Abstract
Polychlorinated biphenyls (PCBs) are persistent organic pollutants associated with non-alcoholic fatty liver disease (NAFLD) in epidemiologic studies. The purpose of this study was to evaluate the hepatic effects of a PCB mixture, Aroclor 1260, whose composition mimics human bioaccumulation patterns, in a mouse model of diet-induced obesity (DIO). Male C57Bl/6J mice were fed control diet or 42% high fat diet (HFD) and exposed to Aroclor 1260 (20 mg/kg or 200 mg/kg in corn oil) for 12 weeks. A glucose tolerance test was performed; plasma/tissues were obtained at necropsy for measurements of adipocytokine levels, histology, and gene expression. Aroclor 1260 exposure was associated with decreased body fat in HFD-fed mice but had no effect on blood glucose/lipid levels. Paradoxically, Aroclor 1260 + HFD co-exposed mice demonstrated increased hepatic inflammatory foci at both doses while the degree of steatosis did not change. Serum cytokines, ALT levels and hepatic expression of IL-6 and TNFα were increased only at 20 mg/kg, suggesting an inhibition of pro-inflammatory cytokine production at the 200 mg/kg exposure. Aroclor 1260 induced hepatic expression of cytochrome P450s including Cyp3a11 (Pregnane-Xenobiotic Receptor target) and Cyp2b10 (constitutive androstane receptor target) but Cyp2b10 inducibility was diminished with HFD-feeding. Cyp1a2 (aryl hydrocarbon Receptor target) was induced only at 200 mg/kg. In summary, Aroclor 1260 worsened hepatic and systemic inflammation in DIO. The results indicated a bimodal response of PCB-diet interactions in the context of inflammation which could potentially be explained by xenobiotic receptor activation. Thus, PCB exposure may be a relevant “second hit” in the transformation of steatosis to steatohepatitis.
Keywords: Aroclor 1260, PCBs, NAFLD, CAR, PXR, AhR
Introduction
Non-alcoholic fatty liver disease (NAFLD) affects up to 46% of the US population and is prevalent in approximately 20% of the population worldwide (Vernon et al., 2011; Williams et al., 2011). NAFLD is a progressive disease, initially manifested by accumulation of lipid droplets in the liver (hepatic steatosis). Following steatosis, in-flammation may occur leading to non-alcoholic steatohepatitis (NASH). NASH may progress to fibrosis, cirrhosis and eventually liver failure or hepatocarcinogenesis. Historically NAFLD was linked to high caloric intake, physical inactivity, genetics, and certain medications’ side effects. Emerging studies have demonstrated that other factors may play a role in the genesis and development of NAFLD or may act as a ‘second hit’ in the progression of hepatic steatosis to steatohepatitis (Dowman et al., 2010). These factors may include exposure to environmental contaminants including polychlorinated biphenyls (PCBs), bromodichloromethane (BDCM) and organochlorine pesticides, such as, dieldrin and DDT (Ruzzin et al., 2010; Seth et al., 2013). Previous work from our laboratory identified NAFLD in highly exposed chemical workers and coined the term toxicant-associated steatohepatitis (TASH) (Cave et al., 2010b). Additionally, we also identified suspected NAFLD/TASH in the National Health and Nutrition Examination Survey (NHANES) participants with chronic low-level exposures to PCBs (Cave et al., 2010a).
PCBs are environmental pollutants that were manufactured and used commercially as dielectric fluids in transformers. Structurally, PCBs are polyhalogenated aromatic hydrocarbons consisting of up to 10 chlorine atoms attached to biphenyl. Depending on the number of chlorine atom substituents, there are 209 possible individual PCB congeners. PCBs were manufactured as mixtures, rather than individual congeners, with a total of 1.3 million tons being produced worldwide (Breivik et al., 2002). In the US, PCB mixtures were sold under the brand name ‘Aroclor’ by the Monsanto Corporation, which manufactured PCBs in Anniston, Alabama. Aroclor 1260 is a PCB mixture that contains 60% chlorine by weight, and was one of the Aroclors marketed in the early stages of PCB production. It was later replaced by other Aroclors (1254, 1248 and 1242). Although their production was banned in the 1970s, PCBs continue to persist in the environment due to their high thermodynamic stability and lack of metabolism by biota, and hence are also known as persistent organic pollutants (POPs). Currently, PCB exposure is thought to occur primarily through ingestion of PCB contaminated food (Schecter et al., 2010).
