote CK, Rea KM, Norris SL, van Rooijen N, Welkos SL. The use of a model of in vivo macrophage depletion to study the role of macrophages during infection with Bacillus anthracis spores. Microb. Pathog. 2004;37:169–175. doi: 10.1016/j.micpath.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht MT, Li H, Williamson ED, Lebutt CS, Flick-Smith HC, Quinn CP, Westra H, Galloway D, Mateczun A, Goldman S, Groen H, Baillie LW. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 2007;75:5425–5433. doi: 10.1128/IAI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim C, Gajendran N, Mittrucker HW, Weiwad M, Song YH, Hurwitz R, Wilmanns M, Fischer G, Kaufmann SH. Human α-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. USA. 2005;102:4830–4835. doi: 10.1073/pnas.0500508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozel TR, Murphy WJ, Brandt S, Blazar BR, Lovchik JA, Thorkildson P, Percival A, Lyons CR. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. USA. 2004;101:5042–5047. doi: 10.1073/pnas.0401351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelat T, Hust M, Laffly E, Condemine F, Bottex C, Vidal D, Lefranc MP, Dubel S, Thullier P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Anti-microb. Agents Chemother. 2007;51:2758–2764. doi: 10.1128/AAC.01528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piris-Gimenez A, Paya M, Lambeau G, Chignard M, Mock M, Touqui L, Goossens PL. In vivo protective role of human group IIa phospholipase A2 against experimental anthrax. J. Immunol. 2005;175:6786–6791. doi: 10.4049/jimmunol.175.10.6786. [DOI] [PubMed] [Google Scholar]
- 30.Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis . Nature. 2002;418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 31.Scorpio A, Chabot DJ, Day WA, O’Brien K, Vietri DNJ, Itoh Y, Mohamadzadeh M, Friedlander AM. Poly-γ-glutamate capsule-degrading enzyme treatment enhances phagocytosis and killing of encapsulated Bacillus anthracis . Antimicrob. Agents Chemother. 2007;51:215–222. doi: 10.1128/AAC.00706-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoop WL, Xiong Y, Wiltsie J, Woods A, Guo J, Pivnichny JV, Felcetto T, Michael BF, Bansal A, Cummings RT, et al. Anthrax lethal factor inhibition. Proc. Natl. Acad. Sci. USA. 2005;102:7958–7963. doi: 10.1073/pnas.0502159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Mulakala C, Ward SC, Jung G, Luong H, Pham D, Waring AJ, Kaznessis Y, Lu W, Bradley KA, Lehrer RI. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J. Biol. Chem. 2006;281:32755–32764. doi: 10.1074/jbc.M603614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drysdale M, Bourgogne A, Hilsenbeck SG, Koehler TM. atxA controls Bacillus anthracis capsule synthesis via acpA and a newly discovered regulator, acpB. J Bacteriol. 2004;186:307–315. doi: 10.1128/JB.186.2.307-315.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green BD, Battisti L, Koehler TM, Thorne CB, Ivins BE. Demonstration of a capsule plasmid in Bacillus anthracis . Infect. Immun. 1985;49:291–297. doi: 10.1128/iai.49.2.291-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steichen CT, Kearney JF, Turnbough CL., Jr Characterization of the exosporium basal layer protein BxpB of Bacillus anthracis . J. Bacteriol. 2005;187:5868–5876. doi: 10.1128/JB.187.17.5868-5876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pestonjamasp VK, Huttner KH, Gallo RL. Processing site and gene structure for the murine antimicrobial peptide CRAMP. Peptides. 2001;22:1643–1650. doi: 10.1016/s0196-9781(01)00499-5. [DOI] [PubMed] [Google Scholar]
- 38.Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J. Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Liu L, Lehrer RI. Identification of a new member of the protegrin family by cDNA cloning. FEBS Lett. 1994;346:285–288. doi: 10.1016/0014-5793(94)00493-5. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg DA, Lehrer RI. Designer assays for antimicrobial pep-tides: disputing the “one-size-fits-all” theory. Methods Mol. Biol. 1997;78:169–186. doi: 10.1385/0-89603-408-9:169. [DOI] [PubMed] [Google Scholar]
