Abstract
UNC-45 is a UCS domain protein that is critical for myosin stability and function. It likely aides in folding myosin during cellular differentiation and maintenance and protects myosin from denaturation during stress. Invertebrates have a single unc-45 gene that is expressed in both muscle and non-muscle tissues. Vertebrates possess one gene expressed in striated muscle (unc-45b) and one that is more generally expressed (unc-45a). Structurally, UNC-45 is composed of a series of alpha-helices connected by loops. It has an N-terminal TPR domain that binds to Hsp90 and a central domain composed of armadillo repeats. Its C-terminal UCS domain, which is also comprised of helical armadillo repeats, interacts with myosin. In this review, we present biochemical, structural and genetic analyses of UNC-45 in Caenorhabditis elegans, Drosophila melanogaster and various vertebrates. Further, we provide insights into UNC-45 functions, its potential mechanism of action and its roles in human disease.
Keywords: UNC-45, TPR domain, UCS protein, myosin, muscle, chaperone, Hsp90
1. Introduction and Overview
UNC-45 (uncoordinated mutant number 45) was identified as a result of a random mutagenesis screen for genes required for motility in the nematode Caenorhabditis elegans (Epstein and Thomson, 1974). Gene mutation and knockdown experiments in various organisms showed UNC-45 to be critical for myosin maturation and accumulation (Barral et al., 1998; Bernick et al., 2010; Etard et al., 2007; Geach and Zimmerman, 2010; Lee et al., 2011b; Melkani et al., 2011; Price et al., 2002; Venolia and Waterston, 1990; Wohlgemuth et al., 2007). Molecular cloning of the C. elegans unc-45 gene (Venolia et al., 1999) defined the encoded ~105 kD protein as a member of the UCS (UNC-45, CRO1, She4p) family of myosin interacting proteins (Barral et al., 1998; Venolia et al., 1999).
UNC-45 molecules exhibit a three domain configuration, with an N-terminal tetratricopeptide repeat (TPR) domain (~115 amino acids), a poorly conserved central domain (~400 amino acids) and a C-terminal UCS domain (~400 amino acids) (Barral et al., 1998; Venolia et al., 1999). The UNC-45 TPR domain, which consists of three consensus TPR repeats, is a co-chaperone helix-turn-helix motif that participates in protein-protein interaction, especially with Hsp70 and Hsp90 (Scheufler et al., 2000). The role of the central domain is unclear. The C-terminal UCS domain is critical for myosin binding (Barral et al., 2002). The structure of UNC-45 and the identities of its interacting proteins are summarized in Figure 1.
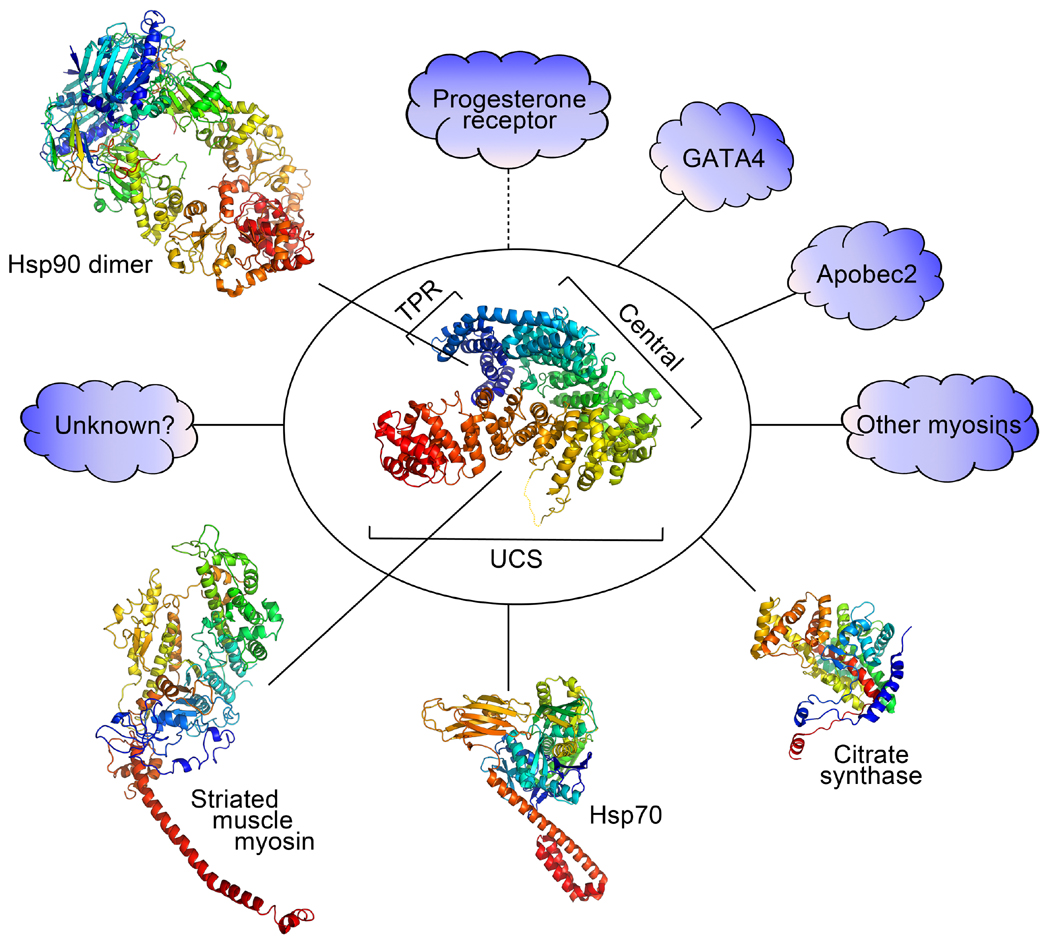
Figure 1. Model showing C. elegans UNC-45 structure domains and interacting proteins.
The large oval encloses the solved crystal structure of C. elegans UNC-45 (PDBID 4I2Z). The UNC-45 UCS domain binds to striated muscle myosin S1 (chicken skeletal muscle myosin, PDBID 2MYS with light chains excluded). The TPR domain binds to Hsp90 (S. cerevisiae Hsp90 dimer in the ATP bound closed conformation, PDBID 2CG9). UNC-45 also binds to Hsp70 (E. coli DnaK, PDBID 4B9Q) and citrate synthase (porcine heart CS, PDBID 1CTS), as well as GATA4, progesterone receptor (directly or indirectly), Apobec2 and other myosins. Additional unknown proteins may serve as UNC-45 co-chaperones or targets. Known protein structures are modeled in PyMOL (PyMOL Molecular Graphics System, Version 1.6.0.0 Schrödinger LLC).
UNC-45 homologues have been found in all Bilateria examined thus far including worms, flies, frogs, fish, mice and humans (Epstein and Thomson, 1974; Etheridge et al., 2002; Geach and Zimmerman, 2010; Lee et al., 2011b; Price et al., 2002) as well as various other species (Hansen et al., 2014). Invertebrates such as worms and flies have one UNC-45 protein (Barral et al., 1998; Lee et al., 2011b; Venolia et al., 1999). In vertebrates, there are two UNC-45 isoforms (Price et al., 2002), one that is expressed in general cell (GC) types (GC UNC-45/UNC-45a/SMAP-1/Kurzschluss (Kus) in zebrafish) and another that is specific to striated muscle (SM) (SM UNC-45/UNC-45b/CMYA4 in mice/Steif in zebrafish). The function of each UNC-45 isoform has been probed in vivo and in vitro. Knock-down experiments in mouse skeletal myogenic C2C12 cells revealed that the general cell type UNC-45 is important for cell proliferation and fusion, while striated muscle UNC-45 is essential for myoblast fusion and sarcomere organization (Price et al., 2002).
In muscle cells, UNC-45 ensures proper folding of myosin to allow its assembly and function in the sarcomere (Ao and Pilgrim, 2000; Barral et al., 1998; Etard et al., 2007; Lee et al., 2011b; Melkani et al., 2011; Price et al., 2002; Srikakulam and Winkelmann, 2004). Partial in vitro folding of myosin by UNC-45 supports this thesis (Liu et al., 2008; Srikakulam et al., 2008). UNC-45 also prevents heat-induced aggregation of myosin in vitro (Barral et al., 2002; Melkani et al., 2010), which may translate to a protective role against stress in vivo. In fact, UNC-45 relocates from the Z-disks to the myosin-containing A-bands during stress in zebrafish muscle (Etard et al., 2008). The roles of UNC-45 in interacting with sarcomeric myosin during development and stress are summarized in Figure 2.
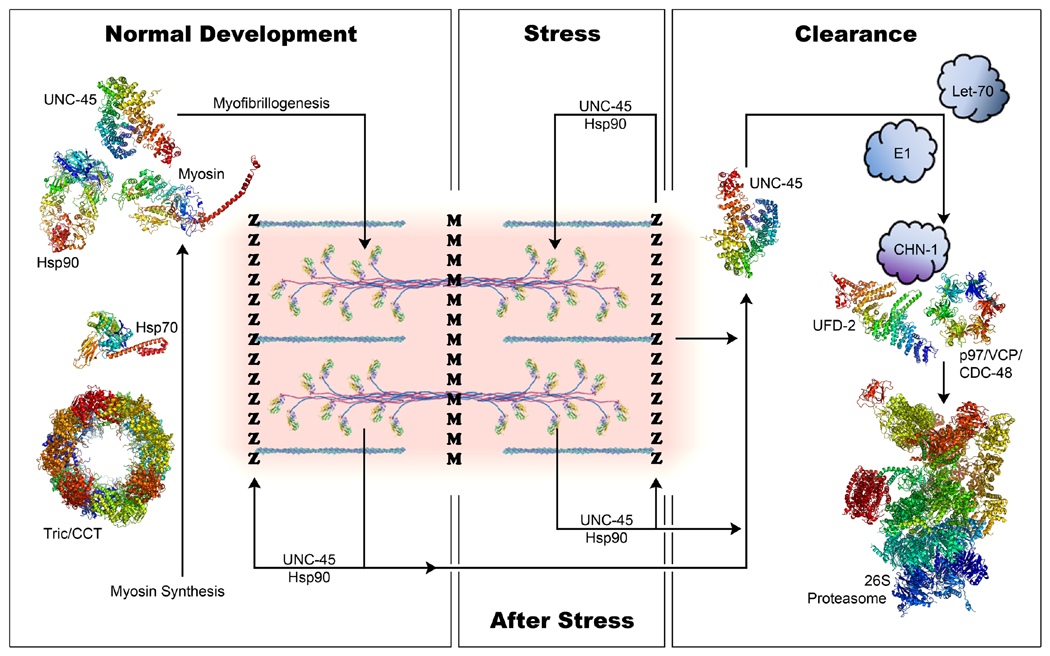
Figure 2. Model of UNC-45 function in assembling and protecting sarcomeric myosin.
During normal development, myosin maturation involves chaperones such as TriC/CCT and Hsp70 (Srikakulam and Winkelmann, 1999; Srikakulam and Winkelmann, 2004). Data from zebrafish studies suggest myosin then forms a complex with UNC-45 and Hsp90 in the cytosol (Etard et al., 2008). The complex remains stable until the end of myofibrillogenesis. Once myosin successfully incorporates into thick filaments, UNC-45 and Hsp90 may dissociate from myosin and move to the Z-disk for storage. Upon stress, UNC-45 and Hsp90 translocate from the Z-disk to the A-band, potentially protecting myosin from denaturation. Since both Drosophila and C. elegans UNC-45 display chaperone function in vitro (Barral et al., 2002; Melkani et al., 2010), it is possible that UNC-45 could protect myosin independently of Hsp90. Upon cessation of stress, UNC-45 and Hsp90 may return to the Z-disk until needed. In cases where UNC-45 needs to be cleared, this is accomplished by the ubiquitin/proteasome protein degradation system. UNC-45 associates with various enzymes such as ubiquitin-activating enzyme E1, ubiquitin-conjugating enzyme E2 (Let-70), and ubiquitin ligases E3/E4 (CHN-1, UFD-2) (Hoppe et al., 2004). Eventually, UNC-45, CHN-1 and UFD-2 form a complex with an AAA ATPase (p97/VCP/CDC-48) (Janiesch et al., 2007) before transiting to the 26S proteasome for degradation.
Pink area represents a single sarcomere. Z, Z-disk; M, M-line. Blue-green actin thin filaments project from the Z-disks, and two myosin thick filaments are pictured across the M-line. Tric/CCT (Bovine, PDBID 4A0O). Hsp70 (E. coli DnaK in the ATP-bound open conformation, PDBID 4B9Q). Hsp90 (S. cerevisiae Hsp90 dimer in the ATP bound closed conformation, PDBID 2CG9). UNC-45 (C. elegans UNC-45, PDBID 4I2Z). Myosin S1 (chicken skeletal muscle myosin, PDBID 2MYS with light chains excluded). UFD-2 (Yeast UFD-2, PDBID 2QIZ). p97/VCP (VCP-like AAA ATPase from Mycobacterium tuberculosis, PDBID 3FP9). 26S proteasome (yeast proteasome, PDBID 4CR2). Known protein structures are modeled in PyMOL (PyMOL Molecular Graphics System, Version 1.6.0.0 Schrödinger LLC).
In yeast and other fungi, there is no UNC-45 protein per se. Instead, these organisms possess related proteins that contain the C-terminal UCS domain but lack the N-terminal TPR domain. These proteins include Rng3p (S. pombe) (Wong et al., 2000), She4p (S. cerevisiae) (Beach and Bloom, 2001; Toi et al., 2003; Wesche et al., 2003), and CRO1 (P. anserine) (Berteaux-Lecellier et al., 1998); disrupted expression of UCS proteins in these organisms causes defects in actin cytoskeleton organization, cytokinesis, and other cellular processes associated with myosins I, V, and non-muscle myosin II. Indeed, the yeast two-hybrid screening method confirmed that the UCS domain of She4p is necessary for its interaction with type V unconventional myosin (Toi et al., 2003). Although it lacks a TPR domain, S. pombe Rng3p is capable of interacting with Hsp90, either directly or indirectly, as demonstrated by genetic, yeast-two hybrid and immunoprecipitation analyses (Mishra et al., 2005). The combined action of Rng3p and Hsp90 is essential for proper function of type II myosin in cytokinesis.
In this article, we review biochemical, genetic, cell biological and developmental aspects of UNC-45 function in several model organisms, with a focus on its role in muscle. We then discuss recent findings from the X-ray crystal structure of UNC-45 and speculate on the mechanism of action of the protein. Given its critical role in myosin folding and protection, understanding how this protein works has implications for basic muscle biology and for human muscle disease etiology and treatment.
