Abstract
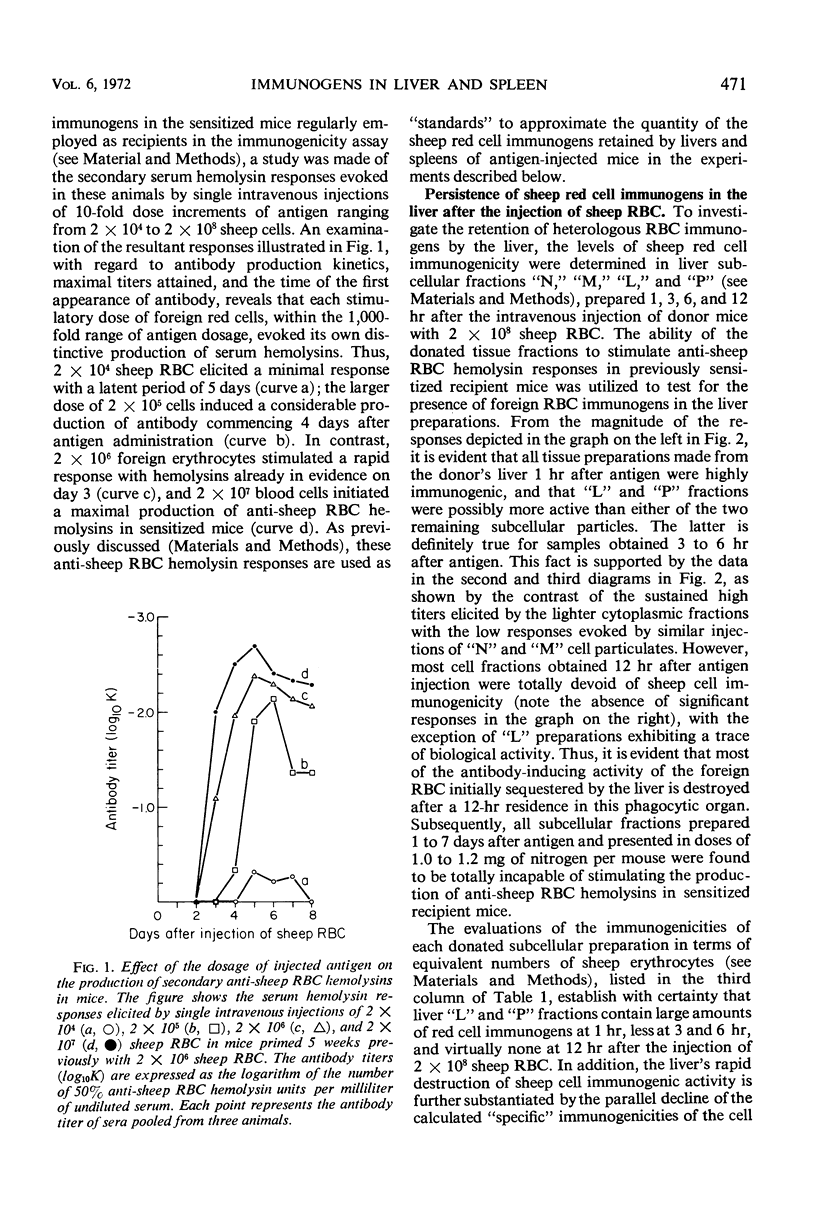
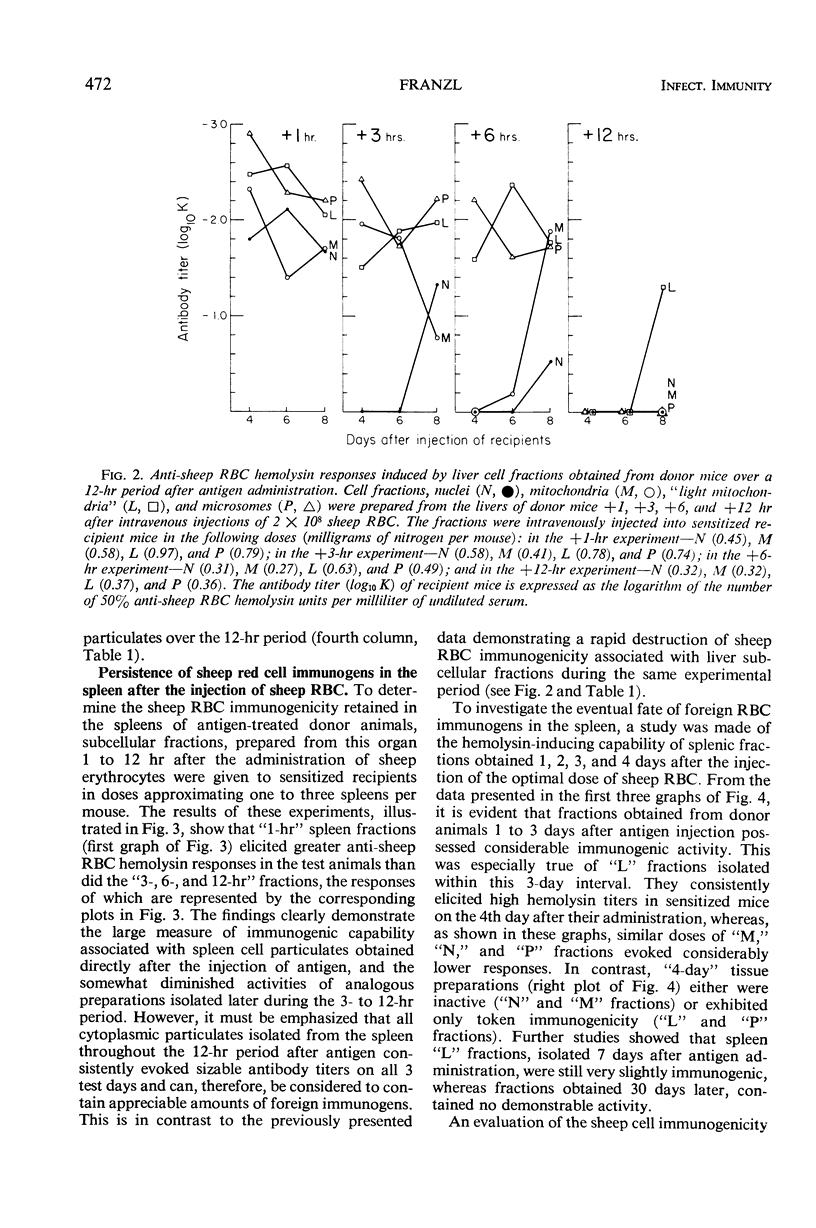
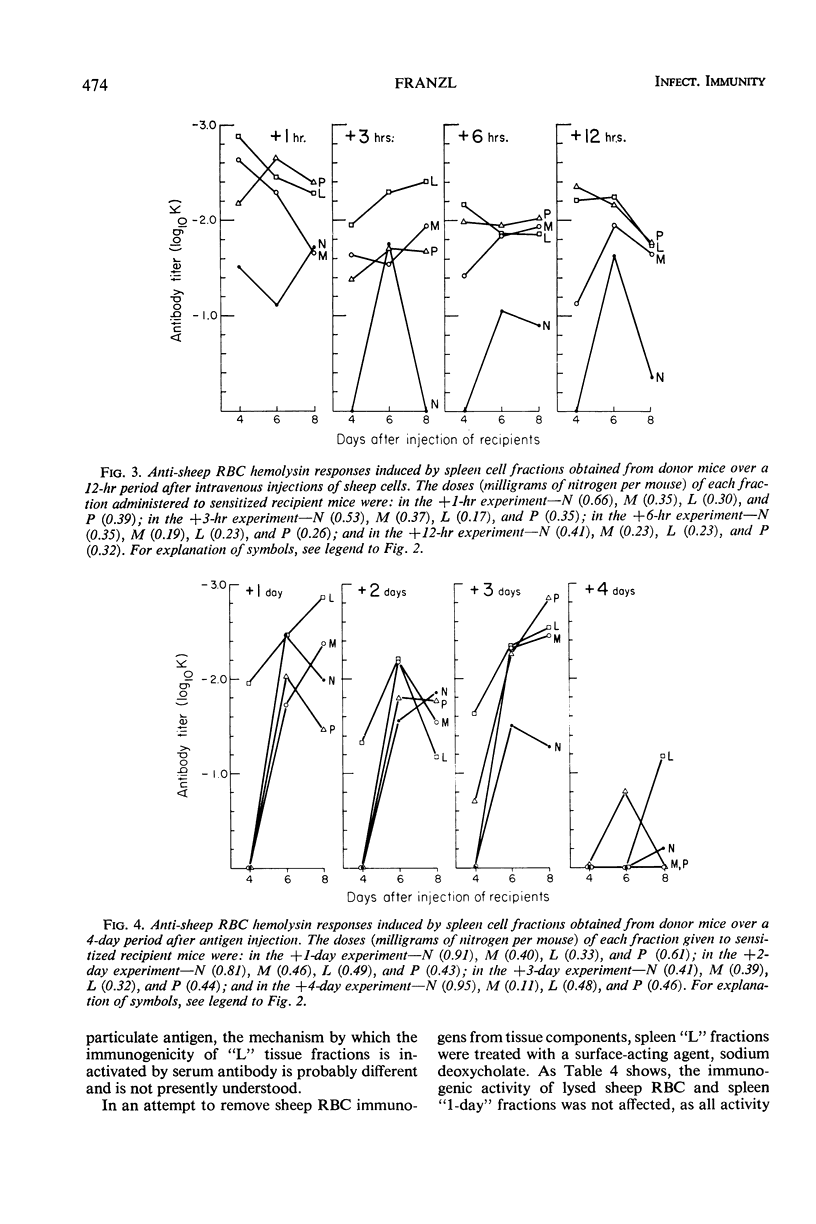
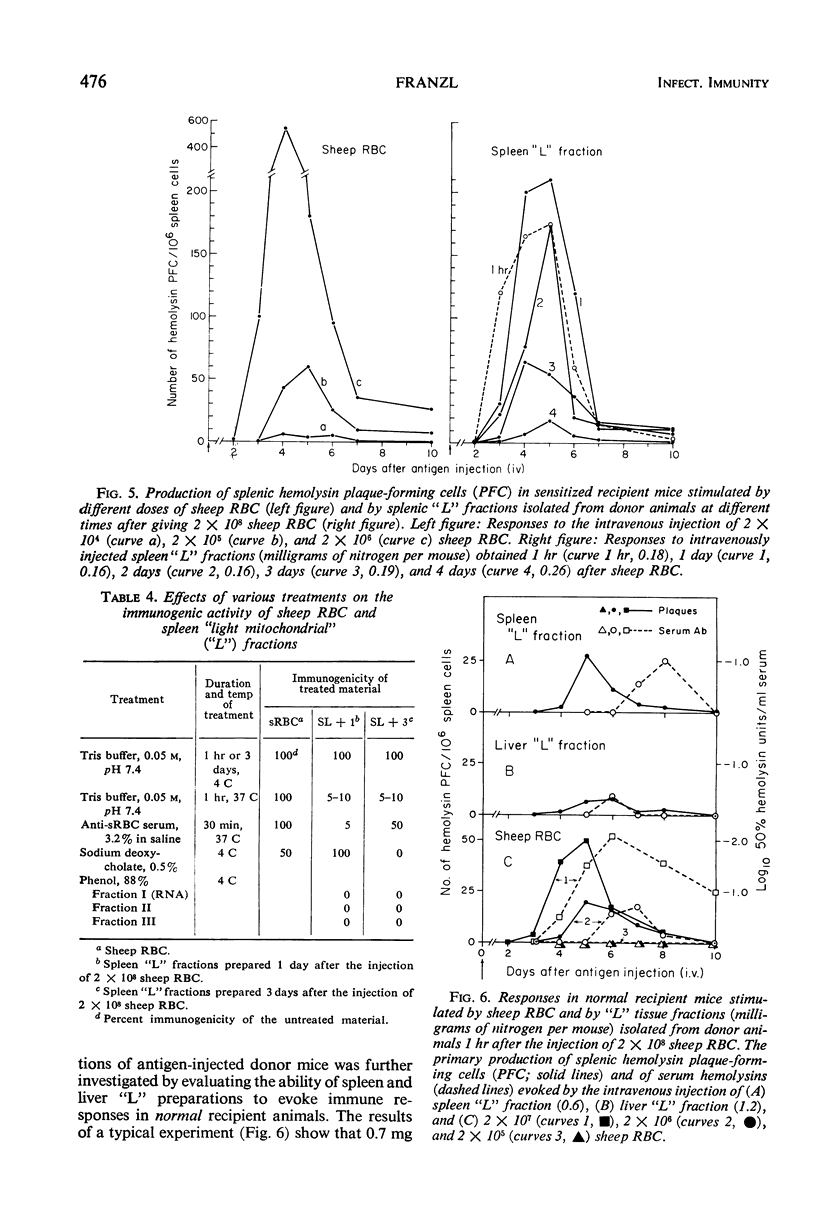
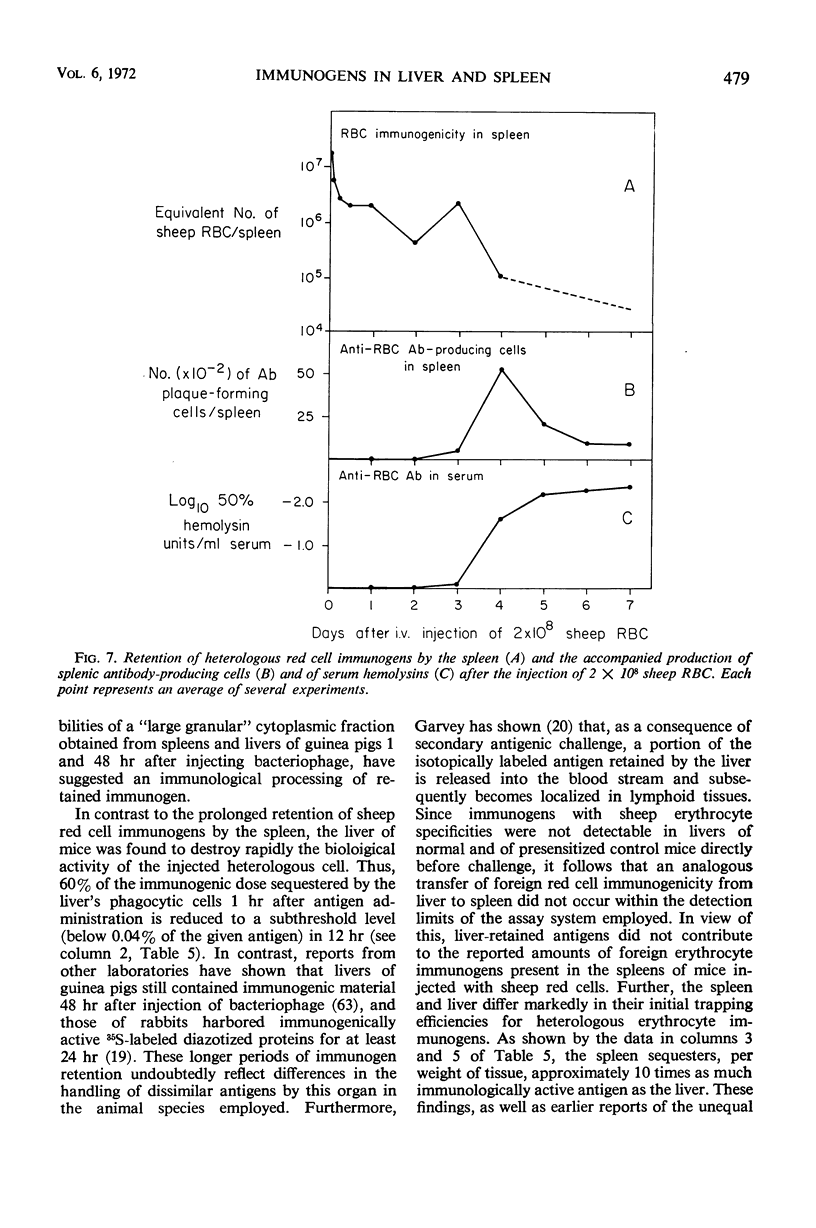
The antibody-inducing activities of foreign red blood cell immunogens sequestered in the spleens and livers of mice injected with sheep erythrocytes were evaluated during the early periods of the immune response. Estimates of immunogenicity, obtained from the magnitudes of anti-sheep red blood cell hemolysin responses evoked in sensitized recipient mice by subcellular tissue fractions prepared from these phagocytic organs, showed that the liver and spleen differ greatly in their handling of this particulate antigen. The liver, functioning primarily as a scavenger organ, destroys completely the immunogenicity of the heterologous erythrocytes in 12 hr. In contrast, the spleen handles foreign erythrocyte immunogens in at least two different ways: approximately 90% of the initially sequestered activity is rapidly destroyed by the spleen in 6 hr, but the remaining activity, associated chiefly with a tissue fraction possessing the sedimentation properties of “light mitochondria,” is retained at a significant level for 3 days, and then progressively decreases to a low level until the 7th day. A correlation of the observed changes in the properties of the immunogenic tissue fraction with known cellular events in the spleen stimulated by antigens indicates that the retention of the degradable erythrocyte immunogen is essential for stimulating and maintaining immune reactions in this antibody-producing organ.