Abstract
Objective
Mutations in nucleotide-binding oligomerisation domain-containing protein 2 (NOD2) remain the strongest genetic determinants for Crohn’s disease (CD). Having previously identified vimentin as a novel NOD2-interacting protein, we aimed to investigate the regulatory effects of vimentin on NOD2 function and the association of variants in Vim to CD susceptibility.
Design
Co-immunoprecipitation, fluorescent microscopy and fractionation were used to confirm the interaction between NOD2 and vimentin. HEK293 cells stably expressing wild-type NOD2 or NOD2-frameshift variant (L1007fs) and SW480 colonic epithelial cells were used alongside the vimentin inhibitor Withaferin-A (WFA) to assess effects on NOD2 function using nuclear factor-kappaB (NF-κB) reporter gene, GFP-LC3-based autophagy, and bacterial gentamicin protection assays. International GWAS meta-analysis data were used to test for association of SNPs in Vim to CD susceptibility.
Results
The leucine rich repeat (LRR) domain of NOD2 contained the elements required for vimentin binding; CD-associated polymorphisms disrupted this interaction. NOD2 and vimentin co-localised at the cell plasma membrane and cytosolic mislocalisation of the L1007fs and R702W variants correlated with an inability to interact with vimentin. Use of WFA demonstrated that vimentin was required for NOD2-dependent NF-κB activation, MDP-induced autophagy induction, and that NOD2 and vimentin regulated the invasion and survival properties of a CD-associated adherent-invasive strain E.coli strain. Genetic analysis revealed an association signal across the haplotype block containing Vim.
Conclusion
Vimentin is an important regulator of NOD2 function and a potential novel therapeutic target in the treatment of CD. Additionally, Vim is a candidate susceptibility gene for CD, supporting the functional data.
Keywords: inflammatory bowel disease, Crohn’s disease, NOD2, vimentin, E.coli, autophagy, genetic association studies
INTRODUCTION
Crohn’s disease (CD), a major form of inflammatory bowel disease (IBD), is a chronic disease of the intestinal tract associated with significant morbidity. To date, a substantial volume of work with regard to CD pathogenesis suggests that disease results from a dysregulated immune response to enteric bacteria in a genetically susceptible host.(1) Major CD susceptibility pathways implicated through recent genome-wide association scanning include the innate immune response (NOD2), the more specific, acquired T cell response (IL23R) and autophagy (ATG16L1, IRGM)(2,3). Examination of the disease-associated microbiome has also implicated several potentially causative agents, most notably E.coli strains with an adherent and invasive phenotype (AIEC), which have been consistently isolated by independent investigators from CD patients with ileal disease(4).
Nucleotide oligomerisation domain protein 2 (NOD2) is a receptor for the bacterial cell wall component muramyl dipeptide (MDP)(5). Although originally characterised as a cytosolic receptor, recent studies have demonstrated that NOD2 is localised to and activated at the cell plasma membrane(6,7). Mutations in NOD2 remain the strongest single genetic determinant for CD with three disease-associated polymorphisms, namely Arg702Trp (R702W), Gly908Arg (G908R) and Leu1007fsinsCys (L1007fs), having been shown to affect the leucine rich repeat (LRR) domain(8). Although carriage of two of the CD-associated NOD2 polymorphisms confers a 20-40-fold risk of developing CD, the mechanisms whereby mutations in this gene influence disease pathogenesis are still unclear.
To date only a small number of NOD2 interacting proteins have been identified,(9) and currently there is limited knowledge with regard to their functional effect on NOD2. In our previous yeast-two-hybrid (Y2H) screen we identified a novel interaction between NOD2 and the cytoskeletal protein vimentin(10). Vimentin is the major intermediate filament (IF) protein present primarily in mesenchymal cells with several lines of research having recently demonstrated its role in bacterial pathogenicity(11,12). Here we aimed to characterise this interaction in mammalian cells and to determine how vimentin impacts upon NOD2 function. Additionally we assessed the association signal of the SNPs in the gene encoding vimentin (Vim) with regard to the genetic susceptibility to CD. We report that vimentin interacts with NOD2 at the cell plasma membrane through the LRR domain, with CD-associated polymorphisms in NOD2 abrogating this interaction. We also demonstrate that vimentin regulates NOD2 activities that are dependent upon its plasma membrane localisation (such as nuclear factor-kappa B [NF-κB] activation, autophagy and bacterial handling) as well as showing that Vim is a candidate susceptibility gene for CD.
METHODS
Plasmids
The generation of hemagglutinin-tagged (HA) NOD2 variants (R702W, G908R and L1007fs) have been described previously(10). For the generation of HA-NOD2 (1-693) lacking the C-terminal LRR domain and HA-NOD2 (1-247) comprising only the two N-terminal CARD domains, site directed mutagenesis (Stratagene, San Diego, CA, USA) was used to introduce a stop codon at amino acids 694 or 248. All primers sequences are available on request. NF-κB-luciferase reporter plasmids were a gift from Dr. Lesley Stark (University of Edinburgh).
Drug Treatments
Withaferin-A (WFA) and dimethyl sulphoxide (DMSO) were purchased from Sigma (Dorset, UK). WFA stock solution (10mg/ml in DMSO) was diluted in Dulbecco’s modified Eagle medium (DMEM) to working concentrations between 2-10μM. Equivalent amounts of DMSO only were used as negative control for WFA. L18-MDP was purchased from InvivoGen (San Diego, CA, USA). L18-MDP stock solution (10mg/ml in H20) was diluted in DMEM to a working concentration of 1μg/ml or 50μg/ml. Tumour necrosis factor-alpha (TNF-α) was purchased from PeproTech (Rocky Hill, NJ, USA). TNF-α stock solution (5μg/ml in H20) was diluted in DMEM to a working concentration of 50ng/ml.
Antibodies
Vimentin, LC3, GAPDH and Histone H1 antibodies were purchased from Abcam (Cambridge, UK), E-cadherin antibody from BD biosciences (San Diego, CA, USA), NOD2 antibody from Cayman Chemical (Ann Arbor, Michigan, USA) and HA-11 antibody from Covance (Emeryville, CA, USA). β-Actin and P65/RelA antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Yeast-two-hybrid spotting assay
Yeast strain AH109 was co-transformed with a plasmid containing the full-length vimentin cDNA, wild-type NOD2 or CD-associated mutant NOD2 R702W using a small-scale LiAc transformation procedure (Yeast Protocols Handbook PT3024-1, Clontech, Santa Clara, CA, USA). Co-transformants were selected on SD/Leu−Trp− plates and spotted onto selective media SD/His−Leu−Trp−.
Cell culture, transfection and immunoblotting
HEK293 cells were grown in DMEM and SW480 cells in Leibovitz’s medium supplemented with 10% fetal calf serum (FCS) (Invitrogen, Carlsbad, CA, USA). For Lipofectamine 2000 transfection (Invitrogen, Carlsbad, CA, USA), 2μl of Lipofectamine was used for every 1μg of DNA. Cells were harvested after 16-18h and lysed in ice-cold extraction buffer (50mM Tris [pH 7.6], 150mM NaCl2, 5mM EDTA, 0.5% NP-40, 5mM NaF, 1mM sodium vanadate, 1 × Protease Inhibitor Cocktail) for 30 min and centrifuged. Protein content of cell extracts was measured using Biorad reagent (Biorad, Hertfordshire, UK). Samples were resolved by denaturing gel electrophoresis, typically 4-12% Novex precast gels (Invitrogen, Carlsbad, CA, USA), electro-transferred to Hybond C-extra nitrocellulose membrane (Amersham, Little Chalfont, UK) and incubated with primary antibody overnight at 4°C. After washing, blots were incubated with secondary antibody, either horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody (Dako, Cambridgeshire, UK), for 1h at room temperature (RT). Proteins were visualised by incubation with ECL western blotting analysis system (Amersham, Little Chalfont, UK) or Immobilon Western chemiluminescent HRP substrate (Millipore, Durham, UK).
Generation of stable cell lines
NOD2 or NOD2-L1007fs were sub-cloned into the p3XFLAG-myc-CMV-26 expression vector (Sigma, Dorset, UK). HEK293 cells were transfected with FLAG-EMPTY, FLAG-NOD2 or FLAG-NOD2-L1007fs vectors using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Following 48h transfection, cells were cultured in DMEM supplemented with 800μg/ml geneticin (Invitrogen, Carlsbad, CA, USA) and resistant colonies picked and screened for NOD2 expression using NOD2-specific antibodies.
Immunoprecipitation
For immunoprecipitation of endogenous vimentin or exogenous HA-NOD2, appropriate antibody bound to protein G beads (Amersham, Little Chalfont, UK) was incubated overnight at 4°C with cell extract (~1mg) diluted to a volume of 500μl in extraction buffer. Bead pellets were then washed before being resuspended in 3× SDS-loading buffer and associated proteins analysed by denaturing gel electrophoresis and immunoblotting.