2. Model Organism-Based Studies of UNC-45 Function
The advantages of well-developed model organisms often include the capacity to produce and screen for mutants, amenability to transgenic approaches, ability to readily view development and a short life cycle. These properties have been used to good advantage in understanding the role of UNC-45 in muscle development and function in the nematode, fly and zebrafish systems.
2.1. Caenorhabditis elegans
Studies in C. elegans set the paradigm by defining the unc-45 mutant phenotype (Epstein and Thomson, 1974) and identifying the gene product (Venolia et al., 1999). The function of UNC-45 in C. elegans has been examined through a combination of genetics (Barral et al., 1998; Venolia and Waterston, 1990), cell biology (Ao and Pilgrim, 2000; Kachur et al., 2004), biochemistry (Barral et al., 2002) and, most recently, structural biology (Gazda et al., 2013).
2.1.1. UNC-45 genetically defined in uncoordinated worms
UNC-45 was first identified through the phenotypic effect of a temperature-sensitive C. elegans mutation (Epstein and Thomson, 1974). When grown at the permissive temperature of 20°C, the mutant worm appeared as wild type. Growth at the non-permissive temperature of 25°C resulted in uncoordinated (UNC) movements and signs of paralysis. This uncoordinated movement phenotype, however, is reversible during larval development. Mutant worms hatched at 25°C and then shifted to 20°C during the larval stage develop into adult worms with wild-type movement, even when later incubated again at 25°C. Embryonic and larval stages are critical for the temperature sensitivity, suggesting that C. elegans UNC-45 is essential for muscle development (Epstein and Thomson, 1974). Examination of the mutant muscle structure by electron microscopy showed that worms grown at the restrictive temperature have decreased thick filament accumulation (Figure 3A) and disrupted distribution of myosin heavy chain isoforms along the thick filament, with MHC A and MHC B mixed throughout the filaments instead of the former being centrally localized (Barral et al., 1998).
Figure 3. Conserved requirement of UNC-45 for worm, fly and vertebrate skeletal muscle structure and function.
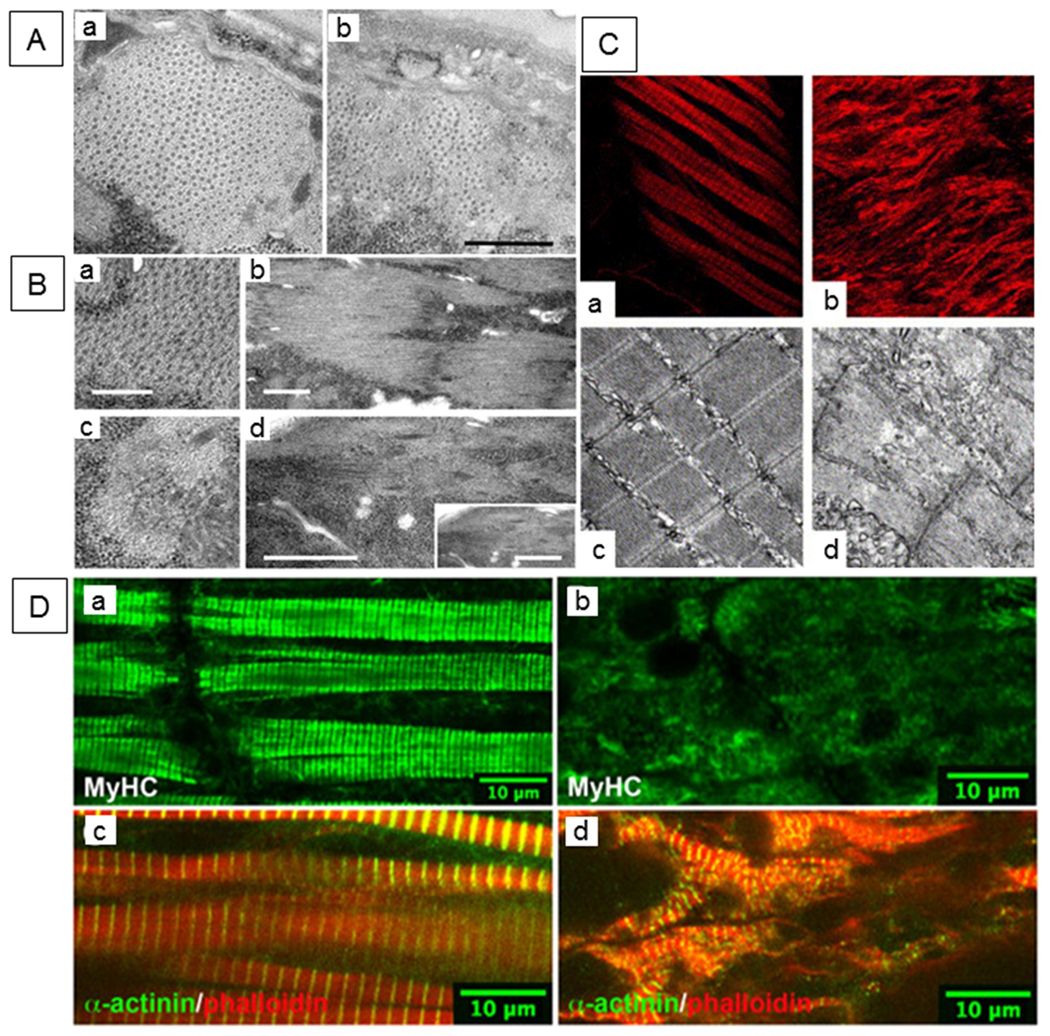
(A) Accumulation of myosin-containing thick filaments is compromised in a heat-sensitive unc-45 UCS domain mutant of C. elegans. Electron micrographs of region II (posterior to the pharynx) cross sections from strain CB286 nematodes grown at (a) 15° or (b) 25°C. Scale bar, 0.5 μm. Figure reprinted, with permission, from Barral et al. (1998) [©1998 Rockefeller University Press. Journal of Cell Biology. 143:1215–1225.] (B) Ultrastructural defects associated with body-wall muscle of 22-hour-old Drosophila unc-45 embryos. Electron micrograph of yw control line (a, b). T-33 embryo (unc-45 null mutant) exhibits reduced level of thick filaments and a loss of the thick-thin filament lattice spacing (c, d). Transverse (a, c) and longitudinal (b, d) orientations are shown. Scale bars: 0.25 μm (a, c); 1.0 μm (b, d). Figure reprinted, with permission, from Lee et al. (2011b). (C) Zebrafish with morpholino (MO)-injected knockdown of unc-45b display lack of trunk muscle striation. Compared to control MO-injected embryos (a, c), unc-45b MO-injected organisms (b, d) display lack of striation. Immunofluorescence images with phalloidin staining of control MO-injected embryos (a) unc-45b MO-injected embryos (b) as well as transmission electron micrographs of control MO-injected (c) and unc-45b MO-injected (d) embryos at 48 hpf are shown. Figure reprinted, with permission, from Wohlgemuth et al. (2007). (D) Missense mutant unc-45 (dicky ticker) results in loss of myosin staining in Xenopus embryonic muscle. Immunofluorescence micrographs of wild-type (left panels) and mutant embryos (right panels) stained with MyHC and α-actinin antibodies. As shown in subpanels a and c, A-bands and Z-disks are well-organized in wild-type somites at stage 43. Muscle myosin staining is greatly reduced in the mutant (b). However, α-actinin-containing sarcomeres are observed (d). Phalloidin-stained thin filaments are present but disorganized in the mutant. Figure reprinted, with permission, from Geach and Zimmerman (2010).
Recessive lethal unc-45 alleles result in embryos that fail to elongate beyond the two-fold stage, lack pharyngeal pumping and often die prior to hatching (Venolia and Waterston, 1990). Interestingly, lethality can be rescued by the presence of a wild-type unc-45 gene in the mother (Venolia and Waterston, 1990). unc-45 RNA and UNC-45 protein are packaged in the egg by the mother and inherited by all embryonic cells, which is apparently sufficient for survival (Kachur et al., 2004). UNC-45 interacts with non-muscle myosin II in these embryos and its absence inhibits cytokinesis (Kachur et al., 2004), suggesting that it plays a similar role to S. pombe UCS protein Rng3p (Wong et al., 2000). Further analysis indicates a role of UNC-45 in permitting non-muscle myosin II function in extrusion of polar bodies, polarity establishment and production of membrane boundaries separating proliferating nuclei in the gonad (Kachur et al., 2008).
2.1.2. Molecular cloning of the unc-45 gene, defining UNC-45 protein domains and determining in vivo protein location
C. elegans unc-45 was the first unc-45 gene to be molecularly cloned and its identity was verified by mutant rescue via injection of a cDNA under the unc-45 promoter (Venolia et al., 1999). Injection of double-stranded RNA for the gene, which yields inhibitory RNA, resulted in an arrested development phenotype comparable to animals carrying unc-45 lethal alleles (Venolia et al., 1999). The gene encodes a protein of 961 amino acids. Its 120 N-terminal amino acids contain the consensus sequence for three TPR motifs. This is followed by an ~400 amino acid central domain and an ~400 amino acid C-terminal region with homology to the fungal proteins CRO1 (Podospora anserine) and She4p (Saccharomyces cerevisiae) (Barral et al., 1998; Venolia et al., 1999). The region of homology shared by UNC-45/CRO1/She4p has been named the UCS domain and proteins that contain it are designated as UCS proteins (Barral et al., 1998). Rng3p from Schizosaccharomyces pombe contains this C-terminal domain as well (Wong et al., 2000). Molecular analyses of several C. elegans unc-45 mutants showed that identified lethal alleles result from the presence of stop codons upstream of the UCS domain, whereas three temperature-sensitive mutations map to sites within the UCS domain, with another one in the central domain (Barral et al., 1998).
A GFP::UNC-45 fusion protein localized to the A-bands of sarcomeres in adult C. elegans body wall muscle (Ao and Pilgrim, 2000), suggesting that it is important for adult muscle structure and/or function. Antibodies raised against an N-terminal peptide of C. elegans UNC-45 also localized to the thick filaments of body wall muscle. However, the central MHC A-containing regions of the thick filaments were not labeled, only the peripheral regions containing MHC B. Interestingly, unc-54 mutant worms that lack MHC B display disorganized MHC A-containing thick filaments and these filaments lack UNC-45. Even when MHC A is over-expressed in unc-54 mutant worms to produce functional thick filaments, UNC-45 does not co-localize and, therefore, it may not be required for MHC A-containing thick filament assembly or maintenance in adults. It thus appears that UNC-45 is a component of thick filaments and functions in stabilizing the myosin isoform outside the central MHC A region in adults (Ao and Pilgrim, 2000). In contrast to earlier observations, however, more recent work suggests that MHC A is in fact reduced in UNC-45 mutants (Landsverk et al., 2007). Further, UNC-45 appears to be required for assembly of MHC A-containing thick filaments in embryos, since it must be present for thick filaments composed solely of MHC A to assemble at this stage (Venolia and Waterston, 1990). Since UNC-45 is mostly diffuse in the cytoplasm at early larval stages (Ao and Pilgrim, 2000), it may be that it assists in folding MHC A prior to assembly into the thick filament. In concordance with this concept, RNAi knockdown of unc-45 results in non-diffusible cytoplasmic patches of MHC A that may arise from its failure to fold and move to a myofibrillar location (Gaiser et al., 2011).
The concept of A-band localization of UNC-45 in nematodes may need to be modified. Although this observation was verified by expressing CFP-labeled UNC-45, the protein was found to localize to the M-band and I-band regions in many muscle cells that lacked A-band localization (Gaiser et al., 2011). It should be noted that these results were obtained in live animals, rather than in the fixed organisms studied in the experiments described above. Interestingly, FRAP experiments showed that UNC-45 localized to the I-band is highly diffusible, whereas A-band protein localization is stable (Gaiser et al., 2011). It is possible that localization to the A-band is induced during muscle stress (including muscle fixation), as appears to be the case in zebrafish (Etard et al., 2008).
2.1.3. UNC-45 partners, chaperone activity, stability and over-expression
Evidence for direct interaction between C. elegans UNC-45 and skeletal muscle myosin was obtained using an in vitro pull-down assay (Barral et al., 2002). Recombinant UNC-45 (His::cUNC-45::Flag) protein was expressed in Sf9 insect cells using engineered baculovirus, allowing for purification of protein for in vitro assays. Through various combinations of pull-downs and co-immunoprecipitations, the direct interactions of UNC-45 with myosin and also with insect Hsp90 and Hsp70 were demonstrated. The interaction with Hsp90 was further validated with C. elegans Hsp90 expressed in Escherichia coli. The Hsp90 and myosin interaction was dependent upon using an elevated temperature of 30°C, suggesting that partial denaturation of one or more of the protein components is required. Interaction with myosin did not require the TPR domain. The recombinant UNC-45 protected citrate synthase and myosin sub-fragment 1 (S1) from heat induced aggregation (Barral et al., 2002). In addition, Barral et al. used truncated versions of UNC-45 to show that the UNC-45 TPR domain interacts with the C-terminal peptide of Hsp90, and to demonstrate that the TPR domain is not required for UNC-45 to interact with myosin S1. These in vitro studies suggest that UNC-45 acts as a chaperone for myosin and as a co-chaperone for Hsp90.
E3/E4 protein ubiquitin ligases appear to precisely control UNC-45 levels, and their disruption leads to dysregulation of UNC-45 levels and abnormal muscle phenotypes. C. elegans UNC-45 stability is regulated through ubiquitylation by the proteins CHN-1 and UFD-2 (Hoppe et al., 2004). CHN-1 (a C. elegans ortholog of CHIP) and UFD-2 contain C-terminal U boxes and function as E3/E4 ubiquitin ligases independently of Hsp70 and Hsp90 chaperones. Either one can add one to three ubiquitin monomers to C. elegans UNC-45. Both are required for UNC-45 to be multi-ubiquitylated, which likely leads to degradation by the 26S proteasome (Figure 2, right). The negative regulation of UNC-45 levels by CHN-1 was genetically confirmed, since a loss-of-function mutation in chn-1 partially rescues muscle defects associated with unc-45 temperature sensitive alleles (Hoppe et al., 2004). Further, over-expression of UNC-45 from extra-chromosomal arrays in a chn-1 mutant background results in severe sarcomere defects (Hoppe et al., 2004). This is true for UNC-45 over-expression in ufd-2, cdc-48.1 and cdc-48.2 mutant backgrounds as well (Janiesch et al., 2007). The CDC-48 proteins are C. elegans homologues of mammalian p97, which is a chaperone that binds ubiquitylated proteins and is implicated in inclusion-body myopathy associated with Paget disease of bone and frontotemporal dementia (Ju and Weihl, 2010). CDC-48 was shown to form a complex with UFD-2 and CHN-1 and hence is involved in UNC-45 stability (Janiesch et al., 2007). This appears to be developmentally relevant in that transcripts for each of these proteins increase in young adults, concomitantly with a reduction in UNC-45 levels (Janiesch et al., 2007).