- 41.Swiecki MK, Lisanby MW, Shu F, Turnbough CL, Jr, Kearney JF. Monoclonal antibodies for Bacillus anthracis spore detection and functional analyses of spore germination and outgrowth. J. Immunol. 2006;176:6076–6084. doi: 10.4049/jimmunol.176.10.6076. [DOI] [PubMed] [Google Scholar]
- 42.Oliva CR, Swiecki MK, Griguer CE, Lisanby MW, Bullard DC, Turnbough CL, Jr, Kearney JF. The integrin Mac-1 (CR3) mediates internalization and directs Bacillus anthracis spores into professional phagocytes. Proc. Natl. Acad. Sci. USA. 2008;105:1261–1266. doi: 10.1073/pnas.0709321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ezzell JW, Jr, Abshire TG, Little SF, Lidgerding BC, Brown C. Identification of Bacillus anthracis by using monoclonal antibody to cell wall galactose-N-acetylglucosamine polysaccharide. J. Clin. Microbiol. 1990;28:223–231. doi: 10.1128/jcm.28.2.223-231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliver AM, Grimaldi JC, Howard MC, Kearney JF. Independently ligating CD38 and FcγRIIB relays a dominant negative signal to B cells. Hybridoma. 1999;18:113–119. doi: 10.1089/hyb.1999.18.113. [DOI] [PubMed] [Google Scholar]
- 45.Kristian SA, Timmer AM, Liu GY, Lauth X, Sal-Man N, Rosenfeld Y, Shai Y, Gallo RL, Nizet V. Impairment of innate immune killing mechanisms by bacteriostatic antibiotics. FASEB J. 2007;21:1107–1116. doi: 10.1096/fj.06-6802com. [DOI] [PubMed] [Google Scholar]
- 46.Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. An-timicrob. Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heninger S, Drysdale M, Lovchik J, Hutt J, Lipscomb MF, Koehler TM, Lyons CR. Toxin-deficient mutants of Bacillus anthracis are lethal in a murine model for pulmonary anthrax. Infect. Immun. 2006;74:6067–6074. doi: 10.1128/IAI.00719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drysdale M, Heninger S, Hutt J, Chen Y, Lyons CR, Koehler TM. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J. 2005;24:221–227. doi: 10.1038/sj.emboj.7600495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brogden KA, Kalfa VC, Ackermann MR, Palmquist DE, McCray PB, Jr, Tack BF. The ovine cathelicidin SMAP29 kills ovine respiratory pathogens in vitro and in an ovine model of pulmonary infection. Antimicrob. Agents Chemother. 2001;45:331–334. doi: 10.1128/AAC.45.1.331-334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 51.Harvill ET, Lee G, Grippe VK, Merkel TJ. Complement depletion renders C57BL/6 mice sensitive to the Bacillus anthracis Sterne strain. Infect. Immun. 2005;73:4420–4422. doi: 10.1128/IAI.73.7.4420-4422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loving CL, Kennett M, Lee GM, Grippe VK, Merkel TJ. Murine aerosol challenge model of anthrax. Infect. Immun. 2007;75:2689–2698. doi: 10.1128/IAI.01875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welkos SL, Friedlander AM. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis . Microb. Pathog. 1988;4:53–69. doi: 10.1016/0882-4010(88)90048-4. [DOI] [PubMed] [Google Scholar]
- 54.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. Impact of LL-37 on anti-infective immunity. J. Leukocyte Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 55.Welkos SL, Trotter RW, Becker DM, Nelson GO. Resistance to the Sterne strain of B. anthracis: phagocytic cell responses of resistant and susceptible mice. Microb. Pathog. 1989;7:15–35. doi: 10.1016/0882-4010(89)90108-3. [DOI] [PubMed] [Google Scholar]