The primary toxicity endpoint of PCB exposure has been an increase in cancer and is thought to be mediated through their interaction with transcription factors namely the aryl hydrocarbon receptor (AhR), the constitutive androstane receptor (CAR) and the pregnane-xenobiotic receptor (PXR). Genotoxic carcinogenic mechanisms are activated via induction of the AhR and the carcinogenicity of a PCB mixture is often defined as its ability to induce the AhR-dependent gene transcription relative to the potent rodent carcinogen and prototypical AhR ligand, tetrachlorodibenzo-p-dioxin (TCDD). In addition “non genotoxic” carcinogenic mechanisms may also be induced including hepatomegaly by the nuclear receptors, CAR and PXR. Although these receptors are primarily involved in xenobiotic detoxification, recent studies have implicated their role in metabolic diseased states such as steatosis, obesity and insulin resistance (Konno et al., 2008; Merrell and Cherrington, 2011; Moya et al., 2010). The role of hepatic receptor–PCB interactions in NASH and the metabolic syndrome is understudied and requires further investigation.
Epidemiological studies have found associations between PCB exposures and metabolic disorders related to NAFLD, including obesity, diabetes/insulin resistance and the metabolic syndrome. Furthermore, 100% of adult NHANES participants had detectable circulating PCB levels with PCB 153 having the highest median serum concentration of any single congener (Cave et al., 2010a). In our recent work, we demonstrated that PCB 153 administration was a relevant ‘second hit’ mechanism that worsened NAFLD/obesity occurring in the context of a high fat diet (HFD) in male C57Bl/6 mice (Wahlang et al., 2013). However, no individual is exposed to a single PCB congener alone and therefore studying a PCB mixture may better simulate human PCB exposure patterns. Moreover, the composition of PCB congeners in Aroclor 1260 mimics human bioaccumulation patterns (McFarland and Clarke, 1989).
Although previous studies on PCBs have focused on carcinogenicity and acute toxicity, effects of chronic low-level exposures to PCBs on other pathologies such as NAFLD, diabetes and obesity are understudied, especially for PCB mixtures. It is therefore pertinent to evaluate the effects of a commercially produced PCB mixture whose composition is relevant to human PCB body burden, in a normal and hyper-caloric state, and investigate nutrient–toxicant interactions that can contribute to liver disease and obesity. We hypothesize that Aroclor 1260, like PCB 153, can act as an obesogen and promote/worsen steatosis and diet-induced obesity (DIO). In this study, we utilized a mouse model to determine if i) Aroclor 1260 exposures alone are sufficient to cause NAFLD and obesity; ii) Aroclor 1260 worsens DIO and NAFLD by exacerbating insulin resistance, adipocytokine dysregulation, alterations in hepatic gene expression, AhR, PXR and CAR activation and other implicated mechanisms; and iii) effects observed with Aroclor 1260 administration are dependent on PCB dose. The results of the current study establish Aroclor 1260 as a “second hit” driving steatosis to steatohepatitis in HFD-fed mice. The results also demonstrate that selective hepatic receptor activation by this PCB mixture was dependent on exposure dose and diet, in part, clarifying the mechanism of PCBs in NAFLD.
Materials and methods
Animals and diets
The animal protocol was approved by the University of Louisville Institutional Animal Care and Use Committee. Male C57Bl/6J mice (8 weeks old; The Jackson Laboratory, Bar Harbor, ME, USA) were divided into 6 study groups (n = 10) based on diet and Aroclor 1260 exposure in this 12 week study utilizing a 2 × 3 design. Mice were fed either a control diet (CD, 10.2% kCal from fat; TD.06416 Harlan Teklad) or a HFD (42% kCal from fat; TD.88137 Harlan Teklad). Diet components are described in Supplemental Table 1. Aroclor 1260 (AccuStandard, CT, USA) was administered in corn oil by oral gavage (vs. corn oil alone) at two doses; a low dose of 20 mg/kg which was designed to mimic the maximum human PCB exposures seen in the Anniston cohort and a high dose of 200 mg/ kg which was similar to that used in rodent carcinogenesis studies (Cave et al., 2010a; National Toxicology Program, 2006b). The 20 mg/kg dose was administered on Week 1 whereas the 200 mg/kg dose was administered as four individual doses of 50 mg/kg each on Weeks 1, 3, 5, and 7 to ensure that acute toxicity was minimized. Mice were housed in a temperature- and light controlled-room (12 h light; 12 h dark) with food and water ad libitum. A glucose tolerance test was performed at Week 11, and the animals were euthanized (ketamine/xylazine, 100/ 20 mg/kg body weight, i.p.) at the end of Week 12. Prior to euthanasia, the animals were analyzed for body fat composition by dual energy X-ray absorptiometry (DEXA) scanning (Lunar PIXImus densitometer, WI, USA). Thus six different treatment groups were evaluated in this fashion: CD + vehicle, CD + Aroclor 1260 (20 mg/kg), CD +Aroclor 1260 (200 mg/kg), HFD + vehicle, HFD + Aroclor 1260 (20 mg/kg), HFD + Aroclor 1260 (200 mg/kg).