Cell fractionation
For sub-cellular fractionation of HA-NOD2-expressing cells the Qproteome cell compartment kit (Qiagen, Valencia, CA, USA) was used according to the manufacturer’s protocol.
Immunofluorescence microscopy
Cells were seeded on 19-mm borosilicate glass cover slips at a density of 2×104 cells. After 24h, if required, cells were transfected with HA-NOD2 DNA constructs for a further 16h. Cells were then fixed with 4% paraformaldehyde, permeabilised with PBS/0.1% triton X-100 and blocked with PBS containing 10% FCS. For protein detection primary antibodies were incubated overnight at 4°C. Cells were then incubated for 1h at RT with fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isothiocyanate (TRITC) conjugated anti mouse or anti rabbit secondary antibodies (Invitrogen, Carlsbad, CA, USA). Where appropriate, cells were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Images were captured using a Carl Zeiss Axioskop 2 fluorescent microscope and analysed using Image J software (National Institutes of Health, Bethesda, MD, USA).
Luciferase Assay
Dual-luciferase reporter assays (Promega, Madison, WI, USA) were performed according to the manufacturer’s protocol. Briefly, SW480 cells were seeded on 24-well plates for 24h prior to transfection with NF-κB-luciferase wild-type (NF-κB-luc-WT) or mutant with NF-κB binding sites deleted (NF-κB-luc-Mut) (both 200ng) and a control for transfection efficiency (renilla, 100ng). After 16h cells were treated for 6h with L18-MDP (1μg/ml) or for 2h with TNF-α (50ng/ml) in the absence or presence of various concentrations of WFA (2h) or DMSO control (2h) and harvested for luciferase assays.
Gentamicin protection assay
For invasion assays, cells were infected with the E.coli strains isolated from the ileum of a patient with CD (CUICD541-10) or healthy ileum (CUH17-1) at a multiplicity of infection (MOI) of 10 for 2h. After the infection period, cells were washed and incubated for a further 2h in medium containing 100μg/ml gentamicin (Invitrogen, Carlsbad, CA, USA) to kill extracellular bacteria; cells were then lysed for 10min. Lysates were serially diluted and plated on LB agar plates; colonies were enumerated following overnight incubation at 37°C. Where appropriate, cells were pre-incubated with WFA or appropriate antibodies for 30min prior to infection. For survival assays, the procedure was performed as above except that, following incubation in 100μg/ml gentamicin, cells were incubated for a further 24h in medium containing 20μg/ml gentamicin. Where appropriate, cells were treated with WFA post infection.
Analysis of gene-wide susceptibility signal for Vim using an international genome-wide association meta-analysis
As members of the United Kingdom Inflammatory Bowel Disease (IBD) Genetics Consortium and the larger International IBD Genetics consortium (IIBDGC) we now have access to novel genetic data generated from large case-control studies. Although a meta-analysis of CD genome-wide association scans (GWAS) has previously been published(2), we were able to interrogate a new improved meta-analysis of GWAS data imputed with the 1000 genomes reference set (http://www.1000genomes.org). To assess variants in Vim (the gene encoding vimentin on chromosome 10 between positions 17,270,258 - 17,279,592 [Ensembl Release 65 -_ENSG00000026025]; Build 37) for association with CD susceptibility we obtained SNP-specific P values generated from these analyses of 7 individual CD datasets encompassing 5,956 CD patients and 14,927 healthy controls (http://www.broadinstitute.org/mpg/ricopili/). P values were generated with PCAs as covariates, therefore correcting the whole analysis for population stratification and inter-study differences. Haploview software version 4.2 (http://www.broad.mit.edu/mpg/haploview)(13) was then used to visualise the haplotype structure (using solid spine of linkage disequilibrium) surrounding Vim to determine the areas of strongest signal.
Statistical analysis
For the functional assays scanning densitometry was performed using Scion Image Software (National Institutes of Health). Results are reported as the mean ±SD assuming normally distributed variables with P values calculated using an unpaired t-test in GraphPad v4.03 (GraphPad Software, CA, USA). R v2.14.1 was used to generate the GWAS SNP scatterplot utilising the ggplot2 package (http://had.co.nz/ggplot2/).
RESULTS
Vimentin is a NOD2-interacting protein in mammalian cells
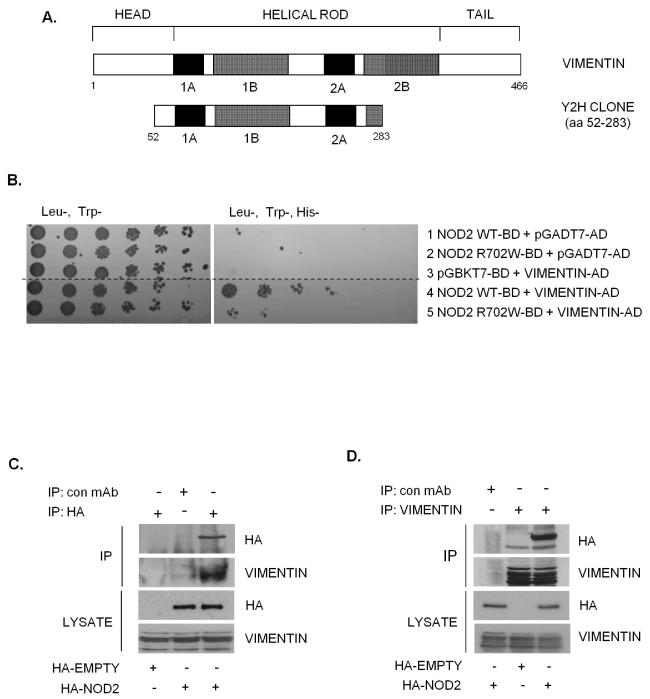
One clone identified by the Y2H screen(10) as a novel NOD2 interactor corresponded with amino acids 52-283 of the IF protein vimentin (Figure 1A). To confirm this interaction, plasmids containing a full-length vimentin open reading frame fused to an activation domain were transformed into yeast along with full length NOD2 or NOD2 with the inactivating polymorphism (R702W). NOD2 interacted with vimentin to activate the reporter gene His3, whereas NOD2-R702W interacted less well with vimentin resulting in reduced activation (Figure 1B). No activation of the reporter gene was observed when vimentin, NOD2 or NOD2-R702W alone were transformed into yeast (Figure 1B). In order to confirm the NOD2-vimentin interaction in a cellular context, cells were transfected with HA-NOD2 and lysates immunoprecipitated with HA-specific antibodies, vimentin-specific antibodies or non-specific antibody as control. Results show that NOD2 and vimentin form a stable protein complex in cell lysates, since the immunoprecipitation of NOD2 enriched vimentin protein (Figure 1C), and conversely the immunoprecipitation of vimentin enriched NOD2 protein (Figure 1D). These results support the Y2H data and demonstrate that vimentin interacts with NOD2 in mammalian cells.
Figure 1. Identification of vimentin as a NOD2 interacting protein.
(a) Schematic showing the structure of vimentin and the vimentin clone identified as binding to NOD2 in our yeast-two-hybrid assay.
(b) Yeast strain AH109 was co-transformed with a plasmid containing the yeast expression vector pGADT7-AD, full-length vimentin cDNA, wild-type NOD2 or CD-associated variant NOD2-R702W as shown. Co-transformants were selected on (Leu-Trp-) plates and spotted onto selective media (His-Leu-Trp-). (Dashed line indicates where irrelevant lanes have been removed).
(c) SW480 cells were transfected with HA-NOD2 or HA-empty vector and extracts immunoprecipitated with HA-11 specific antibodies or non-specific antibody as control. Precipitates were immunoblotted for associated vimentin and NOD2, and direct lysates with HA-11 and vimentin antibodies.
(d) SW480 cells were transfected with HA-NOD2 or HA-empty vector and cell extracts immunoprecipitated with vimentin specific antibodies or non-specific antibody as control. Precipitates were immunoblotted for associated NOD2 and vimentin, and direct lysates with HA-11 and vimentin antibodies.
