Abstract
Heat shock proteins (HSPs), originally identified as heat-inducible gene products, are a family of highly conserved proteins that respond to a wide variety of stress including oxidative stress. Although both acute and chronic oxidative stress have been well demonstrated to induce HSP responses, little evidence is available whether increased HSP levels provide enhanced protection against oxidative stress under elevated yet sublethal temperatures. We studied relationships between oxidative stress and HSPs in a physiological model by using Garra rufa (doctor fish), a fish species naturally acclimatized to different thermal conditions. We compared fish naturally living in a hot spring with relatively high water temperature (34.4±0.6 °C) to those living in normal river water temperature (25.4±4.7 °C), and found that levels of all the studied HSPs (HSP70, HSP60, HSP90, HSC70 and GRP75) were higher in fish living in elevated water temperature compared with normal river water temperature. In contrast, indicators of oxidative stress, including protein carbonyls and lipid hydroperoxides, were decreased in fish living in the elevated temperature, indicating that HSP levels are inversely associated with oxidative stress. The present results provide evidence that physiologically increased HSP levels provide protection against oxidative stress and enhance cytoprotection.
Keywords: Thermal, Oxidation, Stress, Regulation, Garra rufa
Introduction
Heat shock proteins (HSPs) are a highly conserved and ubiquitously expressed family of proteins that respond to a wide variety of physical and metabolic stress, including oxidative and thermal stress [1], [2], [3]. HSP response is usually transcriptionally regulated via the heat shock factor-1 (HSF-1), although post-transcriptional mRNA stabilization provides an additional mode of regulation [4]. Following exposure to various types of stress, the synthesis of HSPs is rapidly initiated [3], [4], [5], [6]. Subsequently, HSPs prevent protein denaturation and aggregation, and also assist in their re-folding [3], [6]. Both acute and chronic oxidative stress induce HSP responses [1]. However, there is little evidence available whether elevated HSP levels provide extra protection against oxidative stress.
HSPs are closely related to thermal tolerance that refers to an ability to survive in elevated temperatures in otherwise lethal acute stress stimuli. In contrast, acclimatization refers to chronic adaptation to altered non-lethal conditions. The desert pupfish living in hot springs in California and Nevada are generally considered as the most heat-tolerant fish species. Indeed, Cyprinodon diabolis lives in Devil's Hole, a hot spring with water temperature as high as 33.9 °C. Moreover, a population of Garra rufa (doctor fish) living in Kangal hot spring in Turkey is dwelling in even more extreme temperatures lethal for most other fish species. Garra rufa is naturally found in the river basins of the Northern and Central Middle East, and they are capable of acclimatizing to live in the warm outdoor springs and pools fed by thermal springs with temperatures ranging from 33 to 35 °C.
Although it is well accepted that oxidative stress induces protective HSP response in tissues, however, the protective role of physiologically elevated levels of HSP to oxidative stress has not been demonstrated. We therefore studied the association of elevated HSPs with oxidative stress markers by utilizing a physiological model of thermal stress adaptation in Garra rufa, acclimatized to different thermal conditions. The presence of a functional HSP system in this fish species has been recently confirmed [7]; thus it was possible to evaluate the relationships between HSPs and oxidative stress by comparing populations of Garra rufa found in elevated water temperature in Kangal hot spring to those found in normal river water temperature. We sought evidence whether sustained elevation of HSPs can decrease oxidative stress and enhance cytoprotection.
Materials and methods
Sample collection
Specimens of Garra rufa were obtained from two different locations in Turkish province of Sivas: Hamam creek representing normal river conditions with water temperature of 25.4±4.7 °C (C, n=20 fish) and Kangal hot spring representing elevated water temperature conditions (34.4±0.6 °C) (HS, n=19 fish). The fish were caught by pulsed DC electrofishing equipment, immediately anesthetized with 100 mg/L of MS222 (Sigma, St. Louis, MO), and sacrificed thereafter. The fish were measured, weighted and dissected fresh on the ice blocks and lateral body wall muscles were immediately frozen in liquid nitrogen and stored at −80 °C until analysis. The fish caught from Hamam creek and Kangal hot spring was aged-matched.