Glucose tolerance test
On the day of the test, mice were fasted for 6 h (7 a.m.–1 p.m.), and fasting blood glucose levels were measured with a hand-held glucometer (ACCU-CHECK Aviva, Roche, Basel, Switzerland) using 1–2 μL blood via tail snip. Glucose was then administered (1 mg glucose/g body weight, sterile saline, i.p.), and blood glucose was measured at 5, 15, 30, 60, 90 and 120 min post injection. Insulin resistance was calculated by homeostasis model assessment using the formula: homeostasis model assessment of insulin resistance (HOMA-IR) = fasting glucose (mg/dL) × fasting insulin (μU/mL)/405.
Histological studies
Liver and adipose sections were fixed in 10% neutral buffered formalin and embedded in paraffin for routine histological examination. Tissue sections were stained with hematoxylin–eosin (H&E) or for chloroacetate esterase activity [CAE, Naphthol AS-D Chloroacetate (Specific Esterase) Kit, Sigma Aldrich, St. Louis, MI, USA] and examined by light microscopy. Photomicrographic images were captured using a high-resolution digital scanner at 10× and 40× magnification. After H&E staining, adipocyte size was measured using Image J software version 1.47 (NIH, Bethesda, MD, USA).
Cytokine and adipokine measurement
Plasma cytokine and adipokine levels were measured using the Milliplex Serum Cytokine and Adipokine Kits (Millipore Corp, Billerica, MA, USA) on the Luminex IS 100 system (Luminex Corp, Austin, TX, USA), as per the manufacturer's instructions. Plasma aspartate transaminase (AST) activity, alanine transaminase (ALT) activity, low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides and cholesterol levels were measured with the Piccolo Xpress Chemistry Analyzer using Lipid Panel Plus reagent disks (Abaxis, Union City, CA, USA).
Measurement of hepatic triglyceride and cholesterol content
Mouse livers were washed in neutral 1× phosphate buffered saline and pulverized. Hepatic lipids were extracted by an aqueous solution of chloroform and methanol, according to the Bligh and Dyer method (Bligh and Dyer, 1959), dried using nitrogen, and resuspended in 5% lipid-free bovine serum albumin. Triglycerides and cholesterol were quantified using the Cobas Mira Plus automated chemical analyzer. The reagents employed for the assay were L-Type Triglyceride M (Wako Diagnostics, Richmond, VA, USA) and Infinity Cholesterol Liquid Stable Reagent (Fisher Diagnostics, Middletown, VA, USA) for triglycerides and cholesterol respectively.
Real-time PCR
Animal liver and adipose tissue samples were homogenized and total RNA was extracted using the RNA-STAT 60 protocol (Tel-Test, Austin, TX, USA). RNA purity and quantity were assessed with the Nanodrop (ND-1000, Thermo Scientific, Wilmington, DE, USA) using the ND-1000 V3.8.1 software. cDNA was synthesized from total RNA using the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA). Polymerase Chain Reaction (PCR) was performed on the Applied Biosystems StepOnePlus Real-Time PCR Systems using the Taqman Universal PCR Master Mix (Life Technologies, Carlsbad, CA, USA). Primer sequences from Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA) were as follows: tumor necrosis factor alpha (TNFα); (Mm00443258-m1), fatty acid synthase (FAS); (Mm00662319-m1), peroxisome proliferator-activated receptor alpha (PPARα); (Mm00440939-m1), carnitine palmitoyl transferase 1A (CPT1A); (Mm01231183-m1), sterol regulatory element binding protein (SREBP-1c); (Mm00550338-m1), cytochrome P450s [Cyp4a10 (Mm02601690-gH), Cyp2b10 (Mm01972453-s1), Cyp3a11 (Mm007731567-m1), Cyp1a2 (Mm00487224-m1)], CD36 (Mm01135198-m1), phosphoenolpyruvate carboxy kinase (PEPCK-1); (Mm01247058-m1), stearoyl coenzyme A desaturase1 (Scd1); (Mm00772290-m1), interleukin 6 (IL-6); (Mm00446190-m1), monocyte inducible protein 1 (MIP1); (Mm00441258-m1), monocyte chemo attractant protein 2 (MCP2); (Mm01297183-m1), transforming growth factor-beta (TGFβ); (Mm01178820_m1), collagen 1α1 (Mm00801666_g1), tissue inhibitor of metalloproteinases-1 (TIMP-1); (Mm00441818_m1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH); (4352932E). The levels of mRNA were normalized relative to the amount of GAPDH mRNA, and expression levels in mice fed control diet and administered vehicle were set at 1. Gene expression levels were calculated according to the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Data are expressed as mean ± SEM. For 2 group comparison, an unpaired t-test was used. Multiple group data were compared using One Way ANOVA followed by Bonferroni's post-hoc test (for parametric data) or Kruskal–Wallis test followed by Dunn's Multiple Comparison Test (for nonparametric data). p < 0.05 was considered statistically significant.
