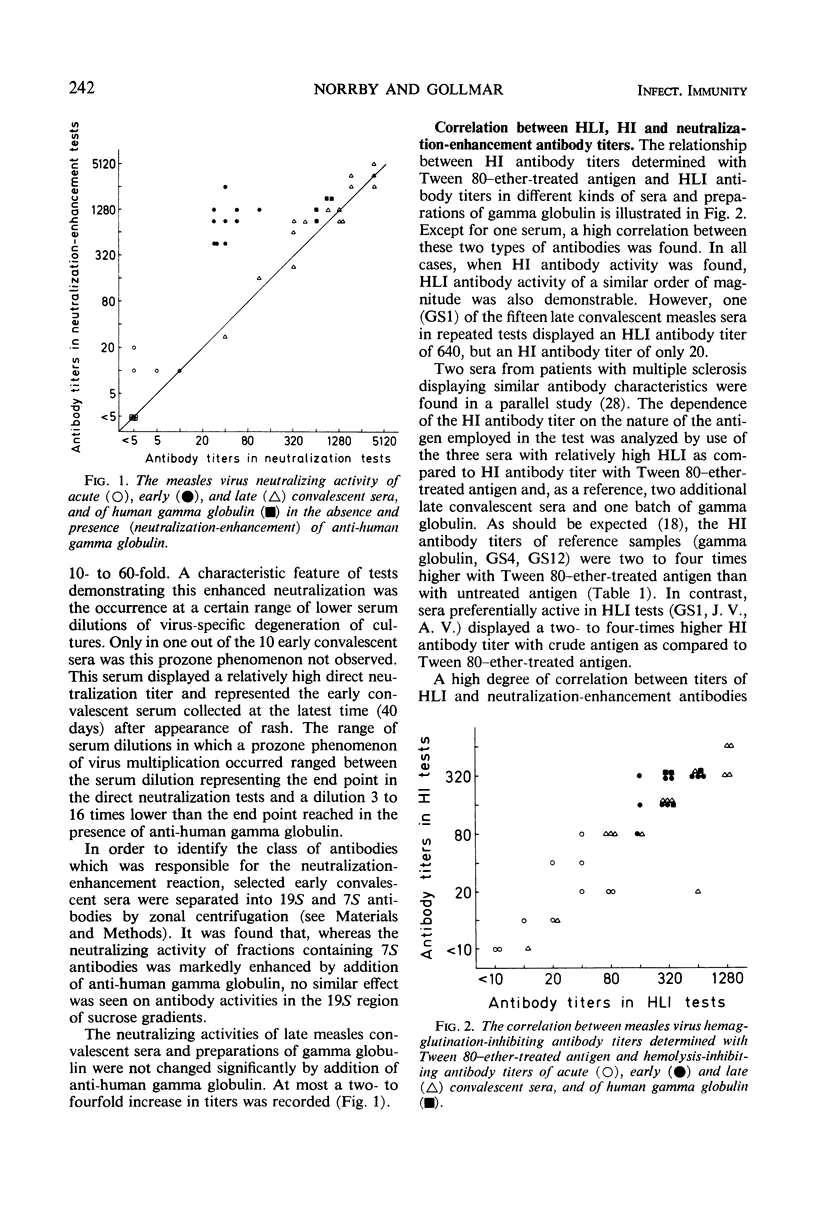
Abstract
Different measles virus-specific antibody activities in acute, early (11 to 40 days after rash) and late (4 to 20 years postinfection) convalescent sera and gamma globulin were determined. Early immunoglobulin G antibodies gave a poor neutralization, which was increased 10- to 60-fold by addition of anti-gamma globulin.