Preparation of samples for Western blot analysis
The frozen fish samples were pulverized with a mortar under liquid nitrogen and sonicated in a buffer containing 25% glycerol, 0.42 mol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 20 mmol/L HEPES, 5 µmol/L DTT and 5 µmol/L PMSF at +4 °C. Protein extracts (30 µg protein per lane) were electrophoresed together with molecular weight markers on SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Millipore, Bedford, MA) [5], [8]. Equal transfer was confirmed by reversible protein staining of the nitrocellulose membrane with Ponceau S (Sigma).
Western blot assay for stress protein expression
Samples were analyzed for expression of stress proteins using standard Western blot techniques, as previously described [5], [8]. After blocking with 5% (w/v) fat-free milk solution at 37 °C for 60 min, the membranes were treated with following monoclonal antibodies with catalog numbers information 60 kDa HSP (HSP60- SPA-806), the 72 kDa inducible form of HSP (HSP70: SPA-810), the 73 kDa constitutive form of HSC (HSC70: SPA-815), the 90 kDa HSP (HSP90: SPA-835), glucose-regulated protein 75 (GRP75: SPA-825) (all from StressGen, Victoria, Canada) or β-actin (Sigma, A5441). Certain antibodies (HSP60, HSP70, HSP90 and β-actin) were tested for reactivity to fish by the manufacturer. In our hands, all primary antibodies used in this study reacted to the target proteins with high sensitivity and specificity. This was confirmed by the correct molecular mass of the protein and comparisons of the Western blot bands with those from other species where the antibody reactivity has already been tested. In addition, horseradish peroxidase-conjugated anti-mouse (StressGen, SAB-100), anti-rabbit (Calbiochem, La Jolla, CA, 401393) and anti-rat immunoglobulins (Zymed Laboratories, San Francisco, CA, 62-9520) were used as secondary antibodies. The membranes were developed with an enhanced chemiluminescence method (NEN Life Sciences, Boston, MA) and quantified using Scion Image software (ScionCorp, Frederick, ML).
Protein carbonyls assay
Protein carbonyls were measured using a modified ELISA method [5]. Briefly, protein derivatization was carried out in 1.5 mL tubes, and 45 µL of dinitrophenylhydrazine solution was added to each 15 µL of sample. The final protein concentration was 1 mg/mL. Absorbance was read at 490 nm using a microplate reader. All absorbances were blanked against PBS. A seven-point standard curve of oxidized BSA was included with each plate.
Lipid hydroperoxides (LPO) assay
Lipid hydroperoxides (LPO) in whole plasma were determined spectrophotometrically based on oxidation of Fe2+ to Fe3+ by lipid hydroperoxides under acidic conditions, followed by complex formation with xylenol orange [2].
Statistics
Student's t-test for independent samples was used for group comparisons. The equality of variances was checked with Levene's test. Statistical significance was set at p<0.05.
Results
Water chemistry
Mean contents of dissolved oxygen content were lower in HS water environment compared to the C environment (4.6±0.8 vs. 7.0±2.1 mg/L, p<0.01), otherwise no significant difference was observed for water chemistry and heavy metal content (Ekmekçi et al., unpublished).
Stress protein expression by Western blot
Higher levels of HSP60 (p<0.001), HSP70 (p<0.001), HSP90 (p<0.001), GRP75 (p<0.001) and HSC70 (p<0.01) were observed in HS muscle tissue compared with C (Fig. 1).
Fig. 1.
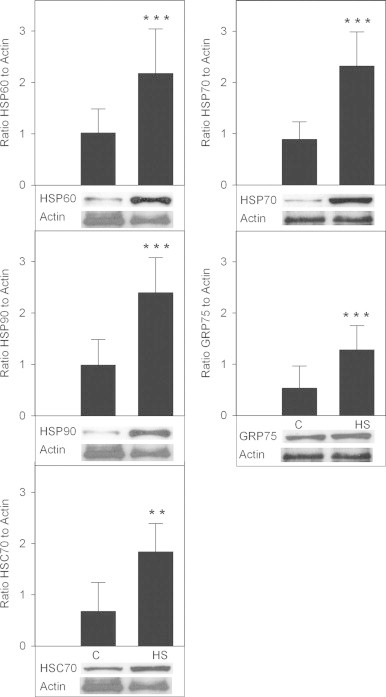
Stress protein levels in Garra rufa fish grown in normal river temperature (C, n=20) or in hot spring representing elevated water temperature conditions (HS, n=19) by Western blot analysis. Data are shown as mean±standard deviation. A representative Western blot is shown. Statistical symbols: ** p<0.01; *** p<0.001.
