Abstract
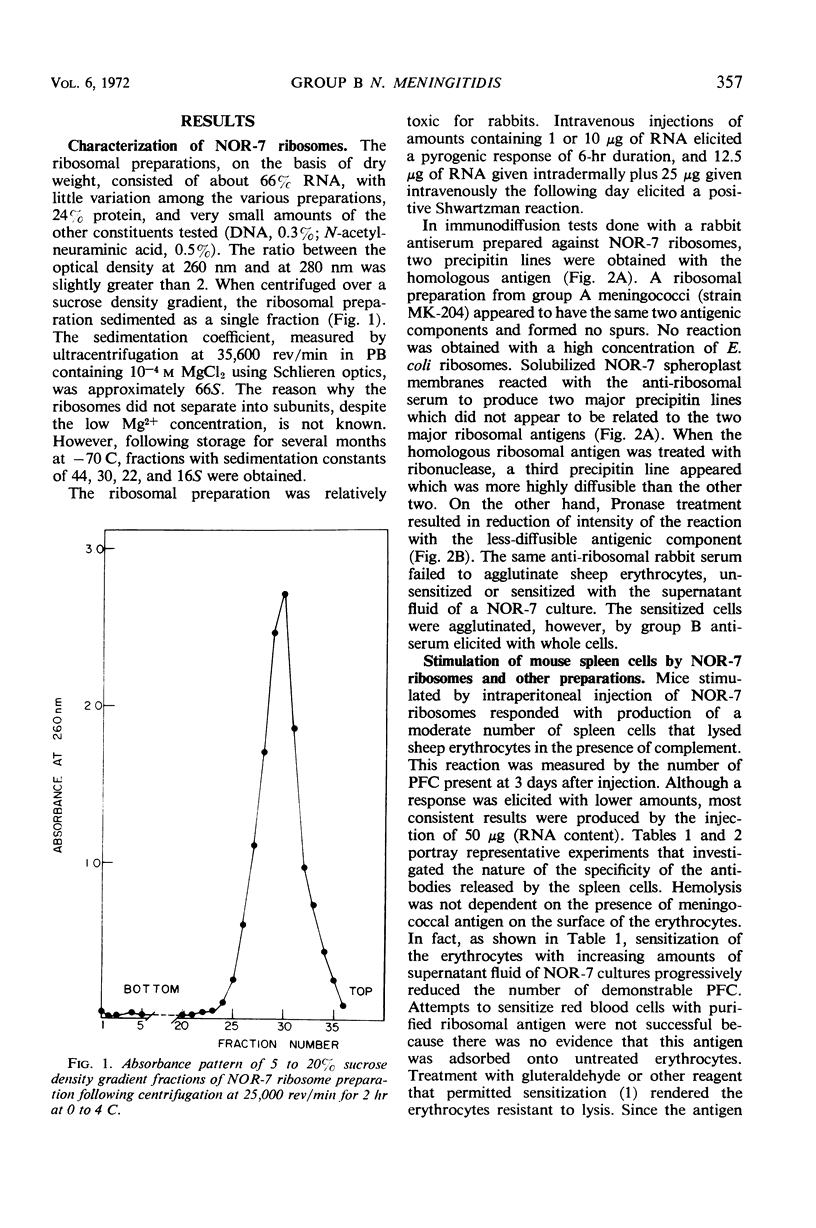
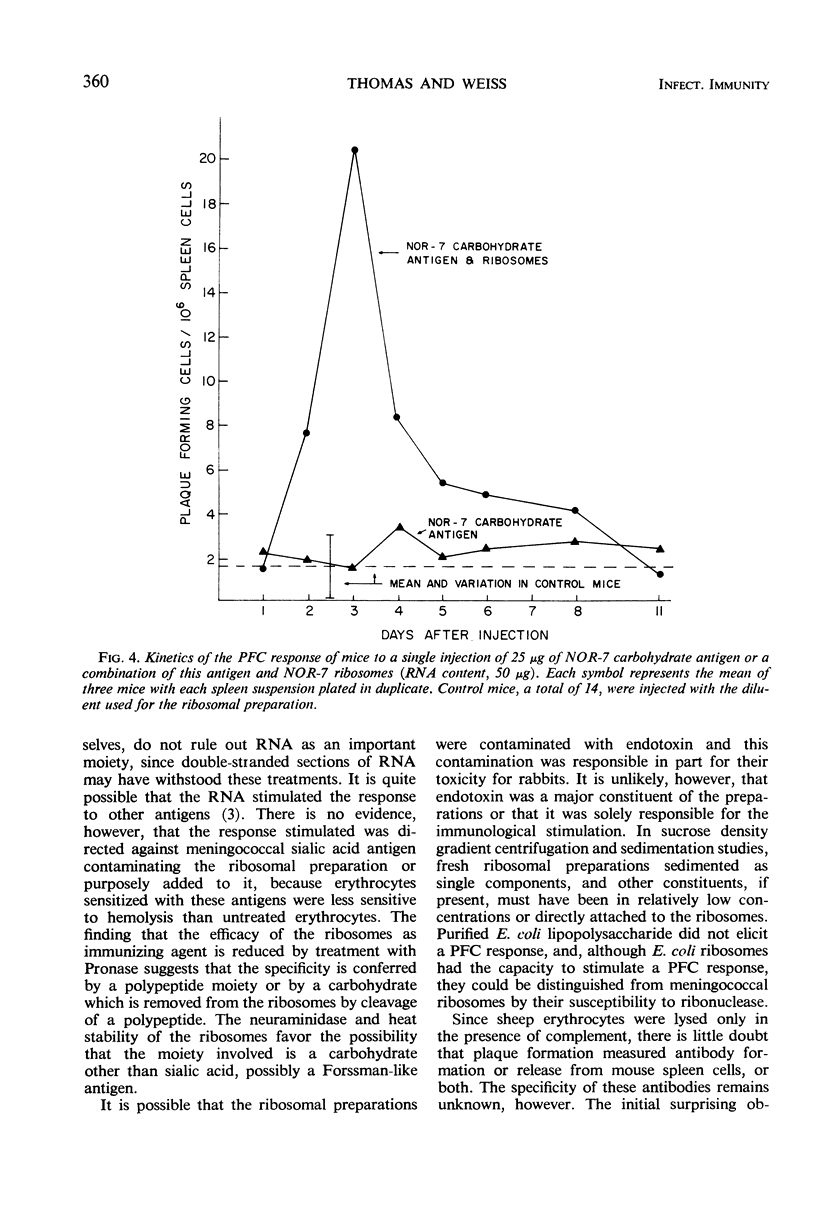
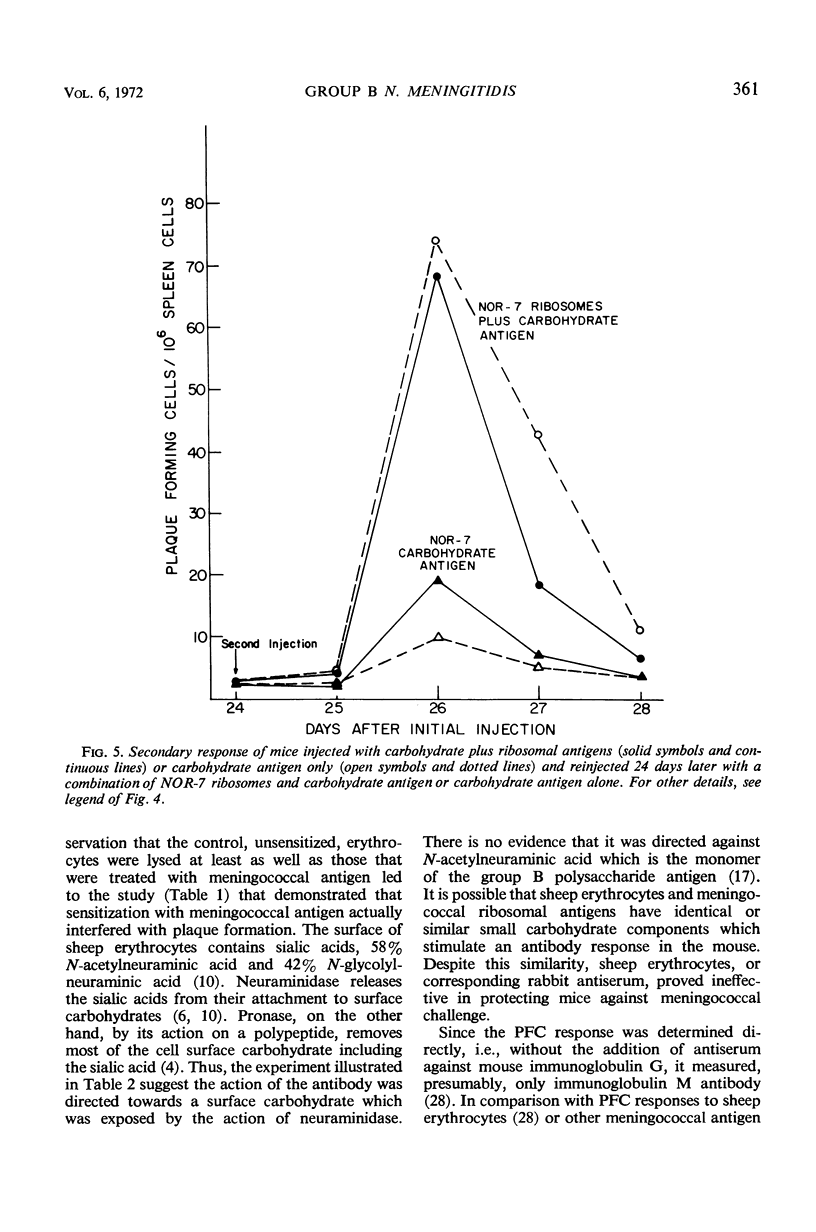
Ribosomes of strain NOR-7 of group B Neisseria meningitidis were isolated by a procedure that included treatment of the cells with sodium dodecyl sulfate, disruption in a French pressure cell, and differential centrifugation. These preparations consisted of 66% ribonucleic acid and 24% protein and sedimented as a single component with a constant of approximately 66S. When used in immunodiffusion tests with homologous rabbit antiserum, untreated ribosomes formed two precipitin lines, when treated with ribonuclease three lines, and when Pronase-digested only one distinct line. Qualitatively indistinguishable reactions were obtained with the same antiserum and ribosomes from group A meningococci, but no precipitation occurred with those of Escherichia coli. When injected into mice, group B ribosomes elicited an increase in the number of antibody-producing spleen cells demonstrable by the hemolytic plaque technique using unsensitized sheep erythrocytes. Sensitization of the erythrocytes with increasing amounts of supernatant fluid of meningococcal cultures progressively reduced the number of demonstrable plaque-forming cells. Neuraminidase treatment of the erythrocytes increased immune hemolysis, whereas Pronase digestion reduced it. Injected mice were protected against homologous and heterologous meningococcal challenge. Both hemolysis and protection-inducing activities of the ribosomes were unimpaired by ribonuclease, but were reduced by Pronase. It is