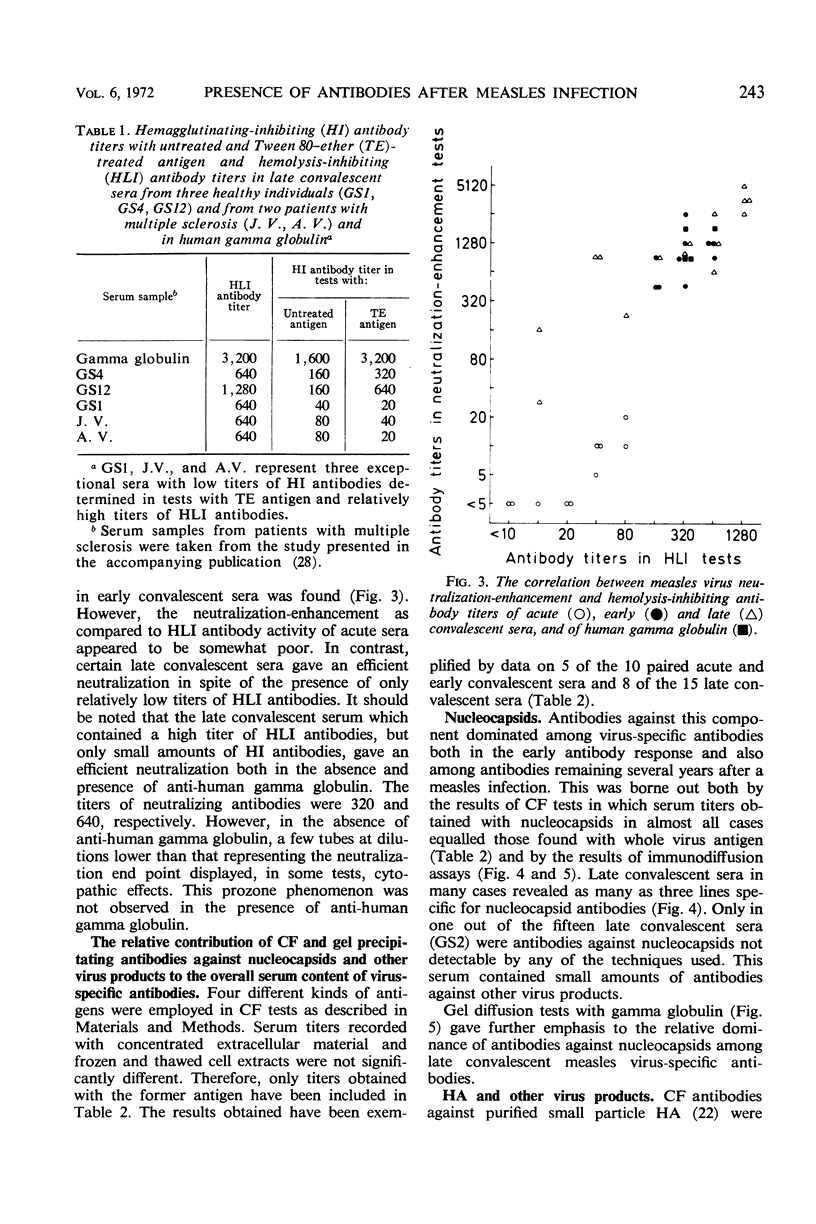
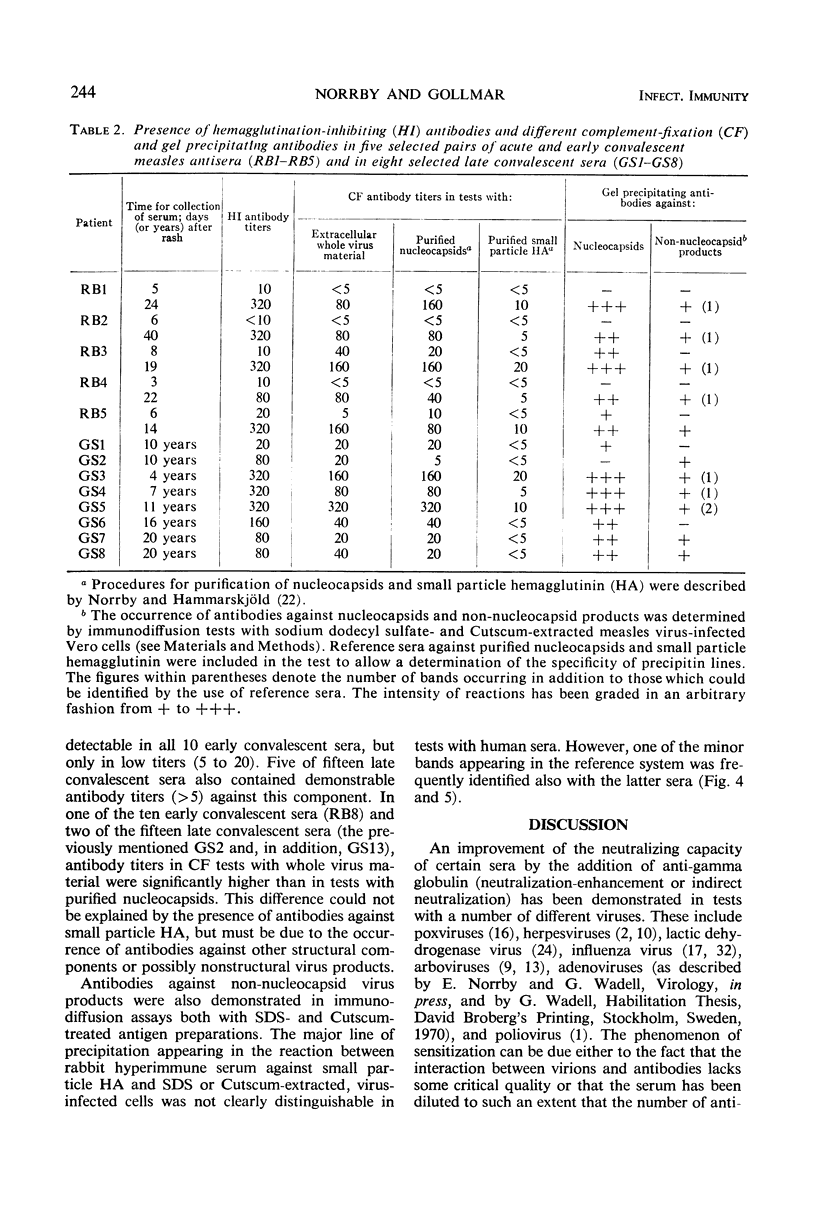
There was a high degree of correlation between titers of hemolysis-inhibiting (HLI) and hemagglutinating-inhibiting (HI) antibodies. However, in one out of fifteen late convalescent sera an HLI antibody titer of 640 in the presence of titer of only 20 in HI tests with Tween 80—either-treated antigen was found. Similar findings were made with sera from two patients with multiple sclerosis included in a parallel study. A somewhat higher titer of HI antibodies was demonstrable in these three sera when untreated material was used as antigen. These findings are interpreted in the following way. Antibodies against the hemagglutinin can block not only virus-specific agglutination but also lysis of red cells. In contrast, antibodies against the hemolysin, besides blocking the biological activity of this component, carry only a slight HI activity. This HI activity can be detected only by use of antigen preparations containing hemagglutinin-associated hemolysin.
Complement-fixation (CF) and immunodiffusion tests (the latter were carried out with antigen preparations treated with 0.25% sodium dodecyl sulfate) demonstrated that, in almost all cases, antibodies against nucleocapsid structures dominated quantitatively among antibodies appearing in connection with and persisting after regular measles infections. Generally, only low titers of antibodies reacting with purified small particle hemagglutinin (HA; 10 to 14S) or additional structural or nonstructural components were identified in CF and immunodiffusion tests.