The LRR domain of NOD2 is the major determinant for binding with vimentin and common CD-associated polymorphisms disrupt this interaction
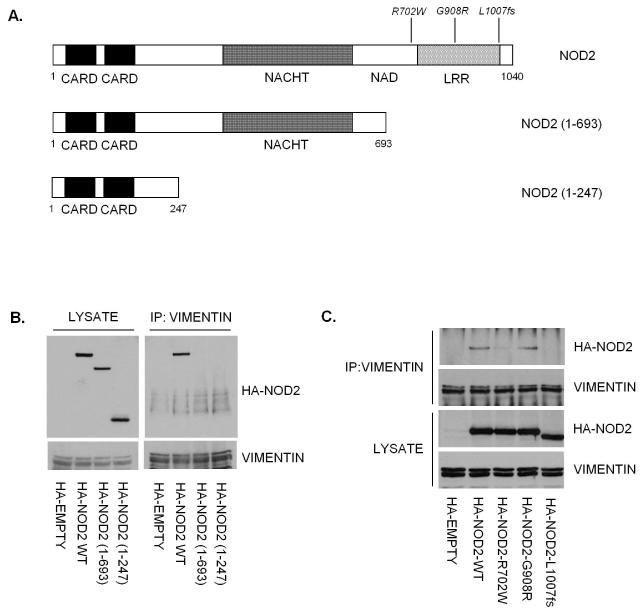
The observation that the NOD2-R702W variant has reduced affinity for vimentin suggests that the LRR domain may be important for mediating this interaction. Using NOD2 mutants, NOD2 (1-693) lacking the C-terminal LRR domain and NOD2 (1-247) comprising only the two N-terminal CARD domains (Figure 2A), we demonstrated by immunoprecipitation that only full-length NOD2 can interact with vimentin (Figure 2B), suggesting that the LRR domain contains the major determinant for binding to vimentin. To further determine whether the CD-associated polymorphisms impact on the interaction with vimentin we used cells expressing NOD2-R702W, NOD2-G908R and NOD2-L1007fs (Figure 2A) to demonstrate that NOD2 and the NOD2-G908R variant can interact with vimentin to a similar degree (Figure 2C). However the NOD2-R702W and NOD2-L1007fs variant proteins seemingly lose their ability to interact with vimentin (Figure 2C).
Figure 2. The LRR domain contains the major determinant for binding to vimentin.
(a) Schematic showing the structure of NOD2 and the location of CD-associated polymorphisms. The NOD2 deletion mutants lacking the LRR domain or lacking both the LRR and NACHT (NAIP, CIITA, HET-E and TP1) domains generated by site-directed mutagenesis are also shown.
(b) HA-NOD2 or the deletion mutants were transfected into HEK293 cells and cell lysates immunoprecipitated with vimentin specific antibodies. Precipitates were immunoblotted for associated NOD2 proteins and vimentin, and direct lysates with HA-11 and vimentin antibodies.
(c) HEK293 cells were transfected with HA-NOD2, HA-NOD2-R702W, HA-NOD2-G908R or HA-NOD2-L1007fs variants, and cell lysates immunoprecipitated with vimentin specific antibodies. Precipitates were immunoblotted for associated NOD2 and vimentin, and direct lysates with HA-11 and vimentin antibodies.
Given that MDP also binds to the LRR domain of NOD2, we investigated whether L18-MDP (a cell permeable derivative of MDP) stimulation would impact on the NOD2-vimentin interaction. Our immunoprecipitation results demonstrate that NOD2 interacts with vimentin to a similar degree in the absence or presence of MDP (Supplementary Figure 1).
NOD2 interacts with vimentin at the plasma membrane and common CD-associated variants are mislocalised to the cytosol
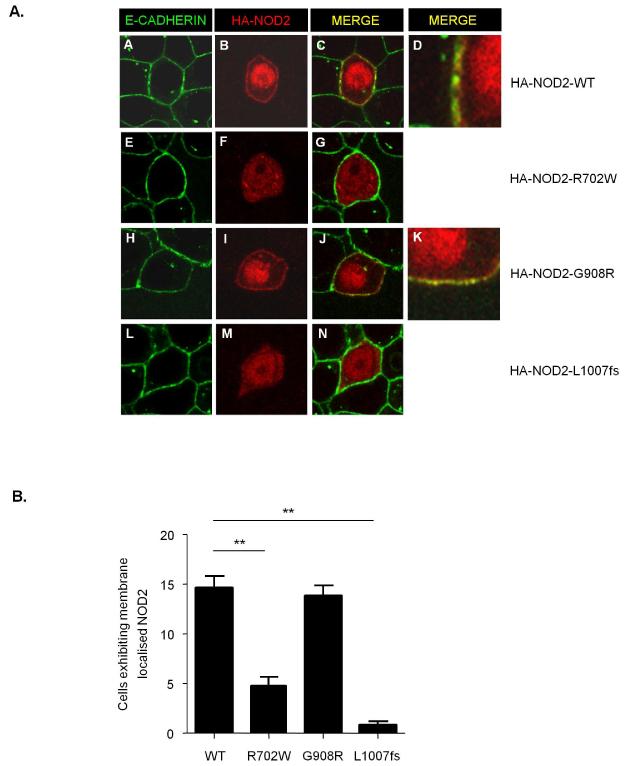
To determine the impact of the LRR-domain polymorphisms on the cellular localisation of NOD2 cells were transfected with HA-NOD2, R702W, G908R or L1007fs variants and immunostained for E-cadherin (basolateral plasma membrane) and HA-NOD2. We found that NOD2 and the G908R variant exhibited diffuse cytosolic staining but with distinct staining clearly observed at the plasma membrane (Figure 3A, panel B, D and I, K respectively and quantified in Figure 3B). In contrast, the R702W and L1007fs variants exhibited diffuse cytosolic staining but little or no distinct staining at the plasma membrane (Figure 3A, panel F and M respectively and quantified in Figure 3B). These results are in agreement with a previous study(14) and confirm the importance of the LRR domain for the correct localisation of NOD2 to the plasma membrane.
Figure 3. Subcellular localisation of CD-associated NOD2 variants.
(a) HEK293 cells were transfected with HA-NOD2, HA-NOD2-R702W, HA-NOD2 - G908R or HA-NOD2-L1007fs variants and immunostained with E-cadherin specific antibodies (green) and exogenous NOD2 with HA-11 antibodies (red). Merged images demonstrate areas where NOD2 and E-cadherin co-localise (yellow) and are highlighted in HA-NOD2 and HA-NOD2-G908R cells (panels d,k).
(b) Quantification of NOD2 membrane localisation in Figure 2A. Twenty cells from three separate fields of view were evaluated for membrane localized NOD2. *** = P<0.001.
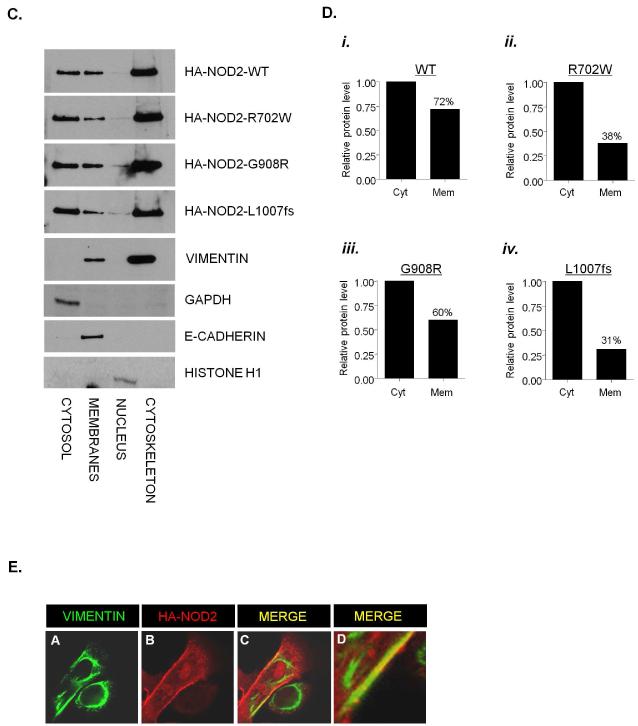
(c) HEK293 cells were transfected with HA-NOD2, HA-NOD2-R702W, HA-NOD2 - G908R or HA-NOD2-L1007fs variants. Following transfection, proteins were separated into specific subcellular fractionations. Each fraction was immunoblotted for NOD2 proteins, vimentin, GAPDH, E-cadherin and Histone H1.
(d) Relative protein membrane fraction of HA-NOD2 (i), HA-NOD2-R702W (ii), HA-NOD2-G908R (iii) and HA-NOD2-L1007fs (iv) in relation to the cytosolic fraction using densitometry. Cyt, cytosol; Mem, membrane.
(e) HEK293 cells were transfected with HA-NOD2 and immunostained with HA-11 antibodies (red) vimentin-specific antibodies (green). Areas where NOD2 and vimentin co-localise are highlighted and appear as yellow.
To further confirm that the R702W and L1007fs variants result in altered cellular distribution of NOD2, cells were transfected with HA-NOD2 or the R702W, G908R or L1007fs variants and cell lysates purified into distinct subcellular compartments (Figure 3C). The cytosolic to membrane ratio of NOD2 and G908W protein was 1: 0.72 and 1: 0.60 respectively (Figures 3D, panel i and iii). In contrast the R702W and L1007fs variant proteins both exhibited decreased membrane localisation with cytosolic to membrane ratios of 1: 0.38 and 1: 0.31 respectively (Figure 3D, panel ii and iv). Each compartment was immunoblotted with a specific marker; vimentin (cytoskeleton), GAPDH (cytosol), E-cadherin (membrane) and Histone-H1 (nucleus) as control. The observation that some L1007fs- and R702W-variant protein remains in the membrane fraction could be due to impurity of the fractionation process, however our positive controls are clean suggesting that NOD2 may localise to other sites within the cell such as the endoplasmic reticulum (ER) and the nuclear or mitochondrial membranes.