concluded that the immunological response elicited by the meningococcal ribosomes does not involve the group-specific carbohydrate antigen. The immunological mechanism by which the mice are protected against meningococcal challenge remains unknown.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Taudou B., Chuilon S. Glutaraldehyde, cyanuric chloride and tetrazotized O-dianisidine as coupling reagents in the passive hemagglutination test. Immunochemistry. 1969 Jan;6(1):67–76. doi: 10.1016/0019-2791(69)90179-7. [DOI] [PubMed] [Google Scholar]
- Braun W., Firshein W. Biodynamic effects of oligonucleotides. Bacteriol Rev. 1967 Jun;31(2):83–94. doi: 10.1128/br.31.2.83-94.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Human erythrocyte membranes: specific labelling of surface proteins. J Mol Biol. 1971 Jun 28;58(3):775–781. doi: 10.1016/0022-2836(71)90039-8. [DOI] [PubMed] [Google Scholar]
- COOK G. M., EYLAR E. H. SEPARATION OF THE M AND N BLOOD-GROUP ANTIGENS OF THE HUMAN ERYTHROCYTE. Biochim Biophys Acta. 1965 Mar 1;101:57–66. doi: 10.1016/0926-6534(65)90030-8. [DOI] [PubMed] [Google Scholar]
- Coppel S., Youmans G. P. Specificity of Acquired Resistance Produced by Immunization with Listeria monocytogenes and Listeria Fractions. J Bacteriol. 1969 Jan;97(1):121–126. doi: 10.1128/jb.97.1.121-126.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- Evans D. G. Detection of Antibody-Producing Cells of Mice Injected with Antigens of Neisseria meningitidis. Infect Immun. 1970 Feb;1(2):151–156. doi: 10.1128/iai.1.2.151-156.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M. A., Feller W. F., Sibal L. R. Methods for detection of antibody to the mammary tumor virus. J Natl Cancer Inst. 1968 Dec;41(6):1395–1400. [PubMed] [Google Scholar]
- GOTTSCHALK A. The influenza virus neuraminidase. Nature. 1958 Feb 8;181(4606):377–378. doi: 10.1038/181377a0. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. C., Peterson N. R., Weiss E. Characterization of spheroplast membranes of Neisseria meningitidis group B. Infect Immun. 1972 Apr;5(4):612–621. doi: 10.1128/iai.5.4.612-621.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Dunne F. T., Jonssen E. K. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J Biol Chem. 1971 Aug 10;246(15):4703–4712. [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol. 1969 Jun;98(3):914–919. doi: 10.1128/jb.98.3.914-919.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Serum-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):374–380. doi: 10.1128/iai.4.4.374-380.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Long J. L. Simplified method for the production of carbohydrate antigen from Neisseria meningitidis. Appl Microbiol. 1969 Nov;18(5):843–847. doi: 10.1128/am.18.5.843-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S. H., Berry L. J. Antibacterial immunity induced by ribosomal vaccines. J Reticuloendothel Soc. 1970 Jul;8(1):13–24. [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation of highly immunogenic ribosomal fractions of Mycobacterium tuberculosis by use of sodium dodecyl sulfate. J Bacteriol. 1966 Jun;91(6):2139–2145. doi: 10.1128/jb.91.6.2139-2145.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]