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L. Antigen binding cells in tolerance and immunity. Transplant Rev. 1970;5:105–129. doi: 10.1111/j.1600-065x.1970.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Ada G. L., Lang P. G., Plymin G. Antigen in tissues. V. Effect of endotoxin on the fate of, and on the immun response to, serum albumin and to albumin-antibody complexes. Immunology. 1968 Jun;14(6):825–836. [PMC free article] [PubMed] [Google Scholar]
- BRANSTER M. V., MORTON R. K. Comparative rates of synthesis of diphosphopyridine nucleotide by normal and tumour tissue from mouse mammary gland; studies with isolated nuclei. Biochem J. 1956 Aug;63(4):640–646. doi: 10.1042/bj0630640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers W. E., de Duve C. Lysosomes in lymphoid tissue. III. Influence of various treatments of the animals on the distribution of acid hydrolases. J Cell Biol. 1967 Feb;32(2):349–364. doi: 10.1083/jcb.32.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton S., Wepsic T., Möller G. Persistence of immunogenicity of two complex antigens retained in vivo. Immunology. 1968 Apr;14(4):491–501. [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Graham R. C., Jr, Schwartz H. J. Ultrastructural identification of possible sites of antigen processing in macrophages. J Immunol. 1969 Sep;103(3):618–621. [PubMed] [Google Scholar]