Landsverk et al. (2007) showed that over-expression of C. elegans UNC-45 in a wild-type background results in reduced numbers of myosin-containing thick filaments and mild paralysis. Their integrated copy of unc-45 expressed about 10 times more robustly than in a previous study, which had shown no effects of over-expression of an integrated gene (Hoppe et al., 2004). Over-expressing UNC-45 apparently leads to increased levels of myosin ubiquitylation and degradation by the proteasome, as reducing proteasomal degradation in vivo improves muscle structure and function in this genetic background (Landsverk et al., 2007). These authors suggest that excess UNC-45 associates with myosin, making it more likely to remain in an unassembled state that is more susceptible to degradation. Ni et al. (2011) used extra-chromosomal arrays expressing UNC-45 fragments to show that reductions in myosin levels, A-band assembly and motility are largely dependent on the presence of the UCS domain. Furthermore, the UCS domain is required to partially rescue lethality of an unc-45 null mutant and the motility and A-band assembly of a temperature-sensitive mutant (Ni et al., 2011). Interestingly, the UCS domain alone appears more effective than full-length UNC-45 on a per molecule basis (Ni et al., 2011). This led to the conjecture that the TPR domain might actually inhibit UNC-45 function, perhaps through presentation of Hsp90. In fact, Hsp90 competes with UNC-45 for binding to myosin in vitro, suggesting that Hsp90 inhibits the myosin chaperone activity of UNC-45 (Ni et al., 2011). This contrasts with the hypothesis that UNC-45 binds to unfolded myosin and presents TPR-bound Hsp90 to perform its chaperone function (Barral et al., 2002). Ni et al. (2011) suggest that Hsp90 binding to myosin inhibits UNC-45 action, serving to regulate myosin folding and/or protection capabilities. The authors further argue that there is no direct evidence for a ternary complex containing UNC-45, Hsp90 and myosin. In contrast, as discussed below, the recent solution of the C. elegans UNC-45 crystal structure suggests a mechanism of coordinated binding of myosin to both the UNC-45 UCS domain and to Hsp90 presented by the UNC-45 TPR domain (Gazda et al., 2013).
2.2. Drosophila melanogaster
UNC-45 was initially defined in Drosophila melanogaster by homology search of its genome using the C. elegans and Danio rerio genes (Etheridge et al., 2002; Hutagalung et al., 2002; Price et al., 2002). The Drosophila gene was subsequently isolated and used to study Drosophila UNC-45 biochemistry (Melkani et al., 2010), expression patterns (Lee et al., 2011b), genetics (Lee et al., 2011b; Melkani et al., 2011) and protein structure (Lee et al., 2011a).
2.2.1. Assessment of UNC-45 chaperone function
His-tagged Drosophila UNC-45 was expressed in E. coli and isolated by Ni2+-affinity chromatography (Melkani et al., 2010). It was found to suppress aggregation of alpha-lactalbumin, citrate synthase and myosin S1 fragment. Further, it is able to refold citrate synthase that has been unfolded by urea treatment (Melkani et al., 2010). Both light scattering and electron microscopy studies demonstrated that two-headed heavy meromyosin sub-fragments of myosin also are protected from aggregation by UNC-45 treatment.
2.2.2. Localization of UNC-45 during development
Antibodies produced against His-tagged Drosophila UNC-45 were used to localize the protein during embryonic development (Lee et al., 2011b). UNC-45 accumulates at the periphery of two hour embryos (blastoderm stage), co-localizing with non-muscle myosin heavy chain II. Expression at later stages of development is most dramatic in developing body wall and pharyngeal muscles. Western blots demonstrated that UNC-45 is expressed during embryonic, larval, pupal and adult stages (Lee et al., 2011b). Examination of various muscles at these stages of development showed that each accumulates UNC-45. Detailed analysis of larval body wall muscles found that UNC-45 is present at Z-disks, co-localizing with alpha-actinin (Lee et al., 2011b). This corresponds to the type of localization observed in some body wall muscles of C. elegans (Gaiser et al., 2011), and suggests that the protein could be re-localized to the A-band to protect myosin during stress, as has been demonstrated in zebrafish (Etard et al., 2008).
2.2.3. Mutant phenotypes in skeletal and cardiac muscles
A null allele for Drosophila unc-45 was produced by imprecise excision of a P transposable element located in the non-coding first exon of the gene (Lee et al., 2011b). The observed phenotype was embryonic lethality. Null mutants showed barely detectable levels of UNC-45 during early muscle development (Lee et al., 2011b), which might be attributed to perdurance of maternal mRNA or protein. Interestingly, levels of muscle myosin in these embryos were essentially normal, suggesting that myosin is translated appropriately. However, late stage mutant (Lee et al., 2011b) or RNAi knockdown embryos (Melkani et al., 2011) displayed a dramatic reduction in myosin heavy chain accumulation on western blots and the null mutant showed near absence of thick filaments by electron microscopy (Lee et al., 2011b) (Figure 3B). Another study found that injection of unc-45 RNAi at the blastoderm stage results in normal muscle fiber patterning but no contractility (Estrada et al., 2006). Thus, as in C. elegans (Barral et al., 1998), Drosophila UNC-45 appears to be critical for myosin stability and function in body wall muscle.
The effect of RNAi knockdown of unc-45 on adult heart development and function was assessed by biochemical, structural and physiological approaches (Melkani et al., 2011). Using the inducible UAS-Gal4 system with the Hand-Gal4 transcriptional driver, RNAi expression was restricted to cardiac cells during all stages of heart development. Third instar larvae displayed increased heart rates, mild cardiac dilation and reduced fractional shortening. Adults at one week of age showed severe cardiac dilation and arrhythmias (Melkani et al., 2011). Detailed physiological studies of adult hearts revealed increased diastolic and systolic intervals and diameters, intermittent heartbeat cessation and decreased fractional shortening. At the structural and biochemical levels, myofibril disarray occurred with an ~75% reduction in myosin levels (Figure 4A). Cardiac unc-45 knockdown organisms displayed dramatically reduced lifespans (Melkani et al., 2011). These phenotypes were recapitulated when regulated Gal4 expression was employed to induce RNAi at either the third instar larval stage or the young pupal stage, but were much less severe when knockdown was initiated during adulthood. This indicates that UNC-45 is critical during metamorphosis, when the heart is remodeled to perform its adult functions (Melkani et al., 2011).
Figure 4. Cardiac defects associated with UNC-45 mutations.
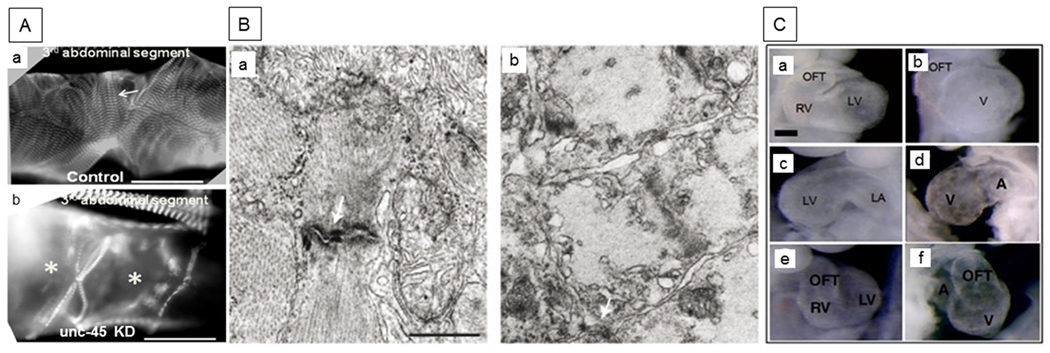
(A) Immunofluorescence micrographs of 1-week-old Drosophila hearts (third segment) from (a) control and (b) unc-45 knockdown adults were probed with antibody against muscle myosin. Control cardiac tubes show typical spiral myofibrillar arrangements within the cardiomyocytes (arrow). Myofibrillar organization is completely disrupted in the conical chamber and third segment of unc-45 knockdown cardiac tubes (indicated by *) with loss of most myosin-containing myofibrils and significant dilation. Scale bars: 75 μm. Figure reprinted, with permission, from Melkani et al. (2011). (B) Electron micrographs of sections through cardiac muscle cells from 5-day-old (a) wild-type and (b) unc-45 steif mutant zebrafish embryos. Arrows show Z-disks of sarcomeres. The mutant lacks proper intercalated disks and myofibrils. Scale bar, 0.5 μm. Figure reprinted, with permission, from Etard et al. (2007). (C) Control (a, c, e) and unc-45b mutant (b, d, f) mouse embryonic cardiac tissue. The mutation results in the blockage of embryonic cardiogenesis at the stage of right heart formation. Frontal, left lateral and right lateral views at E9.5 are shown from top to bottom in the control and mutant hearts. Control hearts (UNC-45bC57/C57, a, c, e) show right and left ventricles (RV, LV), left atrium (LA) and the outflow tract (OFT). In contrast, UNC-45b gene-trap mutant hearts (UNC-45bgt/gt, b, d, f) display a single ventricle (V), one atrium (A) and the OFT. Scale bar, 200 μm (a–f). Figure reprinted, with permission, from Chen et al. (2012).
2.3. Vertebrates
Studies of UNC-45 in vertebrates indicate that they have two UNC-45 genes, one of which is highly expressed in striated muscles (Price et al., 2002). Molecular approaches have demonstrated chaperone activity for mammalian UNC-45 (Liu et al., 2008), whereas cell biological analysis showed that UNC-45 can shuttle between the Z-disk and A-band during cell stress (Etard et al., 2008). Defects arising from mutation or knockout of unc-45 genes in skeletal and/or cardiac muscles have been documented in mouse (Chen et al., 2012), zebrafish (Bernick et al., 2010; Etard et al., 2007; Wohlgemuth et al., 2007) and Xenopus (Geach and Zimmerman, 2010). These studies have obvious implications for understanding the function of UNC-45 in humans.
2.3.1. Two differentially expressed UNC-45 genes in mammals
Mouse and human unc-45 genes were identified by computational analysis and isolated genes were used subsequently to define mammalian UNC-45 expression patterns (Price et al., 2002). Both organisms have two unc-45 genes that are expressed either in striated muscles (SM UNC-45, also known as UNC-45b) or in general cell types (GC UNC-45 or UNC-45a) (Price et al., 2002). While identity within specific UNC-45 isoforms between organisms is high (~95%), UNC-45a and b are only ~55% identical (Price et al., 2002). C2C12 mouse myoblasts express UNC-45a during proliferation and fusion, with higher levels of UNC-45b during fusion and differentiation. Antisense RNA treatments showed that UNC-45a is important for cell proliferation and fusion, whereas UNC-45b is essential for fusion and myofibril development, but not myosin accumulation (Price et al., 2002). Overall it appears that the functions of the single UNC-45 gene of invertebrates have been taken over by two vertebrate genes that are differentially expressed.
2.3.2. Enhancement of in vitro folding of myosin by mouse UNC-45
The cytosolic chaperonin CCT is required for folding of the myosin motor domain, as shown by antibody analysis and immunodepletion studies during the synthesis of the heavy meromyosin subfragment (HMM) in a rabbit reticulocyte lysate system (Srikakulam and Winkelmann, 1999). Although HMM synthesized in this system displayed imperfect folding and defective binding to actin, dimerization of heavy and light chains was accomplished effectively (Srikakulam and Winkelmann, 1999). Moreover, enhanced folding of the motor domain and increased binding to actin occurred when synthesis was performed in the presence of mouse C2C12 muscle cell cytoplasmic extracts, suggesting that factors from muscle are necessary for myosin folding and function (Srikakulam and Winkelmann, 1999). Subsequent studies in C2C12 cells demonstrated that striated muscle cell myosin maturation and assembly proceeds through involvement of a chaperone complex that includes Hsp90 and Hsp70 (Srikakulam and Winkelmann, 2004). However, conformation-dependent antibody binding showed that this chaperone system is not sufficient for complete folding of the striated muscle myosin motor domain, suggesting that additional factors are required (Srikakulam and Winkelmann, 2004). Using the smooth muscle myosin motor domain fused to GFP as a substrate, Liu el al. explored the efficacy of mouse UNC-45 in the myosin in vitro synthesis and folding assay (Liu et al., 2008). They demonstrated that either UNC-45a or UNC-45b dramatically enhanced folding of the myosin head (Liu et al., 2008). This folding is also dependent on the ATPase activity of Hsp90. Flag-tagged UNC-45b expressed in bacteria binds with Hsp90 and targets the unfolded myosin motor domain, but not native myosin (Srikakulam et al., 2008). The UNC-45b-Hsp90 complex is sufficient to enhance folding and maturation of newly synthesized smooth muscle myosin motor domains (Srikakulam et al., 2008). Furthermore, the UNC-45a isoform is more effective in folding the smooth muscle myosin motor domain in the reticulocyte lysate system compared to the striated muscle UNC-45b isoform {Liu, 2008). UNC-45a stimulates the rate of the Hsp90-dependent folding of myosin, thus serving as an activator late in the myosin folding pathway. Neither of the UNC-45 isoforms is sufficient to activate the folding of the striated muscle motor domain on its own (Liu et al., 2008). Minimally, it appears that myosin must interact with the CCT chaperonin, Hsp90 and Hsp70, and UNC-45 in conjunction with Hsp90 to move from the newly synthesized state to a folded and functional molecule (Figure 2).
2.3.3. Defects in mouse cardiac development arising from unc-45b loss-of-function mutation
UNC-45b is expressed in developing heart and somites of mouse embryos and is essential for cardiac development (Chen et al., 2012), which is consistent with other animal models [Drosophila (Melkani et al., 2011), zebrafish (Wohlgemuth et al., 2007) and Xenopus (Geach and Zimmerman, 2010)]. Recessive loss-of-function mutations in mouse unc-45b yielded cardiac developmental arrest with abnormal cardiac looping and heartbeat, resulting in embryonic lethality (Figure 4C); this occurs despite the presence of normal levels of UNC-45a (Chen et al., 2012). As in other systems (Etard et al., 2007; Geach and Zimmerman, 2010; Wohlgemuth et al., 2007), mutant UNC-45b results in decreased accumulation of the cardiac myosins, which impacts contraction of the mutant embryonic hearts. Interestingly, expression of mutant UNC-45b resulted in reduced accumulation of the cardiogenic GATA4 transcription factor (Chen et al., 2012). Furthermore, the blockage of right atrium and ventricle formation in UNC-45b mutants is similar to that seen in GATA4 mutants (Kuo et al., 1997; Zeisberg et al., 2005), raising the possibility that the interaction between UNC-45b and GATA4 is required for right chamber formation (Chen et al., 2012). UNC-45b binds with both α- and β-cardiac myosins and GATA4; however, mRNA levels for neither myosin nor GATA4 were affected by the UNC-45b mutations (Chen et al., 2012). Reduced mRNA expression for Nkx-2.5, Hand1, and Hand2 cardiogenic transcription factors, known target genes of GATA4, was observed in unc-45b mutants, presumably as a result of decreased GATA4 protein levels (Chen et al., 2012). Overall, UNC-45b is an essential chaperone for mouse cardiac sarcomeric myosin accumulation and it also interacts with the cardiogenic GATA4 transcription factor to affect its accumulation, possibly through folding it appropriately.