Markers of oxidative stress
The levels of protein carbonyls and lipid hydroperoxides as indicators of oxidative stress were lower in HS compared with C (p<0.001, p<0.01 respectively) (Fig. 2).
Fig. 2.
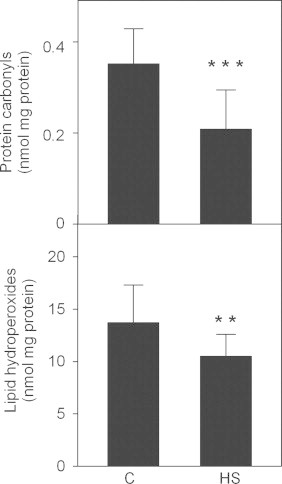
Markers of oxidative stress in Garra rufa fish grown in normal river temperature (C, n=20) or in hot spring representing elevated water temperature conditions (HS, n=19). Data are shown as mean±standard deviation. Statistical symbols: ** p<0.01; *** p<0.001.
Discussion
The present study reports that all the investigated heat shock proteins (HSP70, HSP60, HSP90, HSC70 and GRP75) were increased in fish living in hot spring, characterized by substantially elevated water temperature compared to that found in normal river (mean values of 34.4 vs. 25.4 °C). These increases in HSP levels were parallel to the decrease in protein carbonyls and lipid hydroperoxides, indicating decreased protein and lipid oxidation as a consequence of lower oxidative stress. Therefore, we suggest that acclimatization to elevated temperature results in HSP-mediated adaptation and cytoprotection against oxidative damage.
We observed that increased HSP levels were associated with decreased oxidative stress markers in Garra rufa. This provides a novel fish model for acclimatization studies. The onset temperature for HSP synthesis may not be set for a given species, but may instead vary upon acclimatization conditions [9], [10], [11], [12]. We consider the heat stress in hot spring to be sufficient for HSP response (mean temperature 34.4 °C), since the temperature threshold in fish has been reported to vary from 28 to 33 °C [13], [14]. Moreover, the turnover of HSP70 mRNA seems to be longer in fish than in other species [15] and increased levels of stress proteins in fish living close to their temperature survival may be an adaptive response to maintain correct protein folding under chronic heat stress.
In addition to the major inducible HSP70, our chronic heat stress model also demonstrated increases in the levels of the constitutively expressed HSC70. While HSC70 acts as an important chaperone in non-stressed cells, its role upon exposure to stress is unknown. Our results are in line with previous studies in which HSC70 induction was observed upon exposure of Zebrafish embryos to heat shock [16] and during caudal fin regeneration [17]. Together with the HSP70 family of proteins, HSP90 regulates steroid–hormone response, protein translocation and degradation [18], all crucial systems in chronic stress. The HSP90 and HSP70 proteins have been shown to prevent apoptotic cell death, while HSP60 is linked to pro-apoptotic signaling [19] Thus, the increased HSP levels found in the present study may be crucial for protein repair, regulation of stress–hormone response, protein synthesis and degradation, prevention of apoptosis, and in cellular signaling optimized to the high temperature in Garra rufa.
In the present study, thermal stress increased HSP levels that associated with lower protein and lipid oxidation, providing evidence that physiological increase in HSP levels can protect against oxidative stress. In contrary, other inducers of HSP response, including heavy metals or environmental toxins, hypoxia, handling, crowding, osmotic stress and cold shock induce oxidative stress in fish [20], [21]. Nutrition may also account for the HSP response [22], and adds a potential variable between the two geographical aquatic locations used in the present study. It must be emphasized, however, that since the mean dissolved oxygen content was significantly lower in high aquatic temperature compared to the normal river environment, the fish living in the elevated temperature environment were exposed to relative hypoxia. It has been recently shown that tissue hypoxia is associated with mitochondrial dysfunction and a subsequent increase in the generation of excessive quantities of ROS [23]. This paradoxical increase in ROS production and subsequent development of oxidative stress in hypoxic environment has also been reported in fish [24] and other species [25]. Therefore, the lower levels of oxidative stress observed in those fish with increased HSP levels indicate enhanced HSP-mediated protection against oxidative damage.