- Cohen M. W., Jacobson E. B., Thorbecke G. J. Gamma-globulin and antibody formation in vitro. V. The secondary response made by splenic white and red pulp with reference to the role of secondary nodules. J Immunol. 1966 Jun;96(6):944–952. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., BUKANTZ S. C., DAMMIN G. J., TALMAGE D. W. Fate of I131 labelled bovine gamma globulin in rabbits. Fed Proc. 1951 Jun;10(2):553–557. [PubMed] [Google Scholar]
- ESSNER E. An electron microscopic study of erythrophagocytosis. J Biophys Biochem Cytol. 1960 Apr;7:329–334. doi: 10.1083/jcb.7.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELTON L. D., PRESCOTT B., KAUFFMANN G., OTTINGER B. Pneumococcal antigenic polysaccharide substances from animal tissues. J Immunol. 1955 Mar;74(3):205–213. [PubMed] [Google Scholar]
- FITCH F. W., BARKER P., SOULES K. H., WISSLER R. W. A study of antigen localization and degradation and the histologic reaction in the spleen of normal, x-irradiated, and spleen-shielded rats. J Lab Clin Med. 1953 Oct;42(4):598–620. [PubMed] [Google Scholar]
- FRANZL R. E. Immunogenic sub-cellular particles obtained from spleens of antigen-injected mice. Nature. 1962 Aug 4;195:457–458. doi: 10.1038/195457a0. [DOI] [PubMed] [Google Scholar]
- Franzl R. E., McMaster P. D. The primary immune response in mice. I. The enhancement and suppression of hemolysin production by a bacterial endotoxin. J Exp Med. 1968 Jun 1;127(6):1087–1107. doi: 10.1084/jem.127.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [DOI] [PubMed] [Google Scholar]
- Garvey J. S., Campbell D. H. Autoradiographic investigation of tissues after primary and multiple antigenic stimulation of rabbits. Nature. 1966 Mar 19;209(5029):1201–1202. doi: 10.1038/2091201a0. [DOI] [PubMed] [Google Scholar]