2.3.4. Two differentially expressed UNC-45 isoforms in zebrafish
A zebrafish (Danio rerio) unc-45 gene was identified by computational analysis and shown to encode the canonical TPR, central and UCS domains (Price et al., 2002). This gene encodes the UNC-45b isoform, which is expressed in developing and mature muscles (Etard et al., 2007; Etheridge et al., 2002; Wohlgemuth et al., 2007). In situ hybridization detected unc-45b mRNA lateral to the midline in late gastrulae, followed by expression in muscle precursors (adaxial cells, somites); transcripts are also expressed in developing cardiac and skeletal muscles, but not in most smooth muscles and certain skeletal muscles (Etheridge et al., 2002; Wohlgemuth et al., 2007). A second isoform, UNC-45a, encoded by a different gene, has the standard three-domain structure and displays 54% amino acid identity to zebrafish UNC-45b (Anderson et al., 2008). unc-45a mRNA accumulates globally in early development, likely corresponding to maternal transcription, whereas later stage expression occurs in the midbrain, hindbrain, retina, pharyngeal arches, pharynx, liver, gut, and otic vesicle (Anderson et al., 2008). Thus, in general, UNC-45a is expressed widely whereas UNC-45b is largely restricted to striated muscle precursors and mature muscle cells.
2.3.5. Zebrafish unc-45 mutant and over-expression phenotypes
Wohlgemuth et al. used morpholino-oligonucleotide-mediated knockdown of UNC-45b in zebrafish to demonstrate the requirement of UNC-45 for vertebrate muscle function (Wohlgemuth et al., 2007). This study showed that the unc-45b gene is expressed in the heart and skeletal muscle of zebrafish embryos and that its knockdown results in embryonic paralysis, cardiac dysfunction and embryonic lethality. Further analysis revealed that knockdown of UNC-45b resulted in disorganization of actin-containing myofibrils, dramatically less myosin accumulation and a paucity of myosin-containing filaments in trunk muscles (Figure 3C), similar to the phenotypes seen in invertebrates (Barral et al., 1998; Lee et al., 2011b). While cardiac chamber identity is unaffected and assessed myosin heavy and light chain isoforms accumulate normally in UNC-45b knockdown hearts, the morphants have pericardial edema and lack of cardiac looping (Wohlgemuth et al., 2007). Etard et al. (2007) showed that UNC-45b is required for proper myofibril organization in both cardiac (Figure 4B) and skeletal muscles of zebrafish. Additionally, Hsp90a knockdown yielded similar phenotypes and this protein was shown to interact with UNC-45b in vitro (Etard et al., 2007). Interestingly, transcription of unc-45b is upregulated upon knockdown of Hsp90a and vice versa, suggesting that the genes are co-regulated (Etard et al., 2007). In addition to cardiac and skeletal muscle defects, knockdown of UNC-45b results in cranial structure abnormalities, possibly as a result of defects in muscle morphogenesis or contraction (Wohlgemuth et al., 2007). Bernick et al. (2010) also showed that knockdown of UNC-45b led to defective myofibril organization in skeletal muscles of zebrafish embryos. In addition to thick and thin filament defects, UNC-45b morphants displayed disrupted M-lines and Z-disks in sarcomeres of skeletal muscles, with a significant decrease in myosin protein levels (Bernick et al., 2010). Very recently, Myhre et al. demonstrated that UNC-45b co-localizes with non-muscle myosins in zebrafish myogenic tissue prior to the expression of muscle myosin and that an unc-45b mutation disrupts costamere formation and attachment of muscle fibers to the tendon-like myosepta (Myhre et al., 2014). Thus UNC-45b may be important for non-muscle myosin folding at very early stages of muscle development (Myhre et al., 2014), a role that might have been attributed to UNC-45a due its widespread expression and its necessity for myoblast proliferation (Price et al., 2002).
A recent study demonstrated a functional divergence between zebrafish UNC-45 isoforms (Comyn and Pilgrim, 2012). Double homozygous unc45b−/−; unc45a−/− mutant embryos display cardiac, skeletal muscle and jaw defects corresponding to those of unc45b−/− mutants, suggesting that UNC-45a does not duplicate roles of UNC-45b. Further, these authors were unable to define a role for UNC-45a in myoblast differentiation (Comyn and Pilgrim, 2012). Indeed, Anderson et al. found no skeletal muscle defects in kustr12 UNC-45a mutants that have a stop codon in the unc-45a UCS domain coding region (Anderson et al., 2008). In contrast, they demonstrated a role for UNC-45a in aortic arch development, as the kustr12 mutants display malformations, with organisms unable to form a lumenized connection to the lateral dorsal aorta from aortic arches 5 and 6 (Anderson et al., 2008). The authors speculate that non-muscle myosin (Kachur et al., 2004; Lee et al., 2011b), a progesterone receptor (Chadli et al., 2006) or additional crucial client proteins for UNC-45a fail to be folded appropriately in the kustr12 mutant, resulting in aortic arch malformation.
Over-expression studies of UNC-45b have been carried out in the zebrafish system. An initial investigation suggested that this did not result in observable phenotypes (Etard et al., 2007), but a subsequent analysis using a strong muscle-specific transcriptional promoter found severe defects in thick filament organization and muscle fiber size (Bernick et al., 2010). It was further shown that this effect is dependent upon the presence of the UCS domain, but not the TPR domain (Bernick et al., 2010). This suggests that the TPR-dependent binding of Hsp90 is not required for the detrimental effects observed upon over-expression of UNC-45.
2.3.6. Zebrafish UNC-45b interacting with Hsp90a and Apobec2, and shuttling between Z-disk and A-band
Yeast two-hybrid screening of zebrafish UNC-45b, followed by pull-down assays resulted in identification of Hsp90a, Hsp90a2 and Hsp90b as strongly interacting proteins (Etard et al., 2007). hsp90a and hsp90a2 RNAs accumulate in both skeletal and cardiac muscles, whereas hsp90b RNA accumulates ubiquitously (Etard et al., 2007). Morpholino treatment resulted in a phenotype similar to that seen for unc-45b mutants only when the morpholinos targeted Hsp90a, suggesting that the Hsp90a-UNC-45b interaction is functional in muscle (Du et al., 2008; Etard et al., 2007). Mutants for Hsp90a have a dramatic reduction in myosin-containing thick filaments, but not Z-disks or actin filaments (Du et al., 2008; Hawkins et al., 2008). Myosin reduction occurs after transcription, either from translational inhibition or protein degradation (Du et al., 2008). Mutant defects are largely mimicked by deletion of the MEEVD peptide from the C-terminus of Hsp90a, indicating that interaction with the TPR domain of UNC-45b, which binds to this site, may be essential for normal myofibrillogenesis. Interestingly, myofibrillar disarray associated with the Hsp90a knockdown was restricted to skeletal muscles, suggesting cardiac muscle has another UNC-45b partner, perhaps a different isoform of Hsp90 (Du et al., 2008; Hawkins et al., 2008). However, hsp90a mRNA levels were elevated in both skeletal and cardiac muscles in response to a mutation in unc-45b, suggesting that hsp90a transcription attempts to compensate for reductions in functional UNC-45b (Etard et al., 2007).
Interaction of UNC-45b and Hsp90a with myofibrils of maturing zebrafish muscle occurs at the Z-disks (Etard et al., 2008). During development, zebrafish UNC-45b and Hsp90a are initially co-localized with myosin in the cytoplasm and then a transient association with myosin-containing A-bands is detectable, but only when there are limiting amounts of UNC-45b or Hsp-90a (Etard et al., 2008). Hsp-90a interacts directly with myosin and is not dependent upon its MEEVD UNC-45 binding peptide or the presence of UNC-45b for myosin co-localization (Etard et al., 2008).
Interestingly, UNC-45b association with the Z-disks of mature zebrafish muscle is dynamic and it can be induced to move to the A-bands by heat shock, cold shock, certain fixation conditions or laser-induced membrane lesions (Etard et al., 2008). Hsp90a follows a similar pattern, with the proteins returning to the Z-disk upon the cessation of stress (Etard et al., 2008). UNC-45b truncation experiments showed that Z-disk attachment can be dictated by either the UCS or central domain, whereas binding to myosin is dependent upon the UCS domain or the TPR domain in conjunction with the central domain (Etard et al., 2008). Overall, it appears that UNC-45b and Hsp90a are essential for folding myosin in the cytoplasm and the A-band; thereafter they are localized to Z-disks, where they are held in reserve to protect myosin from unfolding during periods of stress (Figure 2).
A two-hybrid screen identified Apobec2, a possible cytidine deaminase, as a zebrafish UNC-45b binding protein (Etard et al., 2010). Both the central and UCS domains of UNC-45b (but not UNC-45a) interact with two forms of Apobec2 that are co-expressed with UNC-45b in striated muscles (Etard et al., 2010). Knockdown of either form of Apobec2 results in reduced motility, impaired cardiac function, skeletal myofibril disorganization and disrupted muscle fibers and myosepta (tendon equivalents). Apobec2 was shown to co-localize with UNC-45b to the myoseptal boundary and the Z-disks (Etard et al., 2010; Myhre et al., 2014). Although the localization of UNC-45b was not dependent upon the presence of Apobec2, the localization of Apobec2 does depend upon UNC-45b, implicating UNC-45b in muscle fiber attachment to the myoseptum (Etard et al., 2010). At present it is unclear whether the two proteins act as a complex to maintain the myospetum or if Apobec2 is a client of the UNC-45b chaperone.
2.3.7. Disruption of myofibrillogenesis by Xenopus tropicalis unc-45b mutation
Similar to zebrafish and mouse models, mutation of unc-45b (dicky ticker) in the vertebrate Xenopus tropicalis (western clawed frog) results in disrupted skeletal muscle myofibrillogenesis (Figure 3D), paralysis, and lack of heartbeat (Geach and Zimmerman, 2010). The protein is expressed in cardiac and somatic tissue at developmental stage 28, with localized expression in the body wall muscles, jaw and brachial arches at stage 40. Mutants do not respond to vigorous stimulation and they develop cardiac edema, which eventually results in lethality at approximately stage 45. The dicky ticker phenotype arises from a missense mutation in the UCS domain of UNC-45b and it is phenocopied by depletion of UNC-45b using antisense morpholino oligonucleotides (Geach and Zimmerman, 2010). As in other models, Xenopus UNC-45 is required for myosin stability, since mutant embryos do not incorporate myosin into sarcomeres (Figure 3D) (Geach and Zimmerman, 2010). The mutant also displays delayed polymerization of α-actinin, resulting in delayed Z-disk formation (Geach and Zimmerman, 2010). As Z-disk formation is a critical initial step in sarcomerogenesis, this may explain the severe effects of the mutation on myofibril structure.
3. X-Ray Crystal Structures of UCS Proteins
The crystal structures of two UNC-45 proteins and a related UCS-domain-containing protein have been solved: Drosophila melanogaster UNC-45 (Lee et al., 2011a), Caenorhabditis elegans UNC-45 (Gazda et al., 2013) and Saccharomyces cerevisiae She4p (Shi and Blobel, 2010) (Figure 5). Here we draw on the similarities of the currently known structures as a way to validate them and to extrapolate possible functions. A caveat of including the yeast She4 protein in this analysis is that the structure contains a molecule with multiple mutations and a large loop deletion (Shi and Blobel, 2010). Furthermore, since yeast does not have filamentous sarcomeric myosin, it might not be specialized to interact with the muscle myosin II substrate. However, due to the scarcity of UNC-45 structural data, She4p is included here to broaden the scope of our analysis.
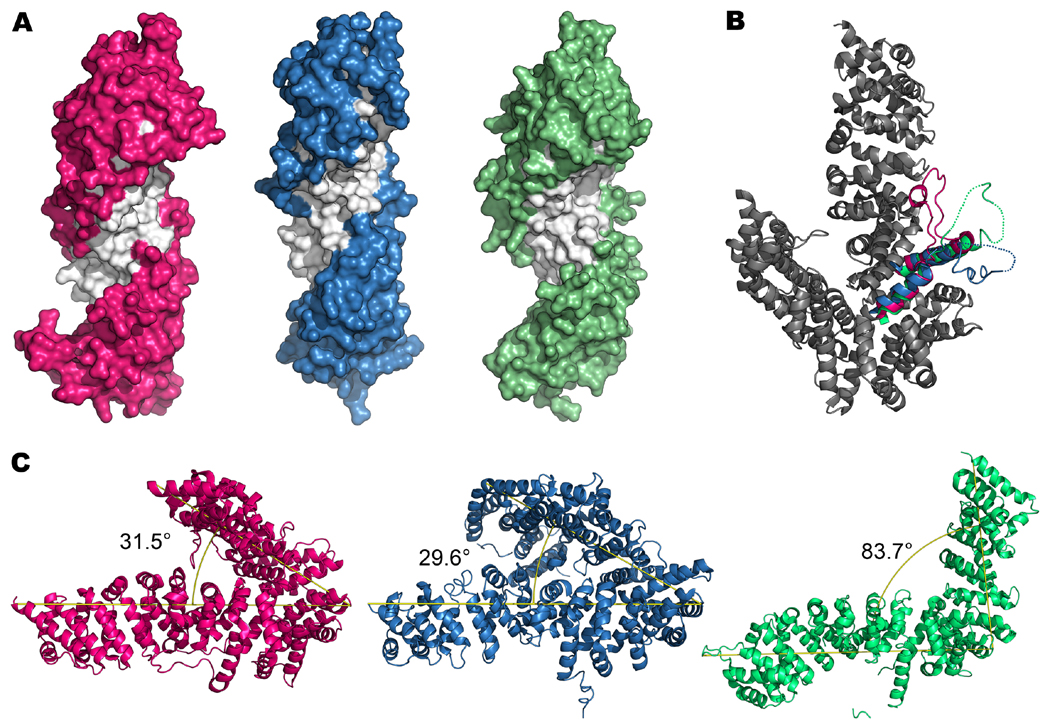
Figure 5. Structural similarities and differences among the three UCS domain proteins, Drosophila UNC-45 (PDBID 3NOW, pink), C. elegans UNC-45 (PDBID 4I2Z, blue), S. cerevisiae She4p (PDBID 3OPB, green).