Two intriguing observations were that the majority of NOD2 resided in the cytoskeletal fraction (Figure 3C), and that vimentin could be detected in both the cytoskeletal and membrane fractions (Figure 3C). These results further demonstrate that NOD2 can interact with cytoskeletal proteins such as vimentin, and suggest that NOD2 and vimentin may interact throughout the cell and at cell membranes. To test this hypothesis cells were transfected with HA-NOD2 and immunostained for vimentin and HA-NOD2. Consistent with our fractionation results vimentin was distributed throughout the cell and exhibited distinct staining around the nuclear and plasma membranes (Figure 3E, panel A) with NOD2 exhibiting diffuse cytosolic staining but also with distinct staining at the plasma membrane (Figure 3E, panel B). Merging of the two images shows clear areas of co-localisation between NOD2 and vimentin at the plasma membrane (Figure 3E, panel C and D).
WFA disrupts the interaction between vimentin and NOD2 and relocalises NOD2 from the plasma membrane to the cytosol
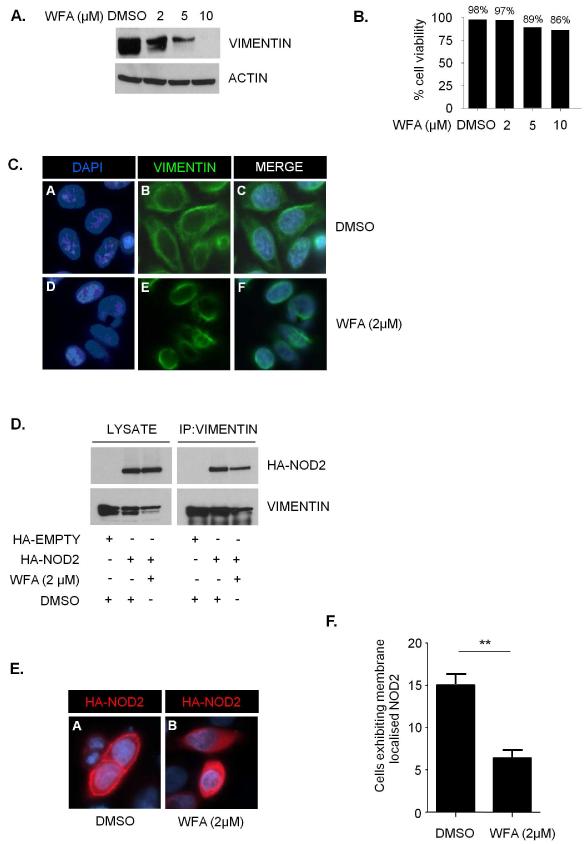
The natural product WFA, a steroidal lactone from the plant Withania Somnifera, binds to and inhibits vimentin(15). To confirm the effects of WFA in our experimental system treatment of cells with 2μM WFA was sufficient to induce vimentin aggregation and cleavage (Figure 4A) with only minor effects on cell viability (Figure 4B). Given that WFA causes vimentin to aggregate and retract from the cell periphery (Figure 4C) we anticipated that WFA would disrupt the interaction between NOD2 and vimentin. Immunoprecipitation demonstrated that WFA treatment significantly reduced the amount of NOD2 protein that interacts with vimentin (Figure 4D). Furthermore, immunofluoresence microscopy demonstrated that WFA treatment resulted in the relocalisation of NOD2 from the plasma membrane to the cytosol (Figure 4E and quantified in Figure 4F). Taken together these results suggest that interaction with vimentin is important for the correct localisation of NOD2 to the plasma membrane.
Figure 4. WFA inhibits vimentin and relocalises NOD2 to the cytosol.
(a) HEK293 cells were treated with WFA or DMSO control as indicated for 2h. Cell extracts were then immunoblotted for vimentin and actin.
(b) The viability of cells treated with WFA or DMSO control for 2h at the indicated concentrations was evaluated using trypan blue exclusion.
(c) HEK293 cells were treated with WFA (2μM) or DMSO control for 2h. Cells were then immunostained with vimentin-specific antibodies and co-stained with DAPI to detect nuclei.
(d) HEK293 cells transfected with HA-NOD2 were treated with WFA (2μM) or DMSO control for 2h, and cell lysates immunoprecipitated with vimentin specific antibodies. Precipitates were immunoblotted for associated NOD2 and vimentin, and direct lysates with HA-11 and vimentin antibodies.
(e) HEK293 cells were transfected with HA-NOD2. Cells were then treated with WFA (2μM) or DMSO control for 2h. Following treatment cells were immunostained with HA-11 antibodies and co-stained with DAPI to detect nuclei.
(f) Quantification of NOD2 membrane localisation in Figure 4E. Twenty cells from three separate fields of view were evaluated for membrane localised NOD2. *** = P<0.001.
WFA inhibits NOD2-dependent NF-κB activation
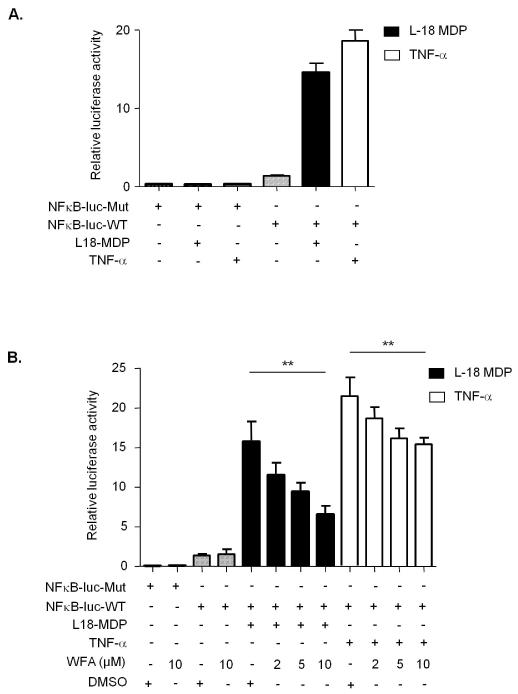
NOD2 has been shown to form a complex with receptor interacting protein 2 (RIP2) and signal downstream to NF-κB from the plasma membrane(6). To assess whether the interaction with vimentin is important for this aspect of NOD2 activity, we performed luciferase reporter assays in SW480 colon epithelial cells which express endogenous NOD2(6). Our results clearly demonstrate that treatment of cells with L18-MDP or TNF-α stimulates the activity of a NF-κB-luciferase reporter plasmid (NF-κB-luc-WT), but not a control reporter plasmid with the NF-κB binding sites deleted (NF-κB-luc-Mut) (Figure 5A). Next, we performed the assay in the absence or presence of increasing concentrations of WFA. Again L18-MDP and TNF-α treatments effectively stimulated NF-κB activity, and pre-treatment with WFA inhibited activation of the reporter in a dose dependent manner (Figure 5B). WFA treatment was able to inhibit NF-κB activation by both L18-MDP and TNF-α, a result that is consistent with a previous report demonstrating that WFA can directly inhibit NF-κB DNA-binding activity and nuclear translocation(16). However, WFA was more effective at inhibiting L18-MDP than TNF-α dependent NF-κB activation (Figure 5B) suggesting some specificity of WFA on NOD2 activity. P65/RelA is a subunit of NF-κB that translocates from the cytosol to the nucleus upon activation(17). To further confirm our findings we monitored P65/RelA translocation in response to L18-MDP and TNF-α treatments in the absence or presence of increasing concentrations of WFA. Pre-treatment with WFA inhibited P65/RelA translocation by both L18-MDP and TNF-α, but again was more effective at inhibiting L18-MDP-dependent NF-κB activation (Supplementary Figure 2). These results suggest that interaction with vimentin and localisation to the plasma membrane are important for NOD2 ability to respond to MDP and signal downstream to NF-κB.
Figure 5. WFA inhibits NOD2-dependent NF-κB signaling.
(a) SW480 cells were transfected with NF-κB-luciferase wild-type (NF-κB-luc-WT) or mutant with NF-κB binding sites deleted (NF-κB-luc-Mut) (200ng) and the internal control renilla (100ng). After 16-18h cells were treated for 6h with L18-MDP (1μg/ml) or for 2h with TNF-α (50ng/ml) and harvested for luciferase assays. The data are derived from triplicate readings.
(b) SW480 cells were transfected with NF-κB-luciferase wild-type (NF-κB-luc-WT) or mutant with NF-κB binding sites deleted (NF-κB-luc-Mut) (200ng) and the internal control renilla (100ng). After 16-18h cells were treated for 6h with L18-MDP (1μg/ml) or for 2h with TNF-α (50ng/ml) in the presence of WFA (2h) or DMSO control (2h) and harvested for luciferase assays. The data are derived from triplicate readings. * = P<0.05, ns = not significant.