Results
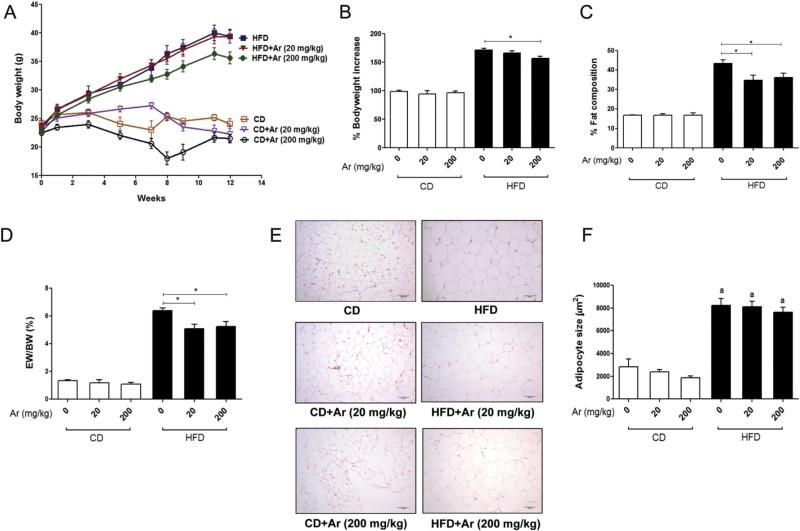
Aroclor 1260 decreased body weight and visceral adiposity in mice fed HFD
During the 12-week study, body weight gain was measured and percent (%) increase in bodyweight was calculated (Fig. 1A&B). All the HFD groups experienced weight gain vs. CD groups. Aroclor 1260 administration did not further increase the body weight of mice consuming HFD. Rather, Aroclor 1260 at 200 mg/kg decreased the body weight gain in HFD-fed mice vs. HFD + vehicle group (171.7 ± 8.9% vs. 156.9 ± 11.3%, p < 0.05). Aroclor 1260 at 20 mg/kg had no effect on body weight in HFD or CD groups. The % body fat composition was evaluated by DEXA scanning prior to harvesting the animals (Fig. 1C). HFD consumption increased total body fat. Aroclor 1260 at both doses diminished the increase in % body fat composition in HFD-fed mice (p < 0.05) but not in CD-fed mice. In fact, mice co-exposed to HFD and Aroclor 1260 (20 mg/kg) appeared to have an increase in lean body mass as compared to HFD-fed mice only (data not shown). There were no differences in % fat composition in CD-fed mice exposed to Aroclor 1260 vs. CD + vehicle group. The pattern of epididymal fat to body weight ratio among the six groups was similar to the pattern of body fat composition obtained by DEXA scanning, further supporting the observation that Aroclor 1260 exposure attenuated the increase in % body fat caused by HFD feeding (Fig. 1D).
Fig. 1.
Effects of Aroclor 1260 exposure on body weight and visceral adiposity. (A). Increase in body weight with time for C57BL/6 mice (n = 10) fed with a 42% milk fat diet (vs. CD). Body weight measurements were taken from Week 1 to Week 12 (12 weeks). (B). The % increase in body weight gain with time was calculated and the body weight at Week 1 was taken as 100%. (C). The % fat composition was measured using a Lunar PIXImus densitometer and Aroclor 1260 (20 and 200 mg/kg) exposure lowered % fat composition in HFD-fed mice vs. HFD + vehicle group. (D). Epididymal weight (EW) to body weight (BW) ratio was calculated for all groups of mice. (E). Epididymal adipose tissue was stained with H&E. (F). Adipocyte size (μm2) was measured and average cell size of >100 cells for each group was calculated. Values are mean ± SEM, p < 0.05, a—Δ due to HFD. CD—control diet, HFD— high fat diet, Ar—Aroclor 1260.
Mean epididymal adipocyte area (μm2) was larger in HFD + vehicle vs. CD + vehicle (p < 0.05). However, Aroclor 1260 had no effect at any of the doses administered, either in mice fed CD or HFD (Fig. 1E&F). Food consumption per mouse per day (g) was calculated over the 12-week period of study. Although all the HFD groups of animals consumed more food (approximately 1.7-fold more calories) as compared to CD groups, Aroclor 1260 did not alter the food consumption rate (data not shown). Thus, Aroclor 1260 exposure at both doses did not increase diet-induced adiposity or adipocyte size.