(A) Putative myosin binding groove. Here, only the UCS domain is shown with helix 3 of armadillo repeat layers 14–20 colored white to highlight the groove. (B) Putative myosin interacting loop. By aligning the crystal structures using the overall protein conformations, the positions of the proposed myosin interacting loops can be compared. Drosophila UNC-45 (in gray) was used as the reference to show relative position of the loops to the UCS domain. The gaps in the loops were filled in manually for better visualization. In addition to the loops, the helices where the loops originate and terminate are also represented in color. (C) The overall bent shape. The angle between the central and the UCS domains was measured using PyMOL. Since the Drosophila and the C. elegans UNC-45 proteins were very similar, the small difference in the bend angle is probably not significant; however, the yeast She4 protein has a drastically larger bend angle. Since all three proteins are composed of layers of armadillo repeats, the difference in the bend angle could suggest flexibility between the central and the UCS domains. Protein structures are modeled in PyMOL (PyMOL Molecular Graphics System, Version 1.6.0.0 Schrödinger LLC).
Yeast She4p contains an N-terminal helix, while the Drosophila and C. elegans UNC-45 proteins contain an N-terminal TPR domain. Each TPR motif is composed of two α-helices. The TPR domain is shared by proteins such as mitochondrial import receptor subunit Tom34, cyclophilin-40 and yeast protein phosphatase T (D’Andrea and Regan, 2003). All three UCS proteins have central and UCS domains. These are composed almost entirely of stacked, α-helical armadillo (ARM) repeat motifs composed of three α-helices per repeat. The stacked ARM repeat solenoid composing the central and UCS domains loops back on itself, to resemble an uneven horseshoe (fly), a proteinous mouth (worm), or an L shape (yeast) (Figure 5C). The continuous stack of ARM repeats makes it difficult to discern the junction between the central and the UCS domain or to attribute function to specific areas of the protein. The lack of obvious domain-like features suggests that ARM repeat proteins, such as UNC-45 and She4p, are versatile scaffolds that allow docking of other proteins (Tewari et al., 2010). An example of such versatility is eukaryotic β-catenin, which plays a structural role in adherens junctions and is a component in the Wnt signal transduction pathway (Tewari et al., 2010). Three additional characteristics are shared by the UCS protein crystal structures: 1) the putative myosin binding groove in the UCS domain, 2) the putative myosin interacting loop in the UCS domain and 3) the overall bent shape. These characteristics are discussed below, followed by consideration of major differences among the crystals.
3.1. Putative myosin binding groove in UCS domain
A groove in the UCS domain (Figure 5A) is formed as a result of the stacked ARM repeat superhelix twisting in a right-handed fashion (Groves and Barford, 1999). This structural arrangement/shape is a signature motif that is conserved in many ARM-repeat containing proteins (Groves and Barford, 1999). Amino acid sequence comparisons among various species also show strong conservation of residues in the UCS domain (Gazda et al., 2013; Lee et al., 2011a). When mapped onto the crystal structure, there is one large patch of conserved, surface amino acid residues around the opening to the groove of the UCS domain (Lee et al., 2011a). To assess the feasibility of this groove serving as a myosin binding surface, and to decipher the particular amino acid residues important for myosin interaction, Gazda et al. (2013) took advantage of strong structural conservation with β-catenin. They mapped the β-catenin ligand onto the C. elegans UNC-45 UCS domain. In doing so, they identified an amino acid position (N758) which, when mutated, abolished myosin binding and failed to rescue a temperature sensitive unc-45 mutant grown at the non-permissive temperature. Interestingly, this particular amino acid residue is in close proximity to the conserved amino acid patch mentioned above.
Though no human UNC-45 structure is available, homology models have been created via in silico molecular modeling and simulations (Fratev et al., 2013). Using Drosophila UNC-45, Fratev et al. (2013) modeled human UNC-45b and examined its possible interaction with a homology model of the human β-cardiac myosin motor domain in the native/folded conformation. This differs from the unfolded conformation that is thought to be necessary for interaction with UNC-45 (Barral et al., 2002). Nonetheless, Fratev et al. identified two areas in the UCS domain that are predicted to interact with the motor domain of myosin. One of these is the groove in the UCS domain, where the human UCS sequence generates a more electronegative surface rather than the hydrophobic surface seen in Drosophila UNC-45. The other putative interaction area between myosin and UNC-45 is a protein loop that is discussed below.
3.2. Putative myosin interacting loop in UCS domain
A second putative myosin interacting region of UCS proteins is an approximately 30 amino acid loop in the UCS domain that is common to all three proteins: amino acid residues 587–613 in Drosophila, 601–632 in C. elegans and 418–447 in S. cerevisiae (Figure 5B). When deleted, it eliminated a protease-sensitive site (Lee et al., 2011a) and caused a loss of myosin binding in pull-down assays (Gazda et al., 2013). Within the three protein crystal structures, only Drosophila UNC-45 contains a fully resolved loop. However, in the C. elegans and S. cerevisiae crystal structures, there is clear evidence of an extended loop at the same position. In the fly structure, the loop is situated near the N-terminus of the UCS domain and extends in parallel along the side of the UCS domain toward the C-terminus. The C. elegans structure suggests that the loop is projecting perpendicularly away from the UCS domain, which is reminiscent of the yeast structure. The difference in the loop position among the currently available structures can be explained by its flexibility. The increased B-factors in this area, the non-hydrophobic amino acid residues present, and the absence of stable surrounding structural moieties support the concept that this loop is capable of transiting through a range of conformations. Using molecular dynamic simulations, Fratev et al. (2013) reported three possible states of the loop, closed, partially open and open. The fly structure could be considered to be in the closed conformation, as it lies closer to the UCS domain, while the worm and yeast structures could be in the open conformation, where the loop projects further from the UCS domain. The location of the putative myosin binding domain on a loop suggests that flexibility is an integral part of its binding function. The loop may act as a feeler to sense and capture denatured myosin, then as a hatch door to secure myosin in close proximity to the UCS groove, or it could act as an opener/wedge to penetrate myosin and further expose the internal peptides for UNC-45 binding.
3.3. Overall bent shape of UCS proteins
Each of the three UCS protein crystal structures presents a molecule with an overall bent structure, although the angle of the bend differs (Figure 5C). They are roughly 32°, 30°, and 84° for Drosophila, C. elegans, and S. cerevisiae respectively. The smaller angles of the fly and worm structures result from the proteins traversing more than 200° on a circular plane back toward the direction of the N-terminus. The bent shape is at odds with previous experiments that suggested a more extended form of the protein, based on the protein envelope obtained through small angle x-ray scattering (SAXS) (Lee et al., 2011a) and electron microscopy of rotary shadowed molecules (Srikakulam et al., 2008). SAXS further suggested that the extended conformation results from significant conformational flexibility, even within the UCS domain. Recent molecular dynamic simulations also suggest a more planar conformation (Fratev et al., 2013). This raises the question as to whether the severe bend is an artifact of the crystallization conditions and does not play a role in UNC-45’s biological function. In addition, even the same protein can show differences in the bend angle; for instance, the crystal structure of the Drosophila Leu63Met mutant agrees with the native protein crystal structure, but its unit cell is slightly different from that of the native construct (Lee et al., 2011a). Their comparison suggests some degree of relative motion between the central and the UCS domains. Overall, investigating whether the protein’s flexibility has functional importance is critical.
3.4. Major differences among crystal structures and their interpretations
The three-domain framework for UNC-45 proteins was proposed based on sequence alignment (Barral et al., 1998) and proteolytic digestion of the UNC-45 protein that yielded three stable fragments (Barral et al., 2002; H.F. Epstein, personal communication). However, analysis of the C. elegans crystal structure suggested that the characteristic bend between the central and the UCS domains may have functional relevance (Gazda et al., 2013). This led to it being labeled as the neck, a newly defined fourth domain. In the crystal lattice of worm UNC-45, a contact between the distal neck domain surface of one molecule and the TPR domain of another was observed (Gazda et al., 2013). This contact resulted in filament formation (see section 4.2) and was deemed “specific” via bioinformatic analysis, due to a calculated PΔG,IF value that satisfies the requirement for hydrophobic/specific interfaces (Gazda et al., 2013). Another possible interface for UNC-45 that results in dimerization was examined but it has a PΔG,IF value that classifies it as a crystal artifact (Gazda et al., 2013). The neck domain contact was not present in the Drosophila or the S. cerevisiae crystal lattices. Instead, the yeast structure shows an N-terminal-helix-mediated dimerization involving the proximal neck domain surface (Shi and Blobel, 2010), while the fly structure does not exhibit evidence of self-association into dimers or filaments (Lee et al., 2011a).
Another difference among the crystal structures pertains to the position of the N-terminal domain. The yeast She4 protein contains only an N-terminal helix that is connected to the central domain by a flexible linker (Shi and Blobel, 2010). In contrast, the N-terminal TPR domain in the worm UNC-45 structure is packed next to the central domain (Gazda et al., 2013). It is connected to the central domain via a long α-helix, which does not suggest flexibility between the two domains. The authors proposed that the interaction between the TPR and central domains is important for UNC-45 function because many amino acid residues lining the interacting region are highly conserved. The fact that the TPR domain makes extensive contact with the central domain in the C. elegans structure is consistent with a proteolysis experiment in which digested C. elegans UNC-45 fragments remained associated as a complex in a pull-down assay (Srikakulam et al., 2008). The fly TPR domain is not resolved in the crystal structure, and the beginning of the central domain projects into a large solvent space (Lee et al., 2011a). Interestingly, the beginning of the fly central domain projects in the same direction as in the worm crystal structure. However, the addition of a seleno-methionine to the TPR domain in the fly Leu63Met mutant did not result in identification of an additional heavy atom peak by Fourier synthesis using diffraction data collected at its absorption peak, suggesting that the TPR domain is connected to the central domain via a linker that is flexible (Lee et al., 2011a). Therefore, the fly UNC-45 and yeast She4 proteins have N-terminal domains that are flexible relative to the rest of the protein, but the worm UNC-45 structure has an N-terminal domain that is fixed relative to the central and the UCS domains. While the variations among the crystal structures may signify functional differences among the proteins, it is also possible that they represent alternative conformations resulting from crystal packing forces.
We compared the locations of the ARM repeats among the three UCS proteins via structural analysis (Figure 6). Overall, as expected, the Drosophila and C. elegans UNC-45 proteins are more similar to each other than they are to the She4 protein. A majority of the differences resides in the central domain (ARM repeat layers 5 to 13), where the α-helices in She4p are much shorter or completely absent, such as helices 7H2 and 8H1. The UCS domains of the three proteins, however, are more similar to each other, with fairly comparable α-helix lengths and more equivalent loop spacings. Two major observations emerge from the three-dimensional crystal structure alignments. First, She4p has longer loops than the UNC-45 proteins in ARM repeat layers 5, 13, and 17. It is unclear what function, if any, is conferred by the different sized loops at these particular locations. Second, helices 12H2 and 12H3 in the UNC-45 proteins are absent from She4p. These helices are located at the apex of the V-shaped protein structure. According to the C. elegans structural analysis, this region makes up the newly defined neck domain that is important for UNC-45 filament formation (Gazda et al., 2013). Such filaments may be key to chaperone action on myosin head domains periodically displayed in thick filaments (see section 4.2). As She4p lacks a TPR domain that is involved in filament formation through its interaction with the neck domain, the absence of the neck helices might be expected.
Figure 6. UNC-45 protein sequence alignment based on the three-dimensional crystal structures.
Crystal structures of C. elegans UNC-45 (PDBID 4I2Z) and the yeast UCS protein She4p (PDBID 3OPB) were aligned to the Drosophila UNC-45 crystal structure (PDBID 3NOW) using the “align to selection” function in PyMOL (PyMOL Molecular Graphics System, Version 1.6.0.0 Schrödinger, LLC). Chain B of the two molecules in the She4 protein structure was utilized for this comparison, because of its higher degree of completeness. Due to the bend in the structures, the N-terminal regions (amino acids 138–439 for Drosophila, 130–452 for C. elegans, and 31–323 for yeast) and the C-terminal regions (amino acids 440–923 for Drosophila, 453–930 for C. elegans, and 324–771 for yeast) were aligned separately. After the three-dimensional structural alignment, the α-helices (Drosophila in pink, C. elegans in blue, and yeast in green) were compared and amino acids occupying comparable positions were aligned manually. Each armadillo repeat layer, designated according to Lee et al. (2011a), is indicated above the helices with the corresponding H1, H2, or H3 labeled. Lower case letters indicate deletions, mutations, or variations from the PubMed sequences.
3.5. Mapping of mutation sites on UNC-45 structure
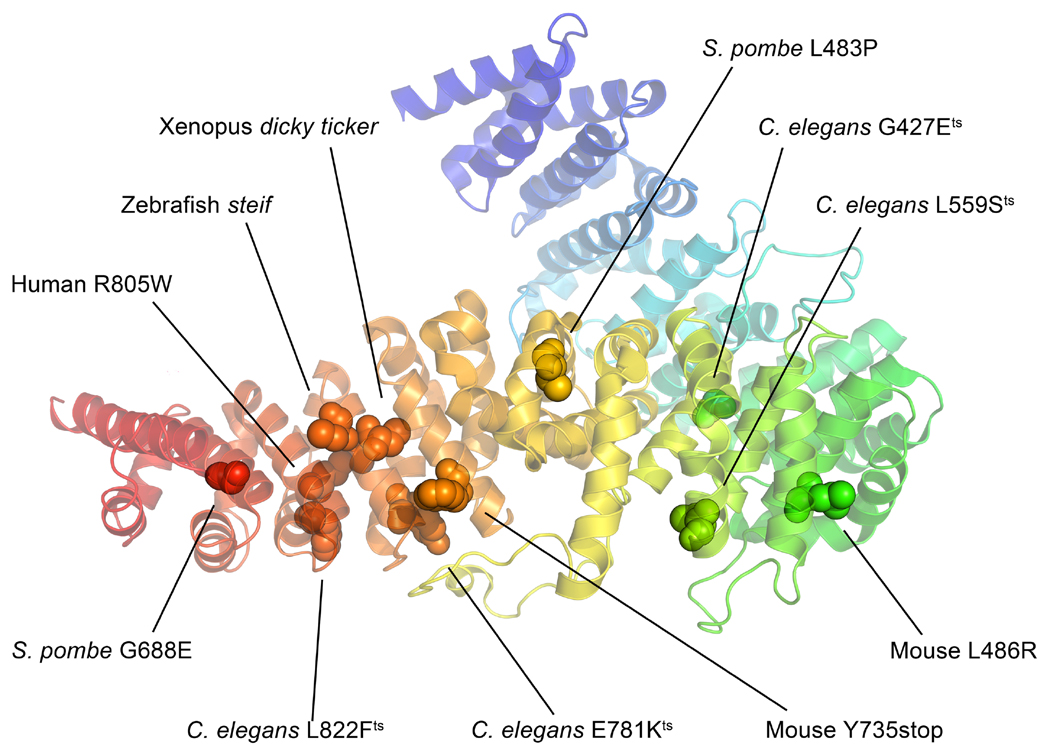
Mapping locations of identified mutations onto the Drosophila UNC-45 crystal structure indicates that they occur in both the central and UCS domains (Lee et al., 2011a) (Figure 7). Two of the C. elegans unc-45 mutations are recessive lethal and result in stop codons within the central domain, suggesting the necessity for the downstream UCS domain (Barral et al., 1998). Four temperature-sensitive C. elegans alleles affect Gly427, Leu559, Glu781 and Leu822 (C. elegans numbering) (Barral et al., 1998). Gly427Glu is predicted to disrupt a tight turn separating ARM repeat layers 10 and 11 within the central domain (Lee et al., 2011a). While the effect of a Leu559Ser mutation near the N-terminus of the UCS domain is unclear, it is predicted that the Glu781Lys mutation in ARM repeat 18 would destabilize the UCS domain by disrupting the ionic interaction with Arg819 in repeat 19 (Lee et al., 2011a). Interestingly, the Leu822Phe C. elegans mutation is located within the vicinity of the Glu781-Arg819 interaction (Lee et al., 2011a). The Xenopus dicky ticker mutation (Cys779Arg) in UNC-45b (Geach and Zimmerman, 2010) maps to the center of helix 3 of ARM repeat 18 in the UCS domain. The introduction of a long charged side group could interfere with substrate binding. Further, the zebrafish steif mutation introduces a stop codon after residue 788 in helix 3 of ARM repeat 18 (Etard et al., 2007), which is two residues C-terminal to the dicky ticker mutation (Hansen et al., 2014). These observations indicate that ARM repeats 18 and 19 in the UCS domain are critical for UNC-45 function.