- HALPERN B. N., BIOZZI G., BENACERRAF B., STIFFEL C. Phagocytosis of foreign red blood cells by the reticulo-endothelial system. Am J Physiol. 1957 Jun;189(3):520–526. doi: 10.1152/ajplegacy.1957.189.3.520. [DOI] [PubMed] [Google Scholar]
- HANNA M. G., Jr GERMINAL CENTER CHANGES AND PLASMA CELL REACTION DURING THE PRIMARY IMMUNE RESPONSE. Int Arch Allergy Appl Immunol. 1965;26:230–251. doi: 10.1159/000229574. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Nettesheim P., Francis M. W. Requirement for continuous antigenic stimulation in the development and differentiation of antibody-forming cells. The effect of passive antibody on the primary and secondary response. J Exp Med. 1969 May 1;129(5):953–971. doi: 10.1084/jem.129.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jílek M., Sterzl J. Influence of the amount of antigen and interval on the secondary reaction. Folia Microbiol (Praha) 1967;12(1):6–20. doi: 10.1007/BF02895084. [DOI] [PubMed] [Google Scholar]
- Kölsch E., Mitchison N. A. The subcellular distribution of antigen in macrophages. J Exp Med. 1968 Nov 1;128(5):1059–1079. doi: 10.1084/jem.128.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNICK J. A., EGDAHL R. H. Ribonucleic acid in "transformation" of lymphoid cells. Science. 1962 Sep 21;137(3534):976–977. doi: 10.1126/science.137.3534.976. [DOI] [PubMed] [Google Scholar]
- MCMASTER P. D., EDWARDS J. L. Impairment of the antigenicity of a protein antigen following its injection into rabbits. J Exp Med. 1957 Aug 1;106(2):219–232. doi: 10.1084/jem.106.