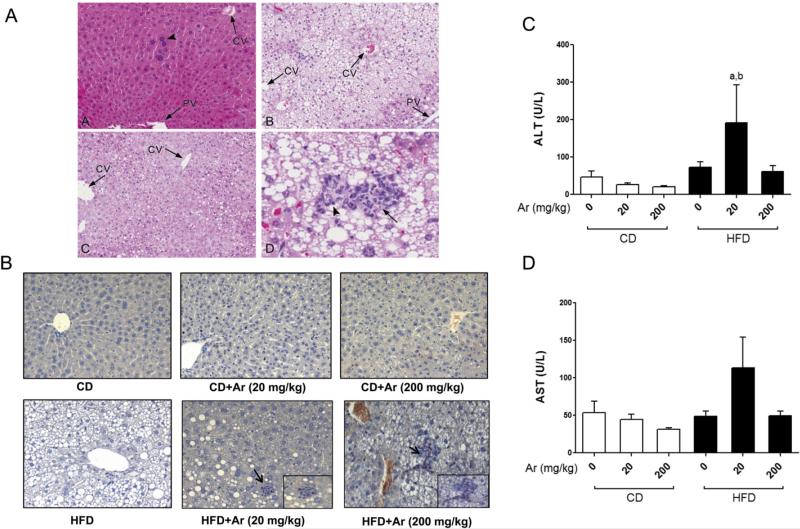
Aroclor 1260 exposure caused increased liver injury in HFD-fed mice
CD-fed mice with or without Aroclor 1260 exposure did not develop steatosis or steatohepatitis as seen with H&E staining of the liver sections (Fig. 2A). Mice fed with HFD developed steatosis in both the un-exposed and Aroclor 1260-exposed groups. The most striking difference between the HFD and HFD + Aroclor 1260 groups was transformation from steatosis to steatohepatitis. HFD + Aroclor 1260 co-exposures resulted in scattered foci of mononuclear cells and neutrophils (confirmed with CAE stained slides) and hepatocyte necrosis which appeared greater in the high dose group (Fig. 2A&B). Aroclor 1260 exposure was associated with centrilobular enlargement of hepatocytes and karyomegaly irrespective of diet type, possibly reflecting hepatic enzyme induction in these mice. Liver to body weight ratio was calculated and there were no differences among the groups (data not shown). No inflammation was observed in Aroclor 1260-exposed mice fed CD.
Fig. 2.
Aroclor 1260 exposure caused steatohepatitis in HFD-fed mice. (A). H&E staining of hepatic sections established the occurrence of centrilobular hepatocellular hypertrophy, karyomegaly, and multinucleate (arrow head) hepatocytes in the CD + Aroclor 1260 (200 mg/kg) group [A]. HFD consumption resulted in variable, centrilobular, microvesicular lipidosis [B–D], while centrilobular hepatocellular hypertrophy was observed in HFD + Aroclor 1260 (20 mg/kg) mice [C]. HFD-fed mice exposed to Aroclor 1260 (200 mg/kg) exhibited occasional, small areas of necrosis and inflammation (steatohepatitis) [D], characterized by neutrophils (arrow head) and pyknotic debris (arrow). (B). CAE staining demonstrated neutrophil infiltration in the HFD + Aroclor 1260 (20 and 200 mg/kg) groups. (C). Serum ALT and (D) AST levels (U/L) were measured (n = 10) using the Piccolo Xpress chemical analyzer. Values are mean ± SEM, p < 0.05, a—Δ due to HFD, b—Δ due to Aroclor 1260 exposure at 20 mg/kg. CD—control diet, HFD—high fat diet, Ar—Aroclor 1260, PV—portal vein, CV—central vein.
Liver injury, as determined by elevated serum ALT levels, was most noticeable in the HFD + Aroclor 1260 (20 mg/kg) group (p < 0.05) while serum AST was unaffected (Fig. 2C&D). Although the higher exposure group (200 mg/kg) fed HFD tended to have more inflammatory foci based on histological assessment, serum ALT levels were not significantly elevated in this group. In the CD-fed mice, Aroclor 1260 exposure did not alter the serum ALT and AST levels at either dose. There was no evidence of fibrosis in the Aroclor 1260-exposed mice as assessed by Sirius Red staining (data not shown). Hepatic expression of fibrotic markers including transforming growth factor-beta (TGFβ), collagen 1α1 and tissue inhibitor of metalloproteinases-1 (TIMP-1) were measured (Supplemental Fig. 1) and there was no increase in mRNA levels among the HFD groups.