Figure 7. Mutations from the various UCS domain containing proteins mapped onto the Drosophila UNC-45 structure (PDBID 3NOW).
Mutations from S. pombe, C. elegans, zebrafish, Xenopus, mouse and human were mapped onto the Drosophila UNC-45 crystal structure using the protein alignment in Figure 6. A majority of the mutations is located in the UCS domain at the bottom of the figure, which underscores the importance of this domain to UNC-45 function. Residue numbers correspond to those of the mutant organisms. Protein structure modeled in PyMOL (PyMOL Molecular Graphics System, Version 1.6.0.0 Schrödinger LLC).
Intriguingly, Arg805Trp, the sole identified human mutation in UNC-45b maps to ARM repeat 19 (helix 2) of the UCS domain (Hansen et al., 2014), which is the equivalent of C. elegans Arg819 discussed above. Hence this would be expected to disrupt the very same ionic interaction that is affected by the C. elegans Glu781Lys mutation, further emphasizing its critical nature. Surprisingly, the human mutation results in dominant juvenile cataracts but does not appear to affect skeletal or cardiac muscle function (Hansen et al., 2014). This is more fully discussed in section 4.3.
Two recessive mouse unc-45b mutations have been characterized as to induced cardiac defects (section 2.3.3) (Chen et al., 2012). Introduction of a stop codon at mouse Tyr735, which is equivalent to an amino acid residue at the beginning of helix 3 of ARM repeat 17 in Drosophila, deletes a large portion of the UCS groove so that it would not be available for substrate binding. The abnormalities induced by this mutation in mouse cardiac muscle further reinforce the observation in C. elegans, Xenopus, zebrafish and human mutations that ARM repeats 18 and 19 are essential. The mouse Leu486Arg unc-45b mutation is mapped to ARM repeat 12H3 on the Drosophila structure, which is located in the central domain, just before the beginning of the UCS domain. Interestingly, helices 12H2 and 12H3 are absent in yeast She4p (Figure 6), suggesting this region of the protein has a function that is specific to higher organisms.
Two temperature-sensitive mutations in the S. pombe UCS protein Rng3p (Wong et al., 2000) affect the UCS domain. Leu483Pro mutates an equivalent amino acid residue in helix H1 of ARM repeat layer 16, which likely results the decreased stability of the UCS domain solenoid ARM repeat stacking (Lee et al., 2011a). Gly688Glu, located in helix 3 of ARM repeat 20, affects the hydrophobic groove arising from the H3 helices of ARM repeats 17–21. By introducing a charged amino acid into the hydrophobic groove, the predicted interactions with myosin may be disrupted (see section 3.1).
A number of mutations were introduced into C. elegans UNC-45 to test putative functions of specific residues based upon the protein’s crystal structure (Gazda et al., 2013). Mutations in the central portion of the hydrophobic groove in the UCS domain, such as in highly conserved helix 3 of ARM repeat 17 (designated repeat 13 by Gazda et al.), were designed to affect either the outer rim (Tyr750Trp) or the central portion (Asn758Tyr) of the groove in order to test predicted interaction with its substrate. Mutated genes were introduced into C. elegans unc-45 temperature-sensitive mutants. The resulting organisms were assessed as to restored muscle function at the restrictive temperature while the mutant proteins’ interactions with myosin were examined by immunoprecipitation (Gazda et al., 2013). While the Tyr750Trp mutation rescued UNC-45 function, Asn758Tyr did not. A third mutation that deleted the putative myosin binding loop (resides 602–630) failed to rescue as well. These results agree with the thesis that the UCS groove and the nearby loop are critical to myosin binding and UNC-45 function.
Additional mutagenesis studies by Gazda et al. (2013) examined the importance of crystal contacts between TPR domains and adjacent neck domains of UNC-45 molecules to yield polar multimers. Computational analyses predicted that such multimers would assemble in solution, but might be transient in nature. To test the formation of multimers in vitro, Gazda et al. incorporated UV-inducible cross-linking amino acids and made deletions of putative interacting regions. This allowed them to define Leu121 (TPR), Val480 (neck) and Val484 (neck) as key residues that mediate multimer formation in solution (Gazda et al., 2013). In vivo experiments showed that Leu121Trp or TPR deletion mutants could rescue myosin binding, but the animals showed reduced motility and fewer A-bands than wild-type organisms. In contrast, expression of Val480Arg/Val484Arg did not restore muscle function or myosin binding, suggesting that this region of the protein may be involved in more aspects of UNC-45 function than multimerization. Each of these mutants also was tested for dominant negative effects by expression in a wild-type background and each caused motility defects and reduced A-band numbers (Gazda et al., 2013). The fact that these phenotypes were not detected when expressing the myosin binding loop deletion or Asn758Tyr argues that the putative multimer formation defects have a dominant effect upon wild-type UNC-45 molecules, while the myosin-binding defects do not (Gazda et al., 2013).
4. Interactions, Mechanisms of Action and Human Disease
4.1. Molecular interactions among UNC-45, myosin and Hsp90
Determining the mechanism of UNC-45 action requires a detailed understanding of its interaction with myosin and with its key partner Hsp90. Assessment of myosin affinity for UNC-45 has been approached using various biochemical techniques. Liu et al. (2008) examined the concentration dependence of UNC-45’s ability to enhance myosin folding and found that the apparent dissociation constant for UNC-45a is stronger than that for UNC-45b (19 vs. 53 nM, respectively). A pull-down assay using C. elegans UNC-45 found a KD value of 1.3 μM (Ni et al., 2011). Assessing UNC-45b binding to myosin attached to a fluorescent reporter yielded an apparent KD of 1.3 μM (Kaiser et al., 2012). Overall, it appears there is strong affinity between UNC-45 and myosin.
Efforts to map the specific residues of myosin that interact with UCS proteins have involved pull-down assays, yeast two-hybrid studies and computational analyses. Initial pull-down assays showed that the myosin S1 head interacts with C. elegans UNC-45 at an elevated temperature of 30°C (Barral et al., 2002) Two-hybrid assays identified a fragment (141 residues in length from HUM-2, a myosin V) in C. elegans non-muscle myosin that starts just upstream of the actin binding site and a second 531 residue fragment from NMY-2 that overlaps the first fragment, extending 19 residues upstream and further downstream into the neck domain (Kachur et al., 2004). Two-hybrid assays with the S. cerevisiae UCS protein She4p and several yeast myosins determined that fragments containing the actin-binding site of myosin interact with She4p (Toi et al., 2003). Further, these interactions occurred more readily at elevated temperatures. Pull-down assays using She4p and yeast Myo4p revealed that a 27-residue fragment of myosin was sufficient to bind She4p with a KD of 1 μM. This region of myosin is located near the cleft between its upper and lower 50 kD domains, with an epitope on the surface and another within the cleft that is inaccessible when the cleft is closed (Shi and Blobel, 2010). The cleft is open when the nucleotide pocket is occupied by ATP or ADP + Pi and closed when actin binds tightly to myosin. The location of these epitopes and their accessibility could influence the binding affinity of She4p (Shi and Blobel, 2010). In contrast to the results with She4p, direct binding of myosin peptides to UNC-45 was not detectable by isothermal titration calorimetry (Gazda et al., 2013). In the future, it may be possible to map specific myosin interactions with UNC-45 using a unique assay that measures the ability of UNC-45 to refold myosin bound to a titin sensor attached to an atomic force microscope probe (Kaiser et al., 2012).
As described in more detail in section 3, crystal structure analysis and molecular dynamics have predicted potential interactions between myosin and UNC-45. Both the Drosophila and C. elegans studies predict that a surface groove (section 3.1) in the UCS domain of UNC-45 acts as a myosin binding site (Gazda et al., 2013; Lee et al., 2011a). In addition to the surface groove, molecular dynamics predicts that an ~30 residue UCS loop also binds to myosin (section 3.2). Molecular dynamics predicts that UNC-45 binds near the region of myosin that interfaces with actin (Fratev et al., 2013), but it must be remembered that this study used folded myosin for its starting point, which may not accurately predict in vivo interactions with the unfolded molecule.
In addition to myosin, UNC-45 interacts with Hsp90, which was shown to bind to the UNC-45 TPR domain using pull-down assays (Barral et al., 2002). Further, Hsp90 appears to be critical, in conjunction with UNC-45, for the partial folding of myosin in an in vitro translation system (Liu et al., 2008). These studies led to the hypothesis that UNC-45 presents TPR-bound Hsp90 to nonfolded myosin that is bound at the UCS domain, allowing Hsp90 to fold the myosin globular domain, perhaps in conjunction with UNC-45’s own chaperone activity (Hutagalung et al., 2002). Interestingly, a recent study suggests that Hsp90 and UNC-45 compete for binding to myosin (Ni et al., 2011). Nonetheless, it is clear that Hsp90 has the capacity to bind to UNC-45. This binding occurs through the C-terminal EDASRMEEVD peptide of Hsp90 with a KD of 14 μM, as determined by isothermal titration calorimetry (Gazda et al., 2013). Moreover, the specific binding configuration of the Hsp90 peptide at the TPR domain has been documented at atomic-level resolution by its co-crystallization with C. elegans UNC-45 (Gazda et al., 2013).
Recently, increased transcription of both unc-45b and hsp90a1 in zebrafish embryos was shown to arise from knockdown of Smyd1b, a protein involved in myosin methylation and stabilization (Li et al., 2013). Myosin accumulation in smyd1b-knockdown embryos was further reduced by over-expression of UNC-45b (Li et al., 2013). Since Smyd1b co-immunoprecipitates with Hsp90a1 and UNC-45 when expressed in HEK293 cells, it may interact with these proteins to facilitate myosin folding and/or modification (Li et al., 2013). Alternatively its interaction could be indirect and mediated, for instance, by myosin. Based on these results, it seems likely that additional interactions, both direct and indirect, for UNC-45 remain to be discovered.
4.2. Mechanism of action of UNC-45
The elucidation of UNC-45’s crystal structure (Gazda et al., 2013; Lee et al., 2011a) suggested that it serves as a scaffold-like protein that can present Hsp90 bound at the TPR domain to myosin bound in the surface groove of the UCS domain, with the myosin possibly held in place by a binding loop (Fratev et al., 2013; Gazda et al., 2013). The TPR domain’s putative attachment to the central domain via a flexible linker (Lee et al., 2011a) may aid in the presentation of Hsp90. The overall flexibility of the molecule is further substantiated by SAXS analysis (Lee et al., 2011a) and electron microscopy (Srikakulam et al., 2008).
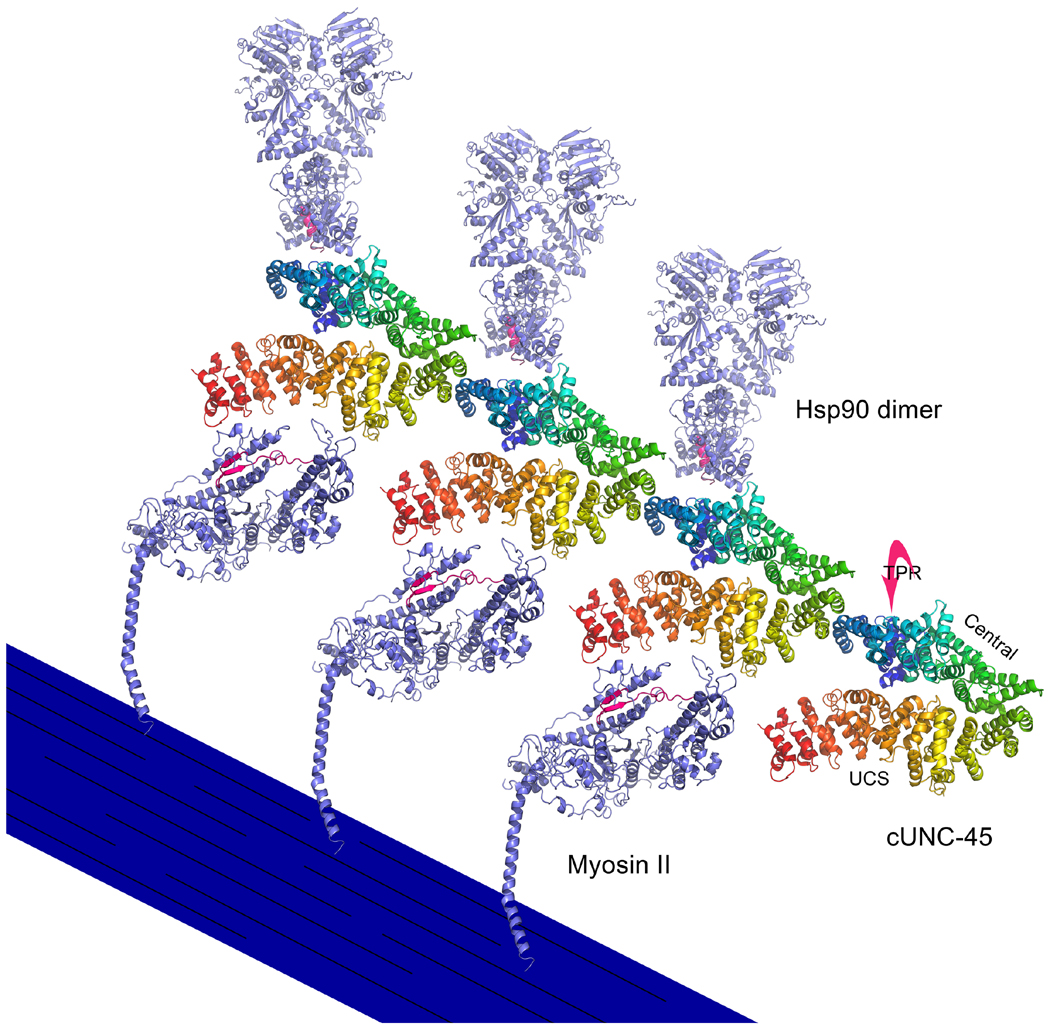
In a refinement of the flexible-linker model, Gazda et al. (2013) proposed that multiple Hsp90-myosin S1 head interactions could be accommodated by the UNC-45 oligomer that was observed in their C. elegans crystal structure. The oligomer forms as a result of interaction of the Hsp90-binding TPR domain with an adjacent UNC-45 molecule at its neck region, which joins the central and UCS domains. Interestingly, the periodicity of oligomeric UNC-45 is similar to that of myosin heads on a thick filament. Hence an oligomer of UNC-45 could fold a series of UCS-bound S1 heads simultaneously through presentation of Hsp90 at appropriate locations on an adjacent thick filament (Gazda et al., 2013) (Figure 8). Gazda et al. (2013) demonstrated that UNC-45 can form oligomers in vitro and that the residues involved in oligomer formation are critical in vivo. Mutation of these residues in C. elegans led to abnormal thick filaments and sarcomeres (Gazda et al., 2013). It is important to note, however, that direct observation of UNC-45 oligomers in vivo has not yet been reported.