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLORS R. C., BRZOSKO W. J. Studies in molecular pathology. I. Localization and pathogenic role of heterologous immune complexes. J Exp Med. 1962 May 1;115:891–902. doi: 10.1084/jem.115.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. Some properties of alkaline phosphatase of cow's milk and calf intestinal mucosa. Biochem J. 1955 Aug;60(4):573–582. doi: 10.1042/bj0600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Cerottini J. C., Dixon F. J. An approach to the quantitation of immunogenic antigen. J Exp Med. 1968 May 1;127(5):1003–1011. doi: 10.1084/jem.127.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster P. D., Franzl R. E. The primary immune response in mice. II. Cellular responses of lymphoid tissue accompanying the enhancement or complete suppression of antibody formation by a bacterial endotoxin. J Exp Med. 1968 Jun 1;127(6):1109–1125. doi: 10.1084/jem.127.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. Recovery from immunological paralysis in relation to age and residual antigen. Immunology. 1965 Aug;9(2):129–138. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J. Antigens in immunity. XIV. Electron microscopic radioautographic studies of antigen capture in the lymph node medulla. J Exp Med. 1968 Feb 1;127(2):263–276. doi: 10.1084/jem.127.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Austin C. M., Ada G. L. Antigens in immunity. VII. Analysis of immunological memory. Immunology. 1965 Oct;9(4):333–348. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Austin C. M., Pye J., Mitchell J. Antigens in immunity. XII. Antigen trapping in the spleen. Int Arch Allergy Appl Immunol. 1966;29(4):368–383. [PubMed] [Google Scholar]