The HFD + vehicle group showed a significant increase in hepatic levels of cholesterol and triglycerides vs. any of the CD groups (Supplemental Fig. 2). However, hepatic cholesterol and triglyceride levels were not significantly increased in the HFD + Aroclor 1260 at 20 or 200 mg/kg vs. any of the CD groups. Likewise, hepatic cholesterol and triglyceride levels were not significantly increased in the HFD + Aroclor 1260 at 20 or 200 mg/kg vs. the HFD group alone. Rather, there was a trend towards decreased hepatic cholesterol and triglycerides with Aroclor 1260 exposure in HFD-fed mice but this was not significant. Taken together, these results suggest that Aroclor 1260 exposure did not worsen steatosis caused by HFD but augmented inflammation and induced steatohepatitis instead.
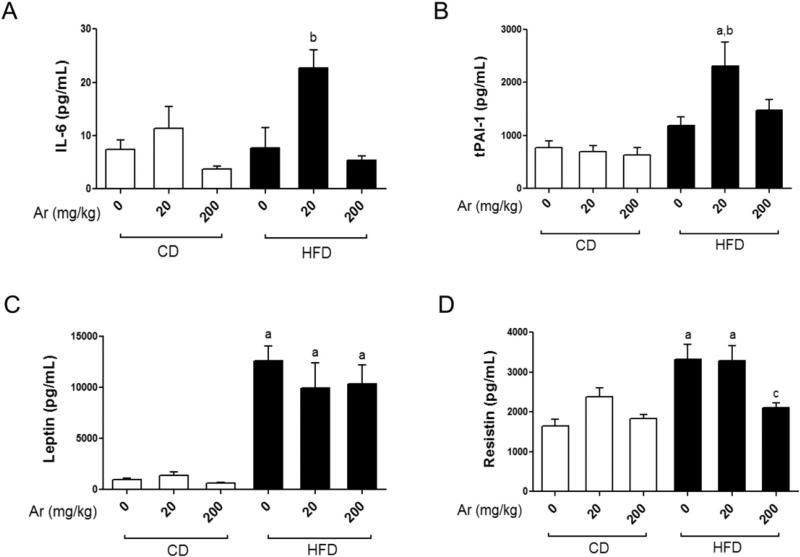
Effects of Aroclor 1260 exposure on serum adipo-cytokines
HFD consumption alone did not increase serum interleukin 6 (IL-6) or tissue plasminogen activator inhibitor (tPAI-1) levels vs. CD (Fig. 3A&B). However, HFD-fed mice exposed to Aroclor 1260 at 20 mg/kg displayed significantly elevated serum IL-6 and tPAI-1 levels (p < 0.05). This exposure-related elevation in pro-inflammatory cytokines was absent in CD-fed mice. In contrast to the lower exposure, Aroclor 1260 at 200 mg/kg did not increase serum IL-6 or tPAI-1 levels in either the HFD or CD groups even though histological examinations indicated higher levels of hepatic macrophage infiltration in HFD fed animals. Serum tumor necrosis factor alpha (TNFα) and monocyte chemo attractant protein-1 (MCP1) levels were not affected by either diet or Aroclor 1260 exposure (Supplemental Fig. 3).
Fig. 3.
Low dose Aroclor 1260 increased serum cytokines in HFD-fed mice. Serum (A) IL-6 (pg/mL), (B) tPAI-1 (pg/mL), (C) leptin (pg/mL) and (D) resistin (pg/mL) levels were measured using the Luminex IS 100 system (n = 10). Values are mean ± SEM, p < 0.05, a—Δ due to HFD, b—Δ due to Aroclor 1260 exposure at 20 mg/kg, c—Δ due to Aroclor 1260 exposure at 200 mg/kg. CD—control diet, HFD—high fat diet, Ar—Aroclor 1260.
All the HFD groups displayed elevated serum leptin levels as compared to CD groups (Fig. 3C, p < 0.05) consistent with the increased adiposity observed in these groups. Exposure to Aroclor 1260 did not affect leptin levels. Interestingly, serum adiponectin levels did not differ between any of the groups, irrespective of the diet type consumed (data not shown), leading to an increased leptin/adiponectin ratio in HFD-fed animals. Similar to leptin, serum resistin levels were increased in mice fed HFD (Fig. 3D, p < 0.05). Aroclor 1260 at 200 mg/kg decreased serum resistin levels in HFD-fed mice vs. HFD + vehicle and HFD + Aroclor 1260 (20 mg/kg) groups (p < 0.05), indicating a dose-dependent interaction between Aroclor 1260 and HFD. In summary, Aroclor 1260 at the lower dose increased serum pro-inflammatory cytokines in HFD-fed mice, while the higher dose caused a decrease in serum resistin levels.