Figure 8. UNC-45 filament structure and its putative interactions with myosin S1 and Hsp90.
Based upon its crystal structure configuration, C. elegans UNC-45 (cUNC-45) forms filaments as a result of interactions between TPR domains and central/UCS neck regions of adjacent molecules (Gazda et al., 2013). The UCS domains of these filaments are proposed to interact with the periodically-spaced S1 heads of myosin thick filaments to facilitate their folding (Gazda et al., 2013). The region of muscle myosin II that may interact with UNC-45 is colored in red, based upon the ability of the homologous region of myosin V to interact directly with the She4p UCS protein in vitro (Shi and Blobel, 2010). The C-terminal peptide (MEEVD) of Hsp90 interacts with the TPR domain of cUNC-45, which lies behind the central domain as depicted (Gazda et al., 2013). The C-terminus of one Hsp90 subunit of the homodimer is colored red to show the area of possible interaction with cUNC-45. It is hypothesized that the UNC-45 filament serves as a scaffold to present Hsp90 chaperone activity to the UCS-bound unfolded myosin S1 domains (Gazda et al., 2013). In this diagram, a stylized thick filament displays folded myosin heads that have detached from cUNC-45. The Hsp90 molecules have detached from cUNC-45 as well. cUNC-45 (PDBID 4I2Z), myosin II (PDBID 2MYS), hsp90 (PDBID 2CG9) are modeled in PyMOL. Protein structures are modeled in PyMOL (PyMOL Molecular Graphics System, Version 1.6.0.0 Schrödinger LLC).
Shi and Blobel (2010) showed that mutated yeast She4p forms homodimers during crystallization and they speculated that the She4p dimer binds to myosin motor domain dimers. They proposed that the She4p dimer could control myosin step size as the motor walks along an actin filament. The affinity of UNC-45 to myosin would be altered during the chemomechanical cycle through the differential binding of UNC-45 to myosin’s surface and cleft binding sites. Cleft interaction might not occur when the cleft closes upon actin binding, and this could affect the dimerization ability of UNC-45 as well as the length of the step that myosin could take (Shi and Blobel, 2010). Although this mechanism of fungal UCS protein action has not yet been experimentally verified, this proposal is in concordance with data showing that addition of fungal UCS proteins can increase the velocity of actin filaments in an in vitro motility system (Lord and Pollard, 2004; Lord et al., 2008). The dimerization model might not be expected to apply to muscle myosin since the TPR domains would likely prevent the sort of dimerization observed for the TPR-less She4p proteins. Further, the low duty ratio of muscle myosin does not facilitate a step-like mechanism. However, one modeling study did suggest that UNC-45b could act as a dimer to link myosin motor domains in muscle (Fratev et al., 2013).
A recent preliminary report provides a sharply contrasting view of UNC-45’s mechanism of action, proposing that it inhibits myosin function by stabilizing an actomyosin conformation that prevents motor translocation (Nicholls et al., 2013). This model suggests that myosin is retained in this inactive conformation so that myofibrils can be assembled without filament sliding. The study shows that Hsp90 releases the translocation block, possibly in conjunction with facilitating myosin folding, suggesting that UNC-45 plays a antithetical role to Hsp90 in its facilitation of myosin function (Nicholls et al., 2013).
From the above discussion, it is clear that the mechanism of action for UNC-45 on myosin remains poorly defined. Determination of the structure of multi-protein complexes (UNC-45, myosin, Hsp90) as well as observation of detailed molecular interactions in vivo during development and during stress may shed light on exactly what role UNC-45 performs when it interacts with its partners and how it facilitates myosin function.
4.3. UNC-45 and human disease
The first human UNC-45b mutation was recently discovered; it was identified by genome-wide linkage analysis and targeted DNA sequencing of a family with autosomal dominant juvenile cataracts (Hansen et al., 2014). In concordance with this observation, UNC-45b was indeed found to be expressed in human embryo eyes (Hansen et al., 2014). While the cataract-causing mutation (Arg805Trp) affects a UCS domain residue that is well conserved in vertebrate UCS proteins, no effects upon muscle or heart function were reported by patients (Hansen et al., 2014). To investigate the effects upon eye development, UNC-45b expression in the developing zebrafish lens was assessed; it was found to localize at cell cortices along with non-muscle myosin II (Hansen et al., 2014; Myhre et al., 2014). In support of the importance of unc-45b expression in the eye, the zebrafish unc-45b steif mutant shows a reduced lens size and lens cells contain ectopic actin fibers and swollen, anteriorly-located nuclei that are absent in the wild-type organism (Hansen et al., 2014). The mutant zebrafish phenotype is partially rescued by embryo injection of RNA encoding the normal human protein, whereas the mutant phenotype is engendered by injection of RNA encoding the human mutant protein into wild-type embryos (Hansen et al., 2014). Overall, it appears that mutant UNC-45b may not permit normal non-muscle myosin II function, thereby inhibiting appropriate lens maturation.
Accumulation of excess UNC-45b is implicated in causing human skeletal muscle myopathy. Mutations in ubiquitin-selective chaperone p97/CDC-48 cause defective degradation of UNC-45. This results in increased levels of UNC-45 and disorganized myofibrils in both C. elegans and humans (Janiesch et al., 2007). The abnormalities arising from stabilization of UNC-45b mirror the sarcomeric disorganization arising from expression of excess UNC-45 in C. elegans (Landsverk et al., 2007) and zebrafish (Bernick et al., 2010). Human mutations in p97/CDC-48 are known to cause inclusion-body myopathy (Janiesch et al., 2007). Based upon the aforementioned connection between p97/CDC-48 and UNC-45, the aggregates that form in p97/CDC-48 mutants may arise from accumulation of abnormally folded myosin and/or defective protein clearance. Recently, it was shown that over-expression of smooth muscle myosin heavy chain mRNA in mouse atria results in decreased levels of unc-45a mRNA and activation of unc-45b mRNA transcription (Kwartler et al., 2014). It is possible that the sporadic thoracic aortic aneurysms and dissections that arise from the smooth muscle myosin gene duplication are caused by the abnormal levels of UNC-45 present in these tissues.
Over-expression of UNC-45a is associated with ovarian and breast cancers. Increased levels of UNC-45a were found in serous carcinomas compared with normal ovarian surface epithelium (Bazzaro et al., 2007). Ectopic expression of UNC-45a resulted in an enhanced rate of ovarian cancer cell proliferation and migration, whereas its knockdown decreased proliferation and migration without altering levels of non-muscle myosin II, a presumed target of UNC-45a (Bazzaro et al., 2007). It thus appears that elevated UNC-45a is linked with ovarian carcinoma cell proliferation and metastasis and that its co-localization with myosin II along the cleavage furrow and at the leading edge of migrating cells may indicate a role in enhancing myosin functions in cell division and cell motility.
Elevated unc-45a mRNA and protein levels were also detected in cultured cell lines obtained from breast carcinoma metastases (Guo et al., 2011). These investigators found that knock-down of the endogenous unc-45a gene significantly suppressed cell proliferation and reduced rates of cell invasion in vitro, possibly due to reduced actomyosin interactions (Guo et al., 2011). Interestingly, two forms of UNC-45a were discovered to be produced by alternative RNA splicing, with one containing an extended proline-rich N terminus. Both show similar affinity for non-muscle myosin IIA and for Hsp90, suggesting that each could serve to fold myosin. However, the stability of the non-extended isoform is reduced in the carcinoma cell lines, suggesting that UNC-45a isoform-specific properties may influence metastatic potential (Guo et al., 2011).
UNC-45a can inhibit retinoic acid signaling, and consequently may be involved in regulating growth, development and tumorigenesis. UNC-45a prevents retinoic acid-mediated cell division arrest; this results in resistance to histone deacetylase inhibitor-based treatment for cancer (Epping et al., 2009). It also has been shown that UNC-45a acts as a modulator for the chaperone activity of Hsp90 on progesterone receptors; it binds to an N-terminal domain on Hsp90 and blocks stimulation of Hsp90 ATPase activity, thereby inhibiting its chaperone activity toward the progesterone receptor (Chadli et al., 2006). Binding of UNC-45a affects Hsp90’s nucleotide binding capacity (Chadli et al., 2008a). Further, UNC-45a is the first co-chaperone identified that can distinguish between two Hsp90 isoforms, and this appears to dictate the cellular distribution of Hsp90β but not Hsp90α in HeLa cells (Chadli et al., 2008b). Enigmatically, UNC-45a can also directly bind to progesterone receptors and appears to stimulate their enhancement of transcription in vivo (Chadli et al., 2006).
Several studies have shown that UNC-45 is required for myosin accumulation in sarcomeres/myofibrils of the myocardium (Geach and Zimmerman, 2010; Melkani et al., 2011; Wohlgemuth et al., 2007). Since UNC-45 is essential for maintaining the structural integrity of the cardiac contractile apparatus, it may be critical for human cardiac function and survival as well. Indeed, proteomic analysis of hearts from patients suffering from ischemic heart failure detected increased levels of UNC-45, supporting the hypothesis that this chaperone is important in human cardiac disease (Stanley et al., 2005). It may prevent myosin unfolding or be required in repair processes, acting to fold and incorporate newly synthesized myosin into the myofibrillar apparatus of damaged cardiomyocytes. Recently Melkani et al. (2013) showed that over-expression of UNC-45 in conjunction with superoxide dismutase reduced amyloid aggregates and ameliorated cardiac dysfunction in a PolyQ-72-induced Huntington’s disease Drosophila model. Thus UNC-45 may have therapeutic potential in preventing aggregate formation as a result of its chaperone properties.
5. Concluding Remarks
Utilization of model organisms has pushed forward the frontiers regarding UNC-45 function at the biochemical, genetic, cell biological, biophysical and structural levels. However, much remains to be learned about UNC-45’s mechanism of action and its role in normal cell behavior and disease. Mutation of residues and sub-domains identified in UNC-45 crystal structures in conjunction with in vitro and in vivo analyses will permit a detailed structure-function relationship to be developed. It is clear that UNC-45, in conjunction with Hsp90, mediates myosin folding and protects it from stress. However, additional co-chaperones and targets for UNC-45 are being elucidated. It will be illuminating to understand how UNC-45’s partners change during development and under stress. Further, new co-crystal structures, particularly with UNC-45 substrates, will clarify UNC-45’s mechanism of action. Probing of human genomes may identify additional mutations within UNC-45 that cause disease. Finally, manipulating UNC-45 levels may prove useful therapeutically in UNC-45-induced disease or in other types of aggregation-based maladies.
Supplementary Material
Acknowledgments
We appreciate comments on the manuscript provided by Dr. Tom Huxford (San Diego State University Chemistry and Biochemistry) and Dr. Daniel Smith (San Diego State University Biology). Work in our laboratory regarding UNC-45 is supported by National Institutes of Health grant 5R01AR055958 to SIB.
Footnotes
Note Added in Proof
Since submission of this article, two important papers regarding UNC-45 have appeared. Bird et al. (2014) demonstrated that UNC-45b in combination with Hsp90 enhance the accumulation of properly folded myosin 15 in an insect cell/baculovirus expression system. Aggregation of the expressed myosin 15 is further obviated through binding of smooth muscle essential and regulatory light chains in lieu of calmodulin, which is myosin 15’s native light chain. The resulting myosin 15 exhibited actin-stimulated ATPase activity and facilitated actin filament sliding. This approach could open the door to studying other difficult-to-express myosins. In the second paper, Bujalowski et al. (2014) found that the central domain of UNC-45b binds skeletal muscle myosin independently of the UCS domain, but with lower affinity. The two domains bind to separate locations on the myosin molecule, but the UCS domain alone confers the chaperone activity of UNC-45b. This was demonstrated using a titin reporter and atomic force microscopy as discussed in section 4.1.
Dedication
We dedicate this article to the memory of Dr. Henry F. Epstein, who founded the field of UNC-45 biology. His interest and support of our efforts is appreciated and missed.