WFA inhibits NOD2-dependent autophagy
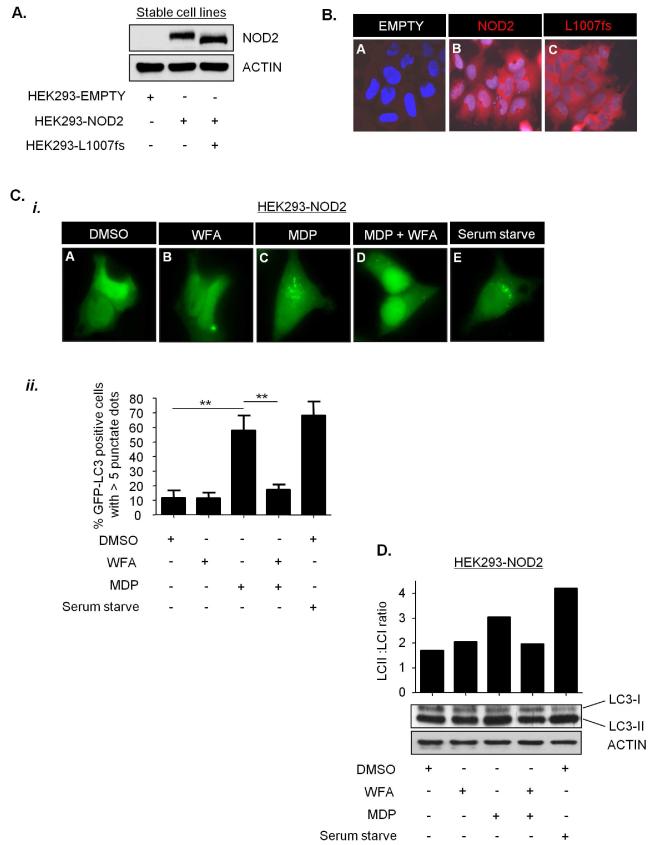
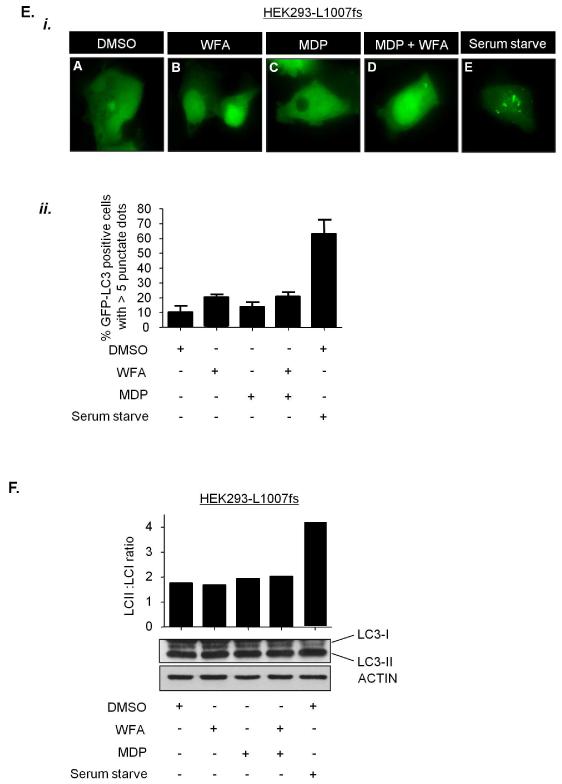
NOD2 has recently been shown to stimulate autophagy in response to MDP, and recruit the critical autophagy protein ATG16L1 to the plasma membrane(18,19). To test whether vimentin regulates the autophagic activity of NOD2 we assessed the effect of WFA on NOD2-dependent autophagy stimulated by L18-MDP. HEK293 cells, which do not express endogenous NOD2,(6) generated to stably express FLAG-EMPTY vector, FLAG-NOD2 or FLAG-NOD2-L1007fs (Figure 6A and 6B) were transiently transfected with a plasmid to express the autophagy marker LC3 fused to GFP. The conversion of LC3-I to its membrane-associated lipidated form LC3-II is a biochemical marker that correlates with the formation of autophagososmes and is commonly used to monitor autophagy levels(20). L18-MDP efficiently stimulated autophagy in HEK293-NOD2 cells and this effect was inhibited by treatment with WFA (Figures 6C and 6D). As a control we assessed the effects of MDP in HEK293-L1007fs cells. As expected, L18-MDP had no effect on autophagy in these cells (Figures 6E and 6F). As a further control serum withdrawal, a potent stimulator of autophagy resulted in a significant increase in LC3 punctae and increase of LC3-II in both HEK293-NOD2 and HEK293-L1007fs cells (Figures 6C-6F). These results are consistent with recent reports demonstrating that plasma membrane localisation is important for NOD2 to activate autophagy and further establish vimentin as an important regulator of NOD2 activity.
Figure 6. WFA inhibits NOD2-dependent autophagy.
(a) HEK293 cells stably expressing FLAG-EMPTY, FLAG-NOD2 or FLAG-NOD2-L1007fs were generated. Cell extracts were immunoblotted with NOD2-specific antibodies.
(b) HEK293 cells stably expressing FLAG-EMPTY, FLAG-NOD2 or FLAG-NOD2-L1007fs were immunostained with NOD2-specific antibodies.
(c) and (e) HEK293 cells stably expressing FLAG-NOD2 (c) or FLAG-NOD2-L1007fs (e) were transfected to express GFP-LC3. Cells were pre-treated for 30min with WFA (2μM) before treatment for 8h with L18-MDP (50μg/ml). Following treatment the percentage of cells exhibiting greater than five distinct LC3 punctate autophagosomes per cell was determined by fluorescence microscopy (i) and quantified in (ii). GFP-LC3 positive cells in 5 separate fields of view were counted for each sample. ** = P<0.01
(d) and (f) HEK293 cells stably expressing FLAG-NOD2 (d) or FLAG-L1007fs (f) were pre-treated for 30min with WFA (2μM) before treatment for 8h with L18-MDP (50μg/ml). Cell extracts were immunoblotted for LC3 and actin. The ratio of LC3-II: LC3-I was measured by densitometry. S/S, serum starved.
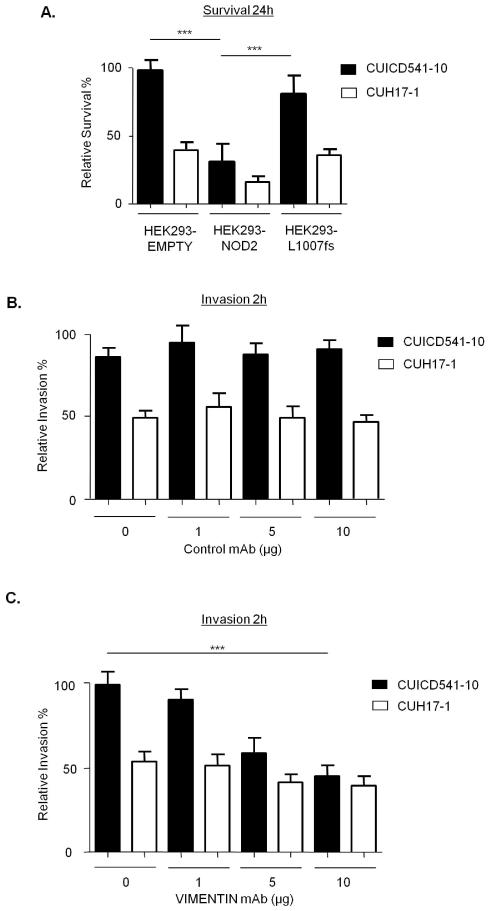
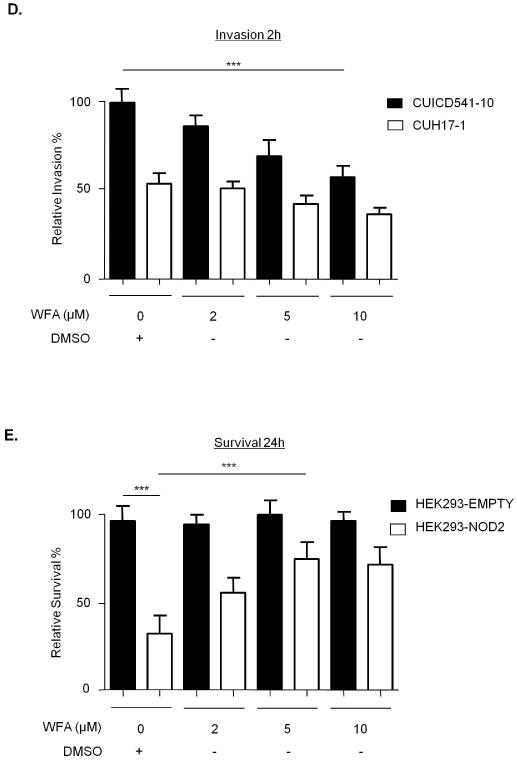
NOD2 and vimentin regulate the invasiveness and survival of AIEC
Recent evidence suggests that AIEC play a putative role in CD(4), and that NOD2 can limit the survival of intracellular bacteria(21). Therefore, we compared the survival of the CD mucosa-associated AIEC strain CUICD541-10(22) in HEK293-NOD2 and HEK293-L1007fs stable cells. As a control we used the non-AIEC strain CUH17-1. Our results show that NOD2 protects host cells by limiting the survival of CUICD541-10 and that this is defective in L1007fs-expressing cells (Figure 7A). In order to assess the role of vimentin in the pathogenicity of the AIEC strain CUICD541-10 we first compared its ability to invade HEK293 cells when vimentin was inhibited by pre-incubation of cells with an antibody that specifically binds to vimentin or with WFA. Our results clearly show that HEK293 express vimentin on the cell surface (Supplementary Figure 3) and that blocking vimentin with specific antibody (compare Figures 7B and 7C) or inhibiting vimentin with WFA (Figure 7D) effectively limit host cell invasion by CUICD541-10 in a dose-dependent manner. In order to assess the importance of vimentin for NOD2 ability to limit bacterial survival, we infected HEK293-NOD2 stable cells with CUICD541-10 or CUH17-1 prior to treatment with increasing concentration of WFA and further incubation. Our results demonstrate that WFA impairs the ability of NOD2 to limit bacterial survival in a dose dependent manner (Figure 7E).