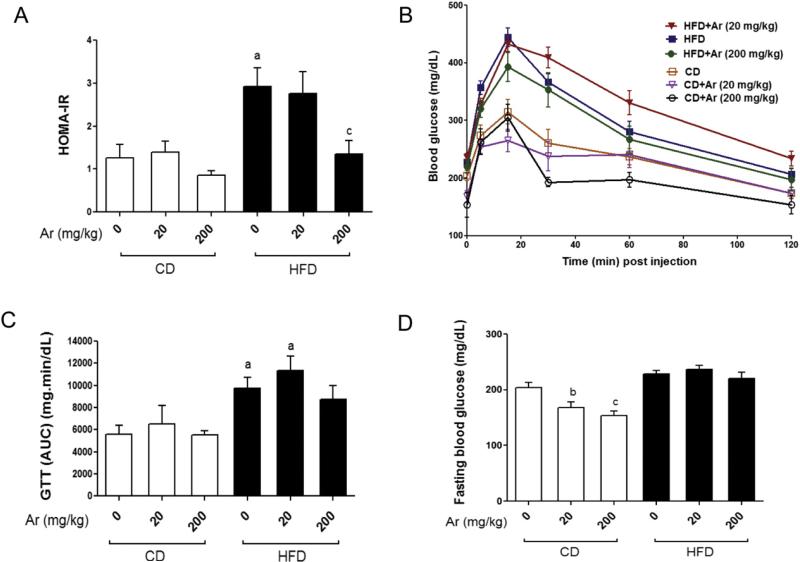
Effects of Aroclor 1260 on insulin resistance, glucose regulation, and serum lipoproteins
A glucose tolerance test was performed and HOMA-IR was calculated to determine if HFD and Aroclor 1260 co-exposure exacerbate HFD-induced insulin resistance, a common hallmark of NAFLD. HFD feeding increased HOMA-IR and Aroclor 1260 exposure (200 mg/kg) decreased HOMA-IR in HFD-fed mice (Fig. 4A). Interestingly, serum insulin levels were lower in the HFD group exposed to high dose Aroclor 1260 (data not shown) which might have resulted in lowered HOMA-IR in this group. The HFD group showed greater area under the curve (AUC) in the glucose tolerance test than any of the CD groups (Fig. 4B&C). Aroclor 1260 though had no additive effect to dietary manipulation alone. Therefore, while Aroclor 1260 exposure (200 mg/kg) improved HOMA-IR, GTT failed to improve. This could indicate impaired glucose-stimulated insulin production with Aroclor 1260 exposure. Fasting blood glucose levels showed that Aroclor 1260 exposure lowered blood glucose levels in the CD-fed mice at both the 20 and 200 mg/kg doses but not in HFD-fed mice (Fig. 4D). This observation is consistent with our earlier study where i.p. injection of PCB 153 resulted in lowered fasting blood glucose levels in mice fed CD (Wahlang et al., 2013).
Fig. 4.
HFD increased insulin resistance, and this was unaffected by Aroclor 1260 co-exposure. (A). HOMA-IR was calculated from fasting blood glucose and insulin levels for all six groups of animals (n = 10). (B). Glucose tolerance test was performed, and blood glucose levels were measured for mice (n = 10) fed with CD or HFD, with or without Aroclor 1260 co-exposure. (C). AUC was calculated, and the HFD groups showed higher AUC levels than the CD groups. (D). Fasting blood glucose levels (mg/dL) were measured, and the CD-fed mice co-exposed to Aroclor 1260 showed lower levels vs. CD + vehicle. Values are mean ± SEM, p < 0.05, a—Δ due to HFD, b—Δ due to Aroclor 1260 exposure at 20 mg/kg, c—Δ due to Aroclor 1260 exposure at 200 mg/kg. CD—control diet, HFD—high fat diet, Ar—Aroclor 1260.
HFD feeding resulted in significantly higher mean serum cholesterol and HDL levels vs. CD feeding (p < 0.05). Aroclor 1260 exposure at 20 or 200 mg/kg did not affect mean cholesterol and HDL levels either in the HFD or CD groups (Table 1). Contrastingly, HFD feeding did not increase mean serum LDL levels vs. CD feeding. However, the HFD + Aroclor 1260 (200 mg/kg) group exhibited significantly higher mean LDL levels vs. CD + vehicle or CD + Aroclor 1260 (200 mg/kg). Mean serum triglyceride levels were unchanged irrespective of diet consumed or level of Aroclor 1260 exposure. In summary, HFD was associated with insulin resistance and serum hypercholesterolemia, but Aroclor 1260 had no observable effect on these parameters.
Table 1.