References
- Anderson MJ, Pham VN, Vogel AM, Weinstein BM, Roman BL. Loss of unc45a precipitates arteriovenous shunting in the aortic arches. Dev Biol. 2008;318:258–67. doi: 10.1016/j.ydbio.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao W, Pilgrim D. Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A. J Cell Biol. 2000;148:375–84. doi: 10.1083/jcb.148.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral JM, Bauer CC, Ortiz I, Epstein HF. Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J Cell Biol. 1998;143:1215–25. doi: 10.1083/jcb.143.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–71. doi: 10.1126/science.1066648. [DOI] [PubMed] [Google Scholar]
- Bazzaro M, Santillan A, Lin Z, Tang T, Lee MK, Bristow RE, Shih Ie M, Roden RB. Myosin II co-chaperone general cell UNC-45 overexpression is associated with ovarian cancer, rapid proliferation, and motility. Am J Pathol. 2007;171:1640–9. doi: 10.2353/ajpath.2007.070325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach DL, Bloom K. ASH1 mRNA localization in three acts. Mol Biol Cell. 2001;12:2567–77. doi: 10.1091/mbc.12.9.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernick EP, Zhang PJ, Du S. Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos. BMC Cell Biol. 2010;11:70. doi: 10.1186/1471-2121-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteaux-Lecellier V, Zickler D, Debuchy R, Panvier-Adoutte A, Thompson-Coffe C, Picard M. A homologue of the yeast SHE4 gene is essential for the transition between the syncytial and cellular stages during sexual reproduction of the fungus Podospora anserina. EMBO J. 1998;17:1248–58. doi: 10.1093/emboj/17.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JE, Takagi Y, Billington N, Strub MP, Sellers JR, Friedman TB. Chaperone-enhanced purification of unconventional myosin 15, a molecular motor specialized for stereocilia protein trafficking. Proc Natl Acad Sci USA. 2014;111:12390–12395. doi: 10.1073/pnas.1409459111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalowski PJ, Nicholls P, Oberhauser AF. UNC-45B chaperone: the role of its domains in the interaction with the myosin motor domain. Biophys J. 2014;107:654–661. doi: 10.1016/j.bpj.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadli A, Bruinsma ES, Stensgard B, Toft D. Analysis of Hsp90 cochaperone interactions reveals a novel mechanism for TPR protein recognition. Biochemistry. 2008a;47:2850–7. doi: 10.1021/bi7023332. [DOI] [PubMed] [Google Scholar]
- Chadli A, Felts SJ, Toft DO. GCUNC45 is the first Hsp90 co-chaperone to show alpha/beta isoform specificity. J Biol Chem. 2008b;283:9509–12. doi: 10.1074/jbc.C800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadli A, Graham JD, Abel MG, Jackson TA, Gordon DF, Wood WM, Felts SJ, Horwitz KB, Toft D. GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Mol Cell Biol. 2006;26:1722–30. doi: 10.1128/MCB.26.5.1722-1730.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Li S, Singh R, Spinette S, Sedlmeier R, Epstein HF. Dual function of the UNC-45b chaperone with myosin and GATA4 in cardiac development. J Cell Sci. 2012;125:3893–903. doi: 10.1242/jcs.106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comyn SA, Pilgrim D. Lack of developmental redundancy between Unc45 proteins in zebrafish muscle development. PLoS One. 2012;7:e48861. doi: 10.1371/journal.pone.0048861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003;28:655–62. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Du SJ, Li H, Bian Y, Zhong Y. Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc Natl Acad Sci U S A. 2008;105:554–9. doi: 10.1073/pnas.0707330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping MT, Meijer LA, Bos JL, Bernards R. UNC45A confers resistance to histone deacetylase inhibitors and retinoic acid. Mol Cancer Res. 2009;7:1861–70. doi: 10.1158/1541-7786.MCR-09-0187. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Thomson JN. Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature. 1974;250:579–80. doi: 10.1038/250579a0. [DOI] [PubMed] [Google Scholar]
- Estrada B, Choe SE, Gisselbrecht SS, Michaud S, Raj L, Busser BW, Halfon MS, Church GM, Michelson AM. An integrated strategy for analyzing the unique developmental programs of different myoblast subtypes. PLoS Genet. 2006;2:e16. doi: 10.1371/journal.pgen.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard C, Behra M, Fischer N, Hutcheson D, Geisler R, Strahle U. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev Biol. 2007;308:133–43. doi: 10.1016/j.ydbio.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Etard C, Roostalu U, Strahle U. Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J Cell Biol. 2008;180:1163–75. doi: 10.1083/jcb.200709128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard C, Roostalu U, Strahle U. Lack of Apobec2-related proteins causes a dystrophic muscle phenotype in zebrafish embryos. J Cell Biol. 2010;189:527–39. doi: 10.1083/jcb.200912125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge L, Diiorio P, Sagerstrom CG. A zebrafish unc-45-related gene expressed during muscle development. Dev Dyn. 2002;224:457–60. doi: 10.1002/dvdy.10123. [DOI] [PubMed] [Google Scholar]
- Fratev F, Jonsdottir SO, Pajeva I. Structural insight into the UNC-45-myosin complex. Proteins. 2013;81:1212–1221. doi: 10.1002/prot.24270. [DOI] [PubMed] [Google Scholar]
- Gaiser AM, Kaiser CJ, Haslbeck V, Richter K. Downregulation of the Hsp90 system causes defects in muscle cells of Caenorhabditis elegans. PLoS One. 2011;6:e25485. doi: 10.1371/journal.pone.0025485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazda L, Pokrzywa W, Hellerschmied D, Lowe T, Forne I, Mueller-Planitz F, Hoppe T, Clausen T. The myosin chaperone UNC-45 is organized in tandem modules to support myofilament formation in C. elegans. Cell. 2013;152:183–95. doi: 10.1016/j.cell.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geach TJ, Zimmerman LB. Paralysis and delayed Z-disc formation in the Xenopus tropicalis unc45b mutant dicky ticker. BMC Dev Biol. 2010;10:75. doi: 10.1186/1471-213X-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves MR, Barford D. Topological characteristics of helical repeat proteins. Curr Opin Struct Biol. 1999;9:383–9. doi: 10.1016/s0959-440x(99)80052-9. [DOI] [PubMed] [Google Scholar]
- Guo W, Chen D, Fan Z, Epstein HF. Differential turnover of myosin chaperone UNC-45A isoforms increases in metastatic human breast cancer. J Mol Biol. 2011;412:365–78. doi: 10.1016/j.jmb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Hansen L, Comyn S, Mang Y, Lind-Thomsen A, Myhre L, Jean F, Eiberg H, Tommerup N, Rosenberg T, Pilgrim D. The myosin chaperone UNC45B is involved in lens development and autosomal dominant juvenile cataract. Eur J Hum Genet. 2014 doi: 10.1038/ejhg.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins TA, Haramis AP, Etard C, Prodromou C, Vaughan CK, Ashworth R, Ray S, Behra M, Holder N, Talbot WS, Pearl LH, Strahle U, Wilson SW. The ATPase-dependent chaperoning activity of Hsp90a regulates thick filament formation and integration during skeletal muscle myofibrillogenesis. Development. 2008;135:1147–56. doi: 10.1242/dev.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe T, Cassata G, Barral JM, Springer W, Hutagalung AH, Epstein HF, Baumeister R. Regulation of the myosin-directed chaperone UNC-45 by a novel E3/E4-multiubiquitylation complex in C. elegans. Cell. 2004;118:337–49. doi: 10.1016/j.cell.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Landsverk ML, Price MG, Epstein HF. The UCS family of myosin chaperones. J Cell Sci. 2002;115:3983–90. doi: 10.1242/jcs.00107. [DOI] [PubMed] [Google Scholar]
- Janiesch PC, Kim J, Mouysset J, Barikbin R, Lochmuller H, Cassata G, Krause S, Hoppe T. The ubiquitin-selective chaperone CDC-48/p97 links myosin assembly to human myopathy. Nat Cell Biol. 2007;9:379–90. doi: 10.1038/ncb1554. [DOI] [PubMed] [Google Scholar]
- Ju JS, Weihl CC. Inclusion body myopathy, Paget’s disease of the bone and fronto-temporal dementia: a disorder of autophagy. Hum Mol Genet. 2010;19:R38–45. doi: 10.1093/hmg/ddq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur T, Ao W, Berger J, Pilgrim D. Maternal UNC-45 is involved in cytokinesis and colocalizes with non-muscle myosin in the early Caenorhabditis elegans embryo. J Cell Sci. 2004;117:5313–21. doi: 10.1242/jcs.01389. [DOI] [PubMed] [Google Scholar]
- Kachur TM, Audhya A, Pilgrim DB. UNC-45 is required for NMY-2 contractile function in early embryonic polarity establishment and germline cellularization in C. elegans. Dev Biol. 2008;314:287–99. doi: 10.1016/j.ydbio.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Kaiser CM, Bujalowski PJ, Ma L, Anderson J, Epstein HF, Oberhauser AF. Tracking UNC-45 chaperone-myosin interaction with a titin mechanical reporter. Biophys J. 2012;102:2212–9. doi: 10.1016/j.bpj.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–60. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Kwartler CS, Chen J, Thakur D, Li S, Baskin K, Wang S, Wang ZV, Walker L, Hill JA, Epstein HF, Taegtmeyer H, Milewicz DM. Overexpression of smooth muscle myosin heavy chain leads to activation of the unfolded protein response and autophagic turnover of thick filament associated proteins in vascular smooth muscle cells. J Biol Chem. 2014;289:14075–88. doi: 10.1074/jbc.M113.499277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsverk ML, Li S, Hutagalung AH, Najafov A, Hoppe T, Barral JM, Epstein HF. The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J Cell Biol. 2007;177:205–10. doi: 10.1083/jcb.200607084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CF, Hauenstein AV, Fleming JK, Gasper WC, Engelke V, Sankaran B, Bernstein SI, Huxford T. X-ray crystal structure of the UCS domain-containing UNC-45 myosin chaperone from Drosophila melanogaster. Structure. 2011a;19:397–408. doi: 10.1016/j.str.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CF, Melkani GC, Yu Q, Suggs JA, Kronert WA, Suzuki Y, Hipolito L, Price MG, Epstein HF, Bernstein SI. Drosophila UNC-45 accumulates in embryonic blastoderm and in muscles, and is essential for muscle myosin stability. J Cell Sci. 2011b;124:699–705. doi: 10.1242/jcs.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhong Y, Wang Z, Gao J, Xu J, Chu W, Zhang J, Fang S, Du SJ. Smyd1b is required for skeletal and cardiac muscle function in zebrafish. Mol Biol Cell. 2013;24:3511–21. doi: 10.1091/mbc.E13-06-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Srikakulam R, Winkelmann DA. Unc45 activates Hsp90-dependent folding of the myosin motor domain. J Biol Chem. 2008;283:13185–93. doi: 10.1074/jbc.M800757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M, Pollard TD. UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J Cell Biol. 2004;167:315–25. doi: 10.1083/jcb.200404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M, Sladewski TE, Pollard TD. Yeast UCS proteins promote actomyosin interactions and limit myosin turnover in cells. Proc Natl Acad Sci U S A. 2008;105:8014–9. doi: 10.1073/pnas.0802874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, Bodmer R, Ocorr K, Bernstein SI. The UNC-45 chaperone is critical for establishing myosin-based myofibrillar organization and cardiac contractility in the Drosophila heart model. PLoS One. 2011;6:e22579. doi: 10.1371/journal.pone.0022579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, Lee CF, Cammarato A, Bernstein SI. Drosophila UNC-45 prevents heat-induced aggregation of skeletal muscle myosin and facilitates refolding of citrate synthase. Biochem Biophys Res Commun. 2010;396:317–22. doi: 10.1016/j.bbrc.2010.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, Trujillo AS, Ramos R, Bodmer R, Bernstein SI, Ocorr K. Huntington’s disease induced cardiac amyloidosis is reversed by modulating protein folding and oxidative stress pathways in the Drosophila heart. PLoS Genet. 2013;9:e1004024. doi: 10.1371/journal.pgen.1004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, D’Souza VM, Chang KC, Huang Y, Balasubramanian MK. Hsp90 protein in fission yeast Swo1p and UCS protein Rng3p facilitate myosin II assembly and function. Eukaryot Cell. 2005;4:567–76. doi: 10.1128/EC.4.3.567-576.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre JL, Hills JA, Jean F, Pilgrim DB. Unc45b is essential for early myofibrillogenesis and costamere formation in zebrafish. Dev Biol. 2014;390:26–40. doi: 10.1016/j.ydbio.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Ni W, Hutagalung AH, Li S, Epstein HF. The myosin-binding UCS domain but not the Hsp90-binding TPR domain of the UNC-45 chaperone is essential for function in Caenorhabditis elegans. J Cell Sci. 2011;124:3164–73. doi: 10.1242/jcs.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P, Bujalowski P, Boehning D, Barral J, Oberhauser AF. Molecular chaperone mediated inhibition of the myosin power stroke may be critical for sarcomere assembly. Mol Biol Cell. 2013;24:57. (abstract 376) [Google Scholar]
- Price MG, Landsverk ML, Barral JM, Epstein HF. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J Cell Sci. 2002;115:4013–23. doi: 10.1242/jcs.00108. [DOI] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Shi H, Blobel G. UNC-45/CRO1/She4p (UCS) protein forms elongated dimer and joins two myosin heads near their actin binding region. Proc Natl Acad Sci U S A. 2010;107:21382–7. doi: 10.1073/pnas.1013038107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikakulam R, Liu L, Winkelmann DA. Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain. PLoS One. 2008;3:e2137. doi: 10.1371/journal.pone.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Myosin II folding is mediated by a molecular chaperonin. J Biol Chem. 1999;274:27265–73. doi: 10.1074/jbc.274.38.27265. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Chaperone-mediated folding and assembly of myosin in striated muscle. J Cell Sci. 2004;117:641–52. doi: 10.1242/jcs.00899. [DOI] [PubMed] [Google Scholar]
- Stanley BA, Liu P, Kirshenbaum LA, Van Eyk JE. Proteomic analysis of ischemic heart failure patients reveals increases in a myosin assembly protein (UNC-45) Circ Res. 2005;97 abstract. [Google Scholar]
- Tewari R, Bailes E, Bunting KA, Coates JC. Armadillo-repeat protein functions: questions for little creatures. Trends Cell Biol. 2010;20:470–81. doi: 10.1016/j.tcb.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Toi H, Fujimura-Kamada K, Irie K, Takai Y, Todo S, Tanaka K. She4p/Dim1p interacts with the motor domain of unconventional myosins in the budding yeast, Saccharomyces cerevisiae. Mol Biol Cell. 2003;14:2237–49. doi: 10.1091/mbc.E02-09-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia L, Ao W, Kim S, Kim C, Pilgrim D. unc-45 gene of Caenorhabditis elegans encodes a muscle-specific tetratricopeptide repeat-containing protein. Cell Motil Cytoskeleton. 1999;42:163–77. doi: 10.1002/(SICI)1097-0169(1999)42:3<163::AID-CM1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Venolia L, Waterston RH. The unc-45 gene of Caenorhabditis elegans is an essential muscle-affecting gene with maternal expression. Genetics. 1990;126:345–53. doi: 10.1093/genetics/126.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche S, Arnold M, Jansen RP. The UCS domain protein She4p binds to myosin motor domains and is essential for class I and class V myosin function. Curr Biol. 2003;13:715–24. doi: 10.1016/s0960-9822(03)00264-1. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth SL, Crawford BD, Pilgrim DB. The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev Biol. 2007;303:483–92. doi: 10.1016/j.ydbio.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Wong KC, Naqvi NI, Iino Y, Yamamoto M, Balasubramanian MK. Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J Cell Sci. 2000;113:2421–32. doi: 10.1242/jcs.113.13.2421. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–31. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.