Figure 7. NOD2 and vimentin regulate the invasion and survival of a CD-associated AIEC.
(a) Bacterial survival was evaluated in HEK293 cells stably expressing FLAG-EMPTY, FLAG-NOD2 or FLAG-NOD2-L1007fs by gentamicin protection assay after 24h using the E.coli strain CUICD541-10 or CUH17-1. ** = P<0.01, * = P<0.05.
(b) Suppression of E.coli strain CUICD541-10 or CUH17-1 invasion of HEK293 cells by pre-incubation of host cells with non-specific control antibodies, (c) antibodies that specifically block vimentin, (d) WFA or DMSO control, was assessed by gentamicin protection assay after 2h. *** = P<0.001.
(e) To assess the effect of WFA on E.coli strain CUICD541-10 survival, bacteria were allowed to invade HEK293 cells stably expressing FLAG-EMPTY or FLAG-NOD2 prior to treatment with WFA or DMSO control. The effect of NOD2 and WFA on the survival of CUICD541-10 was assessed by gentamicin protection assay after 24h. ** = P<0.01, * = P<0.05.
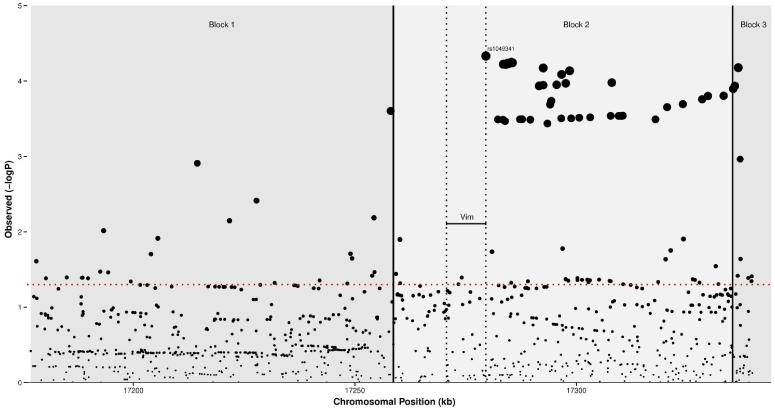
The haplotype block containing Vim is a susceptibility locus for CD
A total of 965 single nucleotide polymorphisms (SNPs) in the haplotype block containing Vim and the two flanking blocks, spanning 163kb, were tested. A total of 51 SNPs attained a P value of <0.001, with 47 of these in the block containing Vim. The strongest SNP (rs1049341) is positioned in the ninth exon (ENSE00001906870) of the Vim gene and attained a P value of 4.67×10−5. A plot of the SNPs in the region is presented in Figure 8.
Figure 8. Results of a meta-analysis of 7 CD genome-wide association studies imputed with the 1000 genomes reference set.
Scatterplot showing −logP values in the 163kb region of Vim for 965 single nucleotide polymorphisms. Vertical dotted lines represent the boundaries of the Vim gene and solid lines the limits of each haplotype block. The −logP value corresponding to P<0.05 is represented by the horizontal (red) dotted line.
DISCUSSION
Since the discovery of NOD2 as a CD susceptibility gene(8) a substantial amount of both basic and clinical research has demonstrated several functions for NOD2, especially with regard to the innate immune response. Studies have now revealed important roles for NOD2 in viral recognition, the regulation of the intestinal microbiome and the regulation of bacterial autophagy(19,23,24). Despite these advances in the understanding of NOD2 function, there is still little known about how NOD2 exerts its effects at the molecular level or how polymorphisms present in the coding region of the LRR domain of NOD2 can lead to the development of CD and its associated complications.
Further to our previous work in a Y2H model(10) we now report that NOD2 and vimentin interact in mammalian cells and demonstrate the LRR domain of NOD2 as the major determinant for binding with vimentin. Importantly, we show that CD-associated polymorphisms in this domain disrupt this interaction. LRR domains are present in a large number of proteins such as Toll-like receptors (TLR) and are important for the regulation of protein-protein interactions(25). The LRR domain of NOD2 is essential for sensing pathogens, with R702W, G908R and L1007fs variants defective in their ability to respond to bacterial lipopolysaccharide (LPS) and peptidoglycan (PGN)(26). Interestingly our study demonstrates that the G908R variant retains the ability to bind vimentin, suggesting that distinct regions within the LRR domain mediate pathogen recognition and vimentin binding. This is consistent with our observation that treatment of cells with the peptidoglycan constituent MDP does not affect the NOD2-vimentin interaction.
LRR domains have also been shown to be important for targeting of proteins to cell membranes(27) and several studies have shown that NOD2 is localised to and functions at the plasma membrane(6,18,28), with the L1007fs protein reported to be mislocalised to the cytosol(6). In agreement with these studies we observe localisation of wild type NOD2 at the plasma membrane and L1007fs to the cytosol, in addition we report the cytosolic mislocalisation of the R702W variant. Importantly, we show that NOD2 and vimentin co-localise at the plasma membrane and that mislocalisation of the L1007fs and R702W variants correlates with an inability to interact with vimentin. It is possible that the L1007fs and R702W polymorphisms cause a conformational change that makes the LRR domain inaccessible thereby preventing the correct localisation of the protein, however our observation that WFA treatment causes the relocalisation of NOD2 to the cytosol, together with our binding studies, strongly suggests that an inability to interact with vimentin is contributing to the mislocalisation of L1007fs and R702W NOD2 variants. Significantly, we demonstrate that NOD2 activities that are dependent upon its plasma membrane localisation, namely NF-κB activation, autophagy induction and bacterial handling are disrupted by interfering with the NOD2-vimentin interaction.
Manipulation of the host cell plasma membrane and associated proteins is an essential step for the uptake, survival and replication of pathogens(29). Several lines of research have demonstrated that vimentin is involved in this process. For example cell-surface-expressed vimentin has been reported as the binding target for several viruses including porcine reproductive respiratory syndrome virus(30) and Japanese encephalitis virus(31). Vimentin structure changes profoundly during bacterial infection and up regulation of vimentin gene expression has been observed during pathogenic H.pylori infection(32). Most relevant to CD pathogenesis are the recent reports that vimentin is expressed on the surface of human brain endothelial cells where it acts as a primary receptor for meningitis associated E. coli (MenPEC) strains expressing the virulence factor IbeA(11), with vimentin-mediated signalling required for IbeA+ E.coli invasion of these cells(11). The IbeA virulence factor is not restricted to MenPEC, and is also present in a variety of other E.coli pathovars, including 18.5% of AIEC strains and 17% of diarrheagenic E.coli(22,33). Significantly, our results demonstrate that vimentin is important for invasion of host cells with a CD-associated AIEC and that inhibiting vimentin interferes with the ability of NOD2 to limit bacterial survival.
Our fractionation results unexpectedly revealed that the majority of NOD2 is bound to the cytoskeleton. NOD2 activity has been shown previously to depend on the integrity of the actin cytoskeleton(28), drugs that disrupt actin have been shown to significantly increase the ratio of soluble to insoluble NOD2(28). Several bacterial pathogens manipulate the host cell cytoskeleton for their own intra- and intercellular motility(34) with the invasion of epithelial cells with AIEC markedly decreased by cytochalasin D and colichicine indicating an important role for actin microfilaments and microtubules(22). An important role for the cytoskeleton in the regulation of autophagy is also emerging(35). Of particular significance is that the proper formation and distribution of autophagosomes depends on the integrity of intermediate filament networks(36), and that autophagic vacuoles are found to be tightly associated with vimentin(37). Our results demonstrating that vimentin is important for NOD2 activity, together with recent studies linking NOD2 and the cytoskeleton with the regulation of autophagy, suggest that NOD2 has evolved to use the cytoskeletal network and in particular cell surface expressed vimentin, as a means to engage with pathogens. NOD2 may then use the intracellular cytoskeleton further to directly transport internalised pathogens via the autophagy machinery to lysosomes for degradation.