Serum levels of cholesterol, high density lipoproteins, low density lipoproteins and triglycerides.
| (mg/dL) | CD | CD + Ar (20 mg/kg) | CD + Ar (200 mg/kg) | HFD | HFD + Ar (20 mg/kg) | HFD + Ar (200 mg/kg) |
|---|---|---|---|---|---|---|
| Cholesterol | 70.1 ± 7.7 | 76.0 ± 3.9 | 76.8 ± 3.8 | 122.1 ± 11.4a | 130.4 ± 8.2a | 130.0 ± 5.3a |
| HDL | 49.1 ± 6.1 | 52.2 ± 3.0 | 56.0 ± 3.0 | 96.8 ± 9.8a | 92.2 ± 4.4a | 93.1 ± 2.7a |
| LDL | 12.3 ± 1.7 | 16.1 ± 1.3 | 12.0 ± 1.6 | 17.3 ± 1.8 | 15.5 ± 2.3 | 21.8 ± 1.4b |
| Triglycerides | 44.1 ± 6.1 | 40.2 ± 3.6 | 43.8 ± 6.3 | 38.6 ± 3.1 | 53.0 ± 3.3 | 50.0 ± 3.1 |
Values are mean ± SEM (mg/dL), p < 0.05, CD-control diet, HFD–high fat diet, Ar–Aroclor 1260, HDL–high density lipoproteins, LDL–low density lipoproteins.
Δ due to HFD.
Δ due to Aroclor 1260 exposure at 200 mg/kg.
Aroclor 1260 exposure modulated hepatic fat metabolism
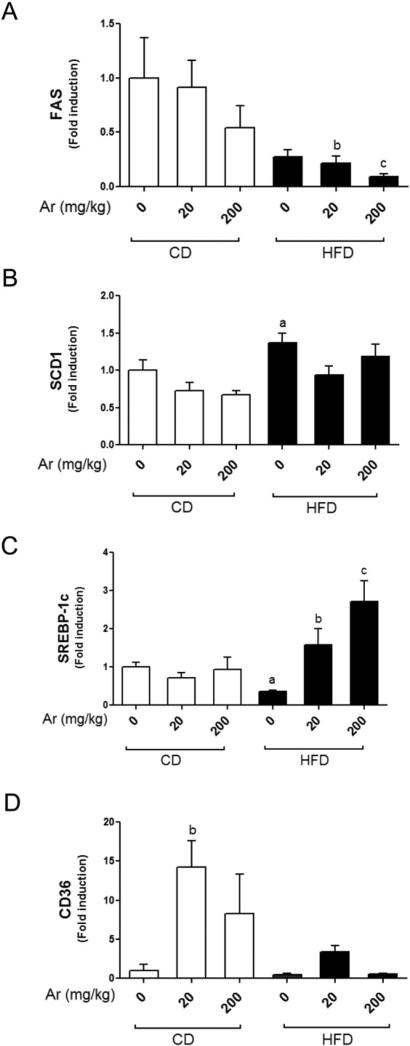
Liver-X-receptor (LXR) is the key transcription factor that drives lipid synthesis and cholesterol metabolism and its over-activation can promote or worsen steatosis (Lima-Cabello et al., 2011). We were interested in evaluating if Aroclor 1260 exposure would increase LXR target gene expression, which in turn, worsens steatosis caused by HFD. There are similarities in the response elements, to which LXR and the receptors that PCBs activate (CAR and PXR), bind. All three receptors have the capacity to bind DR-4 response elements which suggests that some degree of crosstalk may be possible. HFD feeding did not alter FAS hepatic expression vs. CD feeding (Fig. 5A). However, Aroclor 1260 exposure at 20 and 200 mg/kg decreased FAS hepatic expression in HFD-fed mice (p < 0.05). HFD feeding increased SCD1 hepatic expression (Fig. 5B, p < 0.05) and this result was not affected by Aroclor 1260 exposure. Interestingly, HFD feeding alone decreased SREBP-1c mRNA levels but co-exposure to HFD and Aroclor 1260 at both doses resulted in increased SREBP1-C mRNA levels (Fig. 5C, p < 0.05), suggesting an interaction between HFD and Aroclor 1260 exposure. Aroclor 1260 exposure at both doses had no effect on SREBP-1c hepatic expression in CD-fed mice. In contrast, Aroclor 1260 exposure at 20 mg/kg resulted in hepatic upregulation of CD36 in CD-fed mice (Fig. 5D, p < 0.05). Notably, CD36 is an LXR target gene shared by PXR as well (Zhou et al., 2008). In summary, Aroclor 1260 exposure did not increase FAS or SCD1 hepatic expression, suggesting that LXR was not activated by the exposure but that these genes’ expression may have been modulated by CAR or PXR directly or indirectly.
Fig. 5.