Full text
PDF

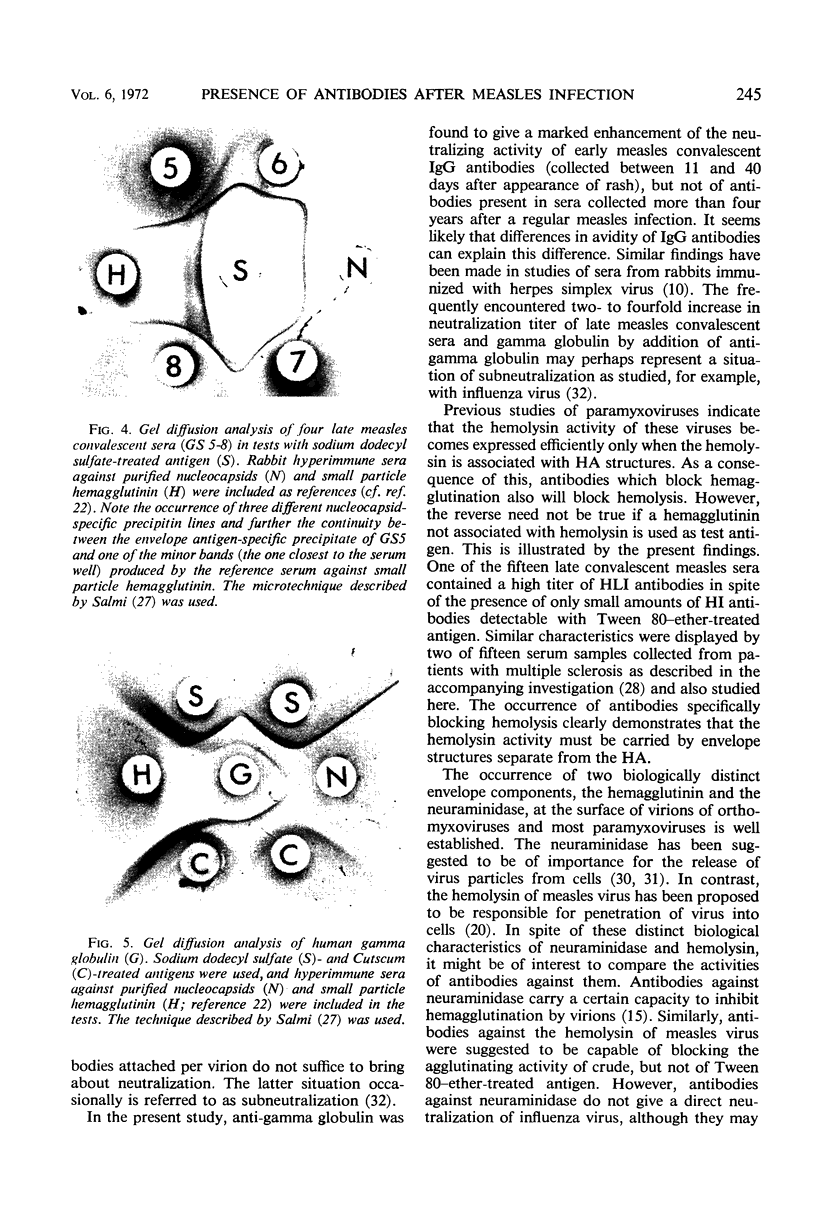


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano A., De Donato S., Jakschik M. Die anwendung von Antiglobulinseren zum Nachweis und zur Titerbestimmung neutralisierender Poliomyelitisantikörper. Zentralbl Bakteriol Orig A. 1971 Jun;217(2):141–147. [PubMed] [Google Scholar]
- Ashe W. K., Notkins A. L. Neutralization of an infectious herpes simplex virus-antibody complex by anti-gamma-globulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):447–451. doi: 10.1073/pnas.56.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRON A. L., MILGROM F., KARZON D. T., WITEBSKY E. DEMONSTRATION OF HUMAN MEASLES ANTIBODY BY MIXED AGGLUTINATION. J Immunol. 1963 Jun;90:908–913. [PubMed] [Google Scholar]
- BLACK F. L., ROSEN L. Patterns of measles antibodies in residents of Tahiti and their stability in the absence of re-exposure. J Immunol. 1962 Jun;88:725–731. [PubMed] [Google Scholar]
- Chiarini A., Norrby E. Separation and characterization of products of two measles virus variants. Arch Gesamte Virusforsch. 1970;29(2):205–214. doi: 10.1007/BF01249306. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport F. M., Minuse E., Hennessy A. V. Antibody response to influenza virus enzyme in man. Arch Environ Health. 1970 Sep;21(3):307–311. doi: 10.1080/00039896.1970.10667243. [DOI] [PubMed] [Google Scholar]
- ENDERS-RUCKLE G. METHODS OF DETERMINING IMMUNITY, DURATION AND CHARACTER OF IMMUNITY RESULTING FROM MEASLES. Arch Gesamte Virusforsch. 1965;16:182–207. doi: 10.1007/BF01253808. [DOI] [PubMed] [Google Scholar]
- Hahon N. Neutralization of residual infectivity of Venezuelan equine encephalomyelitis virus by anti-gamma globulin. J Gen Virol. 1970 Mar;6(3):361–372. doi: 10.1099/0022-1317-6-3-361. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- KASAHARA S., MAKINO S., SASAKI K., NAKAGAWA M. STUDIES ON MEASLES VIRUS. I. HEMADSORPTION TEST AND HEMADSORPTION-INHIBITION TEST IN TISSUE CULTURE INFECTED WITH MEASLES VIRUS. Kitasato Arch Exp Med. 1963 Dec;36:67–75. [PubMed] [Google Scholar]
- Kilbourne E. D., Laver W. G., Schulman J. L., Webster R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968 Apr;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer M., Lik F. Sensitization of influenza virus A2-Singapore by antineuraminidase. J Gen Virol. 1971 Nov;13(2):355–356. doi: 10.1099/0022-1317-13-2-355. [DOI] [PubMed] [Google Scholar]
- Majer M., Link F. Studies on the non-neutralizable fraction of vaccinia virus. Clin Exp Immunol. 1970 Aug;7(2):283–291. [PMC free article] [PubMed] [Google Scholar]