Further studies are now required to address the vimentin-NOD2 interaction within the context of CD patients. Our imputed GWAS results further suggest that Vim is a susceptibility gene for CD, raising the possibility that vimentin is dysregulated within a pathway containing NOD2. Although our previous data demonstrated that variations in Vim were not associated with CD susceptibility,(38) this study only included four SNPs and was likely underpowered to detect an effect. Additional genetic studies will now be needed to confirm this association at a genome-wide significant level, including assessing epistasis between Vim and known susceptibility genes. Additionally, in our previous micro-array study(10) we observed increased levels of vimentin expression in inflamed CD biopsies compared with inflamed control biopsies, although it is important to note that our micro-array data does not discriminate vimentin expression between individual cell types. A focus of our current research is to further evaluate vimentin expression in mucosal biopsy tissue and peripheral blood and lamina propria mononuclear cell populations. As expected, our preliminary immunohistochemical staining of ileal sections using anti-vimentin antibody (Supplementary Figure 4) shows little or no expression of vimentin in intestinal epithelial cells, however vimentin is highly expressed in cells throughout the lamina propria in both control and CD patient material. Significantly, using Optical Projection Tomography (OPT), a new technique for three-dimensional imaging of small biological tissues, vimentin expression is observed at the base of small intestinal crypts (Supplementary Figure 5). Whether this involves the Paneth cells or epithelial stem cells needs to be explored further. However given NOD2 is highly expressed in this location(39) our finding is clearly directly pertinent. It is a well-recognised problem within the field that commercially available antibodies are unable to detect endogenous NOD2. Therefore, we feel that (recognising these limitations) the use of an artificial over expression system adequately answers our experimental questions. Once an antibody that can reliably detect endogenous NOD2 becomes available further studies will be required to validate our key findings in tissue and primary cells from CD patients.
In conclusion, this work describes a novel functional link between NOD2 and vimentin, together with the emerging role of vimentin in bacterial pathogenicity, identify vimentin as a potential molecular target for therapeutic intervention for treating CD. We envisage that WFA could be targeted to a specific subgroup of patients with confirmed ileal CD with known AIEC colonisation either as a prophylactic or therapeutic modality. A major focus of our future research will be to assess whether modulation of vimentin represents a potential therapy for treatment of CD, and in this respect it is encouraging that WFA is already a well-recognised therapy with three clinical trials currently registered on the WHO ICT Registry Platform (http://www.who.int/ictrp/en) looking at various health benefits in other conditions such as pulmonary tuberculosis.
Supplementary Material
SUMMARY BOX.
What is already known about this subject?
Evidence suggests that Crohn’s disease (CD) arises from a defective innate immune response to enteric bacteria.
Mutations in nucleotide oligomerisation domain protein 2 (NOD2) remain the strongest single genetic determinant in CD.
Several lines of research have demonstrated that the intermediate filament vimentin is involved in bacterial pathogenicity.
E.coli strains with an adherent and invasive phenotype (AIEC) have been consistently isolated by independent investigators from CD patients with ileal disease.
What are the new findings?
The leucine rich repeat (LRR) domain of NOD2 contains the functional elements required for the binding of vimentin, with CD-associated polymorphisms in this domain disrupting the interaction.
NOD2 and vimentin co-localise at the cell plasma membrane and mislocalisation of the L1007fs and R702W NOD2 variants to the cytosol correlates with an inability to interact with vimentin.
Vimentin regulates NOD2 activities that are dependent upon its plasma membrane localisation, such as nuclear factor-kappaB (NF-κB) activation, autophagy and handling of CD-associated AIEC.
The gene encoding vimentin (Vim) is a novel candidate susceptibility gene for CD.
How might it impact on clinical practice in the foreseeable future?
This study describes a novel functional link between NOD2, vimentin CD-associated E.coli, identifying vimentin as a potential molecular target for the treatment of CD.
The vimentin inhibitor Withaferin-A, a steroidal lactone from the plant Withania Somnifera, could be potentially used for the treatment of CD either as a prophylactic or therapeutic modality.
Acknowledgements
We wish to thank Dr Charlie Lees and Dr Gwo-Tzer Ho for their critical review of the manuscript. We also thank Dr Lesley Stark for technical assistance and reagents.
Funding: CS is funded by a Medical Research Council project grant (No. G0800759). PH is funded by a Medical Research Council project grant for PICTS (No. G0800675).
Independence of researchers: No author worked with or on behalf of any sources of funding during the preparation of the manuscript.
Appendix
The International Inflammatory Bowel Disease Genetics Consortium consists of the following people:
Tariq Ahmad6, Carl A. Anderson3, Vito Annese15,64, Robert N. Baldassano20, Tobias Balschun8, Murray Barclay10, Jeffrey C. Barrett3, Theodore M. Bayless21, Joshua C. Bis11, Stephan Brand22, Steven R. Brant21, Suzanne Bumpstead3, Carsten Buning23, Judy H. Cho19,63, Albert Cohen24, Jean-Frederick Colombel25, Mario Cottone26, Mauro D’Amato57, Renata D’Inca29, Mark J. Daly65, Ted Denson27, Marla Dubinsky30, Richard H. Duerr47,61, Cathryn Edwards31, David Ellinghaus1, Tim Florin32, Denis Franchimont33, Andre Franke1, Richard Gearry10, Michel Georges13, Jurgen Glas22,34,35, Andre Van Gossum33, Anne M. Griffiths55, Stephen L. Guthery36, Hakon Hakonarson4,20, Talin Haritunians14, Jean-Pierre Hugot39, Dirk J de Jong66, Luke Jostins3, Subra Kugathasan59, Gerd Kullak-Ublick54, Anna Latiano15, Debby Laukens28, Ian Lawrance40, James Lee9, Charlie W. Lees7, Marc Lemann41, Arie Levine42, Cecile Libioulle43, Edouard Louis43, John C. Mansfield60, Christopher G. Mathew16, Dermot P.B. McGovern2,14, Mitja Mitrovic12,67, Grant W. Montgomery17, Craig Mowat44, William Newman45, Orazio Palmieri15, Julián Panés46, Miles Parkes9, Anne Phillips44, C.Y. Ponsioen52, Uros Potočnik67, Natalie J. Prescott16, Deborah D. Proctor19, Graham L. Radford-Smith5, Miguel Regueiro47, John D. Rioux56, Rebecca Roberts10, Jerome I. Rotter14, Paul Rutgeerts48, Jeremy Sanderson49, Miquel Sans46, Stefan Schreiber1,62, Philip Schumm18, Frank Seibold50, Yashoda Sharma19, Mark S. Silverberg51, Lisa A. Simms5, A. Hillary Steinhart51, Stephan R. Targan2, Kent D. Taylor14, Leif Torkvist53, Severine Vermeire48, Jonas Halfvarson37, H.W. Verspaget38, Martine De Vos28, Thomas Walters55, Kai Wang4, Rinse K. Weersma58, David Whiteman17, Cisca Wijmenga12.
(1) Institute of Clinical Molecular Biology, Christian-Albrechts-University Kiel, Schittenhelmstr. 12, D-24105 Kiel, Germany
(2) Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, California, USA.
(3) Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, UK
(4) Center for Applied Genomics, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA
(5) Inflammatory Bowel Disease Research Group, Queensland Institute of Medical Research, Brisbane, Australia.