- Pearlman D. S. The influence of antibodies on immunologic responses. I. The effect on the response to particulate antigen in the rabbit. J Exp Med. 1967 Jul 1;126(1):127–148. doi: 10.1084/jem.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER M., ZIMMERMAN S., HAUROWITZ F. RELATION OF ANTIBODY TITER TO PERSISTENCE OF ANTIGEN. J Immunol. 1965 Jun;94:938–941. [PubMed] [Google Scholar]
- STAVITSKY A. B. Participation of the popliteal lymph node and spleen in the production of diphtheria antitoxin in the rabbit. J Infect Dis. 1954 May-Jun;94(3):306–318. doi: 10.1093/infdis/94.3.306. [DOI] [PubMed] [Google Scholar]
- STERZL J. Presence of antigen; a factor determining the duration of antibody formation by transferred cells. Nature. 1959 Feb 21;183(4660):547–548. doi: 10.1038/183547a0. [DOI] [PubMed] [Google Scholar]
- SWEET L. C., ABRAMS G. D., JOHNSON A. G. THE FATE OF RADIOACTIVE BOVINE GAMMA-GLOBULIN DURING THE PRIMARY ANTIBODY RESPONSE IN THE MOUSE. J Immunol. 1965 Jan;94:105–110. [PubMed] [Google Scholar]
- Schwab J. H., Brown R. R. Modification of antigenic structure in vivo: quantitative studies on the processing of streptococcal cell wall antigens in mice. J Immunol. 1968 Nov;101(5):930–938. [PubMed] [Google Scholar]
- Sercarz E. E., Byers V. S. The X-Y-Z scheme of immunocyte maturation. 3. Early IgM memory and the nature of the memory cell. J Immunol. 1967 Apr;98(4):836–843. [PubMed] [Google Scholar]
- Stejska R., Fitch F. W. Tissue agglutination reaction with normal rat spleen. J Reticuloendothel Soc. 1970 Jan;7(1):121–125. [PubMed] [Google Scholar]