- 56.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 57.Fisher N, Shetron-Rama L, Herring-Palmer A, Heffernan B, Bergman N, Hanna P. The dltABCD operon of Bacillus anthracis Sterne is required for virulence and resistance to peptide, enzymatic, and cellular mediators of innate immunity. J. Bacteriol. 2006;188:1301–1309. doi: 10.1128/JB.188.4.1301-1309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radyuk SN, Mericko PA, Popova TG, Grene E, Alibek K. In vitro-generated respiratory mucosa: a new tool to study inhalational anthrax. Biochem. Biophys. Res. Commun. 2003;305:624–632. doi: 10.1016/s0006-291x(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 59.Thwaite JE, Hibbs S, Titball RW, Atkins TP. Proteolytic degradation of human antimicrobial peptide LL-37 by Bacillus anthracis may contribute to virulence. Antimicrob. Agents Chemother. 2006;50:2316–2322. doi: 10.1128/AAC.01488-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 2004;72:7107–7114. doi: 10.1128/IAI.72.12.7107-7114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. D-Alanylation of teichoic acids promotes group a Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 2005;187:6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, Normark S, Henriques-Normark B. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 2007;9:1162–1171. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 63.Kalfa VC, Jia HP, Kunkle RA, McCray PB, Jr, Tack BF, Brogden KA. Congeners of SMAP29 kill ovine pathogens and induce ultrastructural damage in bacterial cells. Antimicrob. Agents Chemother. 2001;45:3256–3261. doi: 10.1128/AAC.45.11.3256-3261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phos-pholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999;341(3):501–513. [PMC free article] [PubMed] [Google Scholar]
- 65.Ouhara K, Komatsuzawa H, Kawai T, Nishi H, Fujiwara T, Fujiue Y, Kuwabara M, Sayama K, Hashimoto K, Sugai M. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus . J. Antimicrob. Chemother. 2008;61:1266–1269. doi: 10.1093/jac/dkn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- 67.Tran D, Tran P, Roberts K, Osapay G, Schaal J, Ouellette A, Selsted ME. Microbicidal properties and cytocidal selectivity of rhesus macaque θ defensins. Antimicrob. Agents Chemother. 2008;52:944–953. doi: 10.1128/AAC.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, Silvestri C, Mocchegiani F, Licci A, Skerlavaj B, Rocchi M, et al. LL-37 protects rats against lethal sepsis caused by Gram-negative bacteria. Antimicrob. Agents Chemother. 2006;50:1672–1679. doi: 10.1128/AAC.50.5.1672-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein O, Dabach Y, Ben-Naim M, Halperin G, Stein Y. Lower macrophage recruitment and atherosclerosis resistance in FVB mice. Atherosclerosis. 2006;189:336–341. doi: 10.1016/j.atherosclerosis.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 70.Stevenson MM, Kongshavn PA, Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J. Immunol. 1981;127:402–407. [PubMed] [Google Scholar]
- 71.Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. 1956;54:28–36. doi: 10.1017/s0022172400044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lyons CR, Lovchik J, Hutt J, Lipscomb MF, Wang E, Heninger S, Berliba L, Garrison K. Murine model of pulmonary anthrax: kinetics of dissemination, histopathology, and mouse strain susceptibility. Infect. Immun. 2004;72:4801–4809. doi: 10.1128/IAI.72.8.4801-4809.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. Primary involvement of pharynx and peyer’s patch in inhalational and intestinal anthrax. PLoS Pathog. 2007;3:e76. doi: 10.1371/journal.ppat.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown K. Anthrax: a “sure killer” yields to medicine. Science. 2001;294:1813–1814. doi: 10.1126/science.294.5548.1813. [DOI] [PubMed] [Google Scholar]
- 75.Reissman DB, Whitney EA, Taylor TH, Jr, Hayslett JA, Dull PM, Arias I, Ashford DA, Bresnitz EA, Tan C, Rosenstein N, Perkins BA. One-year health assessment of adult survivors of Bacillus anthracis infection. J. Am. Med. Assoc. 2004;291:1994–1998. doi: 10.1001/jama.291.16.1994. [DOI] [PubMed] [Google Scholar]