(6) Peninsula College of Medicine and Dentistry, Barrack Road, Exeter, UK
(7) Gastrointestinal Unit, Molecular Medicine Centre, University of Edinburgh, Western General Hospital, Crewe Road, Edinburgh, UK
(8) popgen Biobank, Christian-Albrechts University Kiel, D-24105 Kiel, Germany
(9) Gastroenterology Research Unit, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK
(10) Department of Medicine, University of Otago, Christchurch 8140, New Zealand
(11) Cardiovascular Health Research Unit, Department of Medicine, University of Washington, Seattle, USA
(12) Department of Genetics, University Medical Center Groningen, Groningen, the Netherlands
(13) Department of Genetics, Faculty of Veterinary Medicine, University of Liège B43, 20 Bd de Colonster, 4000 Liège, Belgium
(14) Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, California, USA
(15) Unit of Gastroenterology, IRCCS-CSS Hospital, San Giovanni Rotondo, Italy
(16) Department of Medical and Molecular Genetics, King’s College London School of Medicine, Floor 8 Tower Wing, Guy’s Hospital, London, UK
(17) Molecular Epidemiology, Queensland Institute of Medical Research, Brisbane, Australia 4006
(18) Department of Health Studies, University of Chicago, Chicago, Illinois, USA
(19) Section of Digestive Diseases, Department of Medicine, Yale University, New Haven, Connecticut, USA
(20) Department of Pediatrics, Center for Pediatric Inflammatory Bowel Disease, The Children’s Hospital of Philadelphia, Philadelphia, USA
(21) Inflammatory Bowel Disease Center, Dept. of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A
(22) Department of Medicine II, University Hospital MunichGrosshadern, Ludwig-Maximilians-University, Munich, Germany
(23) Department of Gastroenterology, Charité, Campus Mitte, Universitätsmedizin Berlin, Berlin, Germany
(24) Montreal Jewish General Hospital, Montréal, Québec, Canada
(25) Registre EPIMAD, Université de Lille, Lille, France
(26) Unit of Gastroenterology, Cervello Hospital, Palermo, Italy
(27) Pediatric Gastroenterology, Cincinnati Children’s Hospital. Medical Center. 3333 Burnet Ave, Cincinnati, USA
(28) Department of Hepatology and Gastroenterology, Ghent University Hospital, Ghent, Belgium
(29) Division of Gastroenterology, University Hospital Padua, Italy
(30) Department of Pediatrics, Cedars Sinai Medical Center, Los Angeles, CA, USA
(31) Torbay Hospital, Torbay, Devon, UK
(32) Department of Gastroenterology, Mater Health Services, Brisbane, Australia 4101
(33) Department of Gastroenterology, Erasmus Hospital, Free University of Brussels, Brussels, Belgium
(34) Department of Preventive Dentistry and Periodontology, Ludwig-Maximilians-University, Munich, Germany
(35) Department of Human Genetics, RWTH Aachen, Germany
(36) Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT, USA
(37) Department of Medicine, Örebro University Hospital, Örebro, Sweden
(38) Dept of Gastroenterology, Leiden University Medical Center, Leiden, The Netherlands
(39) Université Paris Diderot, Paris, France
(40) School of Medicine and Pharmacology, The University of Western Australia, Fremantle, Australia 6160
(41) GETAID group, Université Paris Diderot, Paris, France
(42) Pediatric Gastroenterology Unit, Wolfson Medical Center and Sackler School of Medicine, Tel Aviv University, Israel
(43) Division of Gastroenterology, CHU, Université de Liège, Liège, Belgium
(44) Dept of Medicine, Ninewells Hospital and Medical School, Dundee, UK
(45) Department of Medical Genetics, University of Manchester, Manchester, UK
(46) Department of Gastroenterology, Hospital Clínic/IDIBAPS. CIBER EHD. Barcelona, Spain
(47) Division of Gastroenterology, Hepatology and Nutrition, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
(48) Division of Gastroenterology, University Hospital Gasthuisberg, Leuven, Belgium
(49) Dept Gastroenterology, Guy’s & St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, London, UK
(50) Division of Gastroenterology, Inselspital, University of Bern, Bern, Switzerland
(51) Mount Sinai Hospital Inflammatory Bowel Disease Centre, University of Toronto, Canada
(52) Department of Gastroenterology, Academic Medical Center, Amsterdam, the Netherlands
(53) Department of Clinical Science Intervention and Technology, Karolinska Institutet, Stockholm, Sweden
(54) Division of Clinical Pharmacology and Toxicology University Hospital Zurich, CH-8091 Zurich, Switzerland
(55) The Hospital for Sick Children, University of Toronto, Ontario, Canada
(56) Université de Montréal and the Montreal Heart Institute, Research Center, Montréal, Québec, Canada
(57) Department of Biosciences and Nutrition, Karolinska Institute, Stockholm, Sweden
(58) Department of Gastroenterology, University Medical Center Groningen, Groningen, The Netherlands
(59) Department of Pediatrics; Emory University School of Medicine, Atlanta, GA, USA
(60) Institute of Human Genetics, Newcastle University, Newcastle upon Tyne, UK
(61) Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
(62) Department for General Internal Medicine, Christian-Albrechts-University, Schittenhelmstr. 12, D-24105 Kiel, Germany
(63) Department of Genetics, Yale School of Medicine, New Haven CT, USA
(64) Unit of Gastroenterology, University Hospital Careggi Florence, Italy
(65) Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA
(66) Department of Gastroenterology and Hepatology, Radboud University Medical Center Nijmegen, the Netherlands
(67) University of Maribor, Faculty of Medicine, Center for human molecular genetics and pharmacogenomics, Slomskov trg 15, 2000 Maribor
Footnotes
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) No authors have support from any commercial company for the submitted work; (2) all authors have no relationships with any company that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) all authors have no non-financial interests that may be relevant to the submitted work.
Copyright/licence for publication: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its licensees, to permit this article (if accepted) to be published in BMJ editions and any other BMJPG products and to exploit all subsidiary rights, as set out in our licence. (http://resources.bmj.com/bmj/authors/checklists-forms/licence-for-publication)
Ethics approval: Not required.
Access of researchers to data: All authors had open access to the raw dataset and statistical methods used during the preparation of the manuscript.
REFERENCES
- 1.Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–53. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 2.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42(12):1118–25. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson P, Van Limbergen J, Wilson DC, et al. Genetics of childhood-onset inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):346–61. doi: 10.1002/ibd.21283. [DOI] [PubMed] [Google Scholar]
- 4.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127(2):412–21. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 5.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 6.Barnich N, Aguirre JE, Reinecker HC, et al. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-κB activation in muramyl dipeptide recognition. J Cell Biol. 2005;170(1):21–6. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philpott DJ, Girardin SE. Nod-like receptors: sentinels at host membranes. Curr Opin Immunol. 2010;22(4):428–34. doi: 10.1016/j.coi.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 9.Van Limbergen J, Philpott D, Griffiths AM. Genetic profiling in inflammatory bowel disease: from association to bedside. Gastroenterology. 2011;141(5):1566–71. e1. doi: 10.1053/j.gastro.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Nimmo ER, Stevens C, Phillips AM, et al. TLE1 modifies the effects of NOD2 in the pathogenesis of Crohn’s disease. Gastroenterology. 2011;141(3):972–81. e1–2. doi: 10.1053/j.gastro.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Chi F, Jong TD, Wang L, et al. Vimentin-mediated signalling is required for IbeA+ E. coli K1 invasion of human brain microvascular endothelial cells. Biochem J. 2010;427(1):79–90. doi: 10.1042/BJ20091097. [DOI] [PubMed] [Google Scholar]
- 12.Mor-Vaknin N, Punturieri A, Sitwala K, et al. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5(1):59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 13.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 14.Lecine P, Esmiol S, Metais JY, et al. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007;282(20):15197–207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- 15.Bargagna-Mohan P, Hamza A, Kim YE, et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14(6):623–34. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol. 2009;9(5):614–9. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 18.Travassos LH, Carneiro LA, Ramjeet M, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11(1):55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 19.Cooney R, Baker J, Brain O, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–7. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 20.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4(2):151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hisamatsu T, Suzuki M, Reinecker HC, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124(4):993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 22.Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. Isme J. 2007;1(5):403–18. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 23.Petnicki-Ocwieja T, Hrncir T, Liu YJ, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106(37):15813–8. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabbah A, Chang TH, Harnack R, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10(10):1073–80. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci. 1994;19(10):415–21. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Bonen DK, Ogura Y, Nicolae DL, et al. Crohn’s disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 2003;124(1):140–6. doi: 10.1053/gast.2003.50019. [DOI] [PubMed] [Google Scholar]
- 27.Legouis R, Jaulin-Bastard F, Schott S, et al. Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep. 2003;4(11):1096–102. doi: 10.1038/sj.embor.7400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legrand-Poels S, Kustermans G, Bex F, et al. Modulation of Nod2-dependent NF-kappaB signaling by the actin cytoskeleton. J Cell Sci. 2007;120(Pt 7):1299–310. doi: 10.1242/jcs.03424. [DOI] [PubMed] [Google Scholar]
- 29.Cossart P, Roy CR. Manipulation of host membrane machinery by bacterial pathogens. Curr Opin Cell Biol. 2010;22(4):547–54. doi: 10.1016/j.ceb.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JK, Fahad AM, Shanmukhappa K, et al. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J Virol. 2006;80(2):689–96. doi: 10.1128/JVI.80.2.689-696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang JJ, Yu CY, Liao CL, et al. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell Microbiol. 2011 doi: 10.1111/j.1462-5822.2011.01624.x. [DOI] [PubMed] [Google Scholar]
- 32.Bryant AE, Bayer CR, Huntington JD, et al. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685–92. doi: 10.1086/504261. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Medina M, Mora A, Blanco M, et al. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. J Clin Microbiol. 2009;47(12):3968–79. doi: 10.1128/JCM.01484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature. 2007;449(7164):827–34. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- 35.Monastyrska I, Rieter E, Klionsky DJ, et al. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc. 2009;84(3):431–48. doi: 10.1111/j.1469-185X.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000;69:303–42. doi: 10.1146/annurev.biochem.69.1.303. [DOI] [PubMed] [Google Scholar]
- 37.Doherty FJ, Wassell JA, Mayer RJ. A putative protein-sequestration site involving intermediate filaments for protein degradation by autophagy. Studies with microinjected purified glycolytic enzymes in 3T3-L1 cells. Biochem J. 1987;241(3):793–800. doi: 10.1042/bj2410793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimmo ER, Stevens C, Phillips AM, et al. TLE1 Modifies the Effects of NOD2 in the Pathogenesis of Crohn’s Disease. Gastroenterology. 2011;141(3):972–81. e2. doi: 10.1053/j.gastro.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 39.Ogura Y, Lala S, Xin W, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52(11):1591–7. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.