- Sulitzeanu D. Affinity of antigen for white cells and its relation to the induction of antibody formation. Bacteriol Rev. 1968 Dec;32(4 Pt 2):404–424. [PMC free article] [PubMed] [Google Scholar]
- Szakal A. K., Hanna M. G., Jr The ultrastructure of antigen localization and viruslike particles in mouse spleen germinal centers. Exp Mol Pathol. 1968 Feb;8(1):75–89. doi: 10.1016/0014-4800(68)90007-5. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G. The dynamics of hemolysin formation in intact and splenectomized rabbits. J Infect Dis. 1950 Jul-Aug;87(1):37–62. doi: 10.1093/infdis/87.1.37. [DOI] [PubMed] [Google Scholar]
- UHR J. W., WEISSMAN G. INTRACELLULAR DISTRIBUTION AND DEGRADATION OF BACTERIOPHAGE IN MAMMALIAN TISSUES. J Immunol. 1965 Apr;94:544–550. [PubMed] [Google Scholar]
- Uchitel' I. Ia, Khasman E. L., Zaitseva L. G., Shumakova G. V. Sokhrannost' antigena v lizosomnykh i RNK-fraktsiiakh selezenki i pecheni krolikov vo vremiia antiteloobrazovaniia. Zh Mikrobiol Epidemiol Immunobiol. 1969 Aug;46(8):12–17. [PubMed] [Google Scholar]
- Unanue E. R., Cerottini J. C. The immunogenicity of antigen bound to the plasma membrane of macrophages. J Exp Med. 1970 Apr 1;131(4):711–725. doi: 10.1084/jem.131.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle W. O. Persistence of immunological memory to soluble protein antigens. Immunology. 1966 Apr;10(4):377–382. [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Dukor P. The role of lysosomes in immune responses. Adv Immunol. 1970;12:283–331. doi: 10.1016/s0065-2776(08)60172-8. [DOI] [PubMed] [Google Scholar]