In conclusion, our results provide evidence that physiological increases in HSP levels protect against oxidative stress. Moreover, our results underline the use of Garra rufa as a useful fish model to study mechanisms of environmental acclimatization.
Acknowledgements
This work has been supported by the grants from the Finnish Ministry of Education and Culture, Juho Vainio and Yrjö Jahnsson Foundations, Helsinki, Finland, High Technology Foundation of eastern Finland, University of Hacettepe, Unit of Scientific Researches (0202601006) and COST actions CM1001 “Chemistry of non-enzymatic protein modification – modulation of protein structure and function” and TD1304 “Zinc-Net”. We thank Taina Vihavainen and Taija Hukkanen for the skillful technical assistance.
References
- 1.Kalmar B., Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Advanced Drug Delivery Reviews. 2009;61(4):310–318. doi: 10.1016/j.addr.2009.02.003. 19248813 [DOI] [PubMed] [Google Scholar]
- 2.Kinnunen S., Oksala N., Hyyppä S., Sen C.K., Radak Z., Laaksonen D.E., Szabó B., Jakus J., Atalay M. Alpha-lipoic acid modulates thiol antioxidant defenses and attenuates exercise-induced oxidative stress in standardbred trotters. Free Radical Research. 2009;43(8):697–705. doi: 10.1080/10715760903037673. 19548154 [DOI] [PubMed] [Google Scholar]
- 3.Van Oosten-Hawle P., Porter R.S., Morimoto R.I. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell. 2013;153(6):1366–1378. doi: 10.1016/j.cell.2013.05.015. 23746847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaarniranta K., Elo M., Sironen R., Lammi M.J., Goldring M.B., Eriksson J.E., Sistonen L., Helminen H.J. Hsp70 accumulation in chondrocytic cells exposed to high continuous hydrostatic pressure coincides with mRNA stabilization rather than transcriptional activation. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(5):2319–2324. doi: 10.1073/pnas.95.5.2319. 9482883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oksala N.K., Lappalainen J., Laaksonen D.E., Khanna S., Kaarniranta K., Sen C.K., Atalay M. Alpha-lipoic acid modulates heat shock factor-1 expression in streptozotocin-induced diabetic rat kidney. Antioxidants & Redox Signaling. 2007;9(4):497–506. doi: 10.1089/ars.2006.1450. 17280490 [DOI] [PubMed] [Google Scholar]
- 6.Richter K., Haslbeck M., Buchner J. The heat shock response: Life on the verge of death. Molecular Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. 20965420 [DOI] [PubMed] [Google Scholar]
- 7.Tutar Y., Oksan S. Heat shock protein 70 purification and characterization from Cyprinion macrastomus macrastomus and Garra rufa obtusa. Journal of Thermal Biology. 2010;37:95–99. [Google Scholar]
- 8.Atalay M., Oksala N.K., Laaksonen D.E., Khanna S., Nakao C., Lappalainen J., Roy S., Hänninen O., Sen C.K. Exercise training modulates heat shock protein response in diabetic rats. Journal of Applied Physiology. 2004;97(2):605–611. doi: 10.1152/japplphysiol.01183.2003. 15075301 [DOI] [PubMed] [Google Scholar]
- 9.Dietz T.J. Acclimation of the threshold induction temperatures for 70-kDa and 90-kDa heat shock proteins in the fish Gillichthys mirabilis. Journal of Experimental Biology. 1994;188:333–338. doi: 10.1242/jeb.188.1.333. 7964381 [DOI] [PubMed] [Google Scholar]
- 10.Buckley B.A., Owen M.E., Hofmann G.E. Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. Journal of Experimental Biology. 2001;204(20):3571–3579. doi: 10.1242/jeb.204.20.3571. 11707506 [DOI] [PubMed] [Google Scholar]
- 11.Deane E.E., Woo N.Y. Cloning and characterization of the hsp70 multigene family from silver sea bream: modulated gene expression between warm and cold temperature acclimation. Biochemical and Biophysical Research Communications. 2005;330(3):776–783. doi: 10.1016/j.bbrc.2005.03.039. 15809064 [DOI] [PubMed] [Google Scholar]
- 12.Lund S.G., Ruberté M.R., Hofmann G.E. Turning up the heat: The effects of thermal acclimation on the kinetics of hsp70 gene expression in the eurythermal goby, Gillichthys mirabilis. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 2006;143(4):435–446. doi: 10.1016/j.cbpa.2005.12.026. 16466955 [DOI] [PubMed] [Google Scholar]
- 13.Dyer S.D., Dickson K.L., Zimmerman E.G., Sanders B.M. Tissue-specific patterns of synthesis of heat-shock proteins and thermal tolerance of the fathead minnow (Pimephales promelas) Canadian Journal of Zoology. 1991;69(8):2021–2027. doi: 10.1139/z91-282. [DOI] [Google Scholar]
- 14.Dietz T.J., Somero G.N. The threshold induction temperature of the 90-kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys) Proceedings of the National Academy of Sciences of the United States of America. 1992;89(8):3389–3393. doi: 10.1073/pnas.89.8.3389. 1565632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiDomenico B.J., Bugaisky G.E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. 7159929 [DOI] [PubMed] [Google Scholar]
- 16.Santacruz H., Vriz S., Angelier N. Molecular characterization of a heat shock cognate cDNA of zebrafish, hsc70, and developmental expression of the corresponding transcripts. Developmental Genetics. 1997;21(3):223–233. doi: 10.1002/(SICI)1520-6408(1997)21:3<223::AID-DVG5>3.0.CO;2-9. 9397538 [DOI] [PubMed] [Google Scholar]
- 17.Tawk M., Joulie C., Vriz S. Zebrafish Hsp40 and Hsc70 genes are both induced during caudal fin regeneration. Mechanisms of Development. 2000;99(1–2):183–186. doi: 10.1016/S0925-4773(00)00478-0. 11091090 [DOI] [PubMed] [Google Scholar]
- 18.Young J.C., Agashe V.R., Siegers K., Hartl F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nature Reviews. Molecular Cell Biology. 2004;5(10):781–791. doi: 10.1038/nrm1492. 15459659 [DOI] [PubMed] [Google Scholar]
- 19.Kregel K.C. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. Journal of Applied Physiology. 2002;92(5):2177–2186. doi: 10.1152/japplphysiol.01267.2001. 11960972 [DOI] [PubMed] [Google Scholar]
- 20.Deane E.E., Kelly S.P., Luk J.C., Woo N.Y. Chronic salinity adaptation modulates hepatic heat shock protein and insulin-like growth factor I expression in black sea bream. Marine Biotechnology. 2002;4(2):193–205. doi: 10.1007/s10126-001-0091-5. 14961280 [DOI] [PubMed] [Google Scholar]
- 21.Iwama G.K., Afonso L.O., Todgham A., Ackerman P., Nakano K. Are hsps suitable for indicating stressed states in fish? Journal of Experimental Biology. 2004;207(1):15–19. doi: 10.1242/jeb.00707. 14638828 [DOI] [PubMed] [Google Scholar]
- 22.Sagstad A., Sanden M., Haugland Ø, Hansen A.C., Olsvik P.A., Hemre G.I. Evaluation of stress- and immune-response biomarkers in Atlantic salmon, Salmo salar L., fed different levels of genetically modified maize (Bt maize), compared with its near-isogenic parental line and a commercial suprex maize. Journal of Fish Diseases. 2007;30(4):201–212. doi: 10.1111/j.1365-2761.2007.00808.x. 17394522 [DOI] [PubMed] [Google Scholar]
- 23.Gorenkova N., Robinson E., Grieve D.J., Galkin A. Conformational change of mitochondrial complex I. Increases ROS sensitivity during ischemia. Antioxidants & Redox Signaling. 2013;19(13):1459–1468. doi: 10.1089/ars.2012.4698. 23419200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lushchak V.I., Bagnyukova T.V. Hypoxia induces oxidative stress in tissues of a goby, the rotan Perccottus glenii. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2007;148(4):390–397. doi: 10.1016/j.cbpb.2007.07.007. 17822934 [DOI] [PubMed] [Google Scholar]
- 25.Chandel N.S., Budinger G.R. The cellular basis for diverse responses to oxygen. Free Radical Biology & Medicine. 2007;42(2):165–174. doi: 10.1016/j.freeradbiomed.2006.10.048. 17189822 [DOI] [PubMed] [Google Scholar]