- NORRBY E. C., MAGNUSSON P., FALKSVEDEN L. G., GROENBERG M. SEPARATION OF MEASLES VIRUS COMPONENTS BY EQUILIBRIUM CENTRIFUGATION IN CSCL GRADIENTS. II. STUDIES ON THE LARGE AND THE SMALL HEMAGGLUTININ. Arch Gesamte Virusforsch. 1964 Mar 13;14:462–473. doi: 10.1007/BF01555078. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc Soc Exp Biol Med. 1962 Dec;111:814–818. doi: 10.3181/00379727-111-27930. [DOI] [PubMed] [Google Scholar]
- NORRBY E., LAGERCRANTZ R., GARD S., CARLSTROEM G. MEASLES VACCINATION. I. SEROLOGIC RESPONSES TO AN INACTIVATED VACCINE. Arch Gesamte Virusforsch. 1963 Aug 26;13:548–558. [PubMed] [Google Scholar]
- Norrby E., Hammarskjöld B. Structural components of measles virus. Microbios. 1972 Jan;5(17):17–29. [PubMed] [Google Scholar]
- Norrby E. The effect of a carbobenzoxy tripeptide on the biological activities of measles virus. Virology. 1971 Jun;44(3):599–608. doi: 10.1016/0042-6822(71)90374-6. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Mage M., Ashe W. K., Mahar S. Neutralization of sensitized lactic dehydrogenase virus by anti-gammglobulin. J Immunol. 1968 Feb;100(2):314–320. [PubMed] [Google Scholar]
- Salmi A. A. Gel precipitation reactions between alkaline extracted rubella antigens and human sera. Acta Pathol Microbiol Scand. 1969;76(2):271–278. doi: 10.1111/j.1699-0463.1969.tb03257.x. [DOI] [PubMed] [Google Scholar]
- Salmi A. A., Norrby E., Panelius M. Identification of different measles virus-specific antibodies in the serum and cerebrospinal fluid from patients with subacute sclerosing pancencephalitis and multiple sclerosis. Infect Immun. 1972 Sep;6(3):248–254. doi: 10.1128/iai.6.3.248-254.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. L., Khakpour M., Kilbourne E. D. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968 Aug;2(8):778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto J. T., Rott R. Functional significance of sialidose during influenza virus multiplication. Virology. 1966 Dec;30(4):731–737. doi: 10.1016/0042-6822(66)90178-4. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Preparation and properties of antibody directed specifically against the neuraminidase of influenza virus. J Immunol. 1967 Jul;99(1):49–55. [PubMed] [Google Scholar]
- Zalan E., Borman E., Labzoffsky N. A. Infectious influenza virus-antibody complex. Arch Gesamte Virusforsch. 1971;34(3):209–213. doi: 10.1007/BF01242994. [DOI] [PubMed] [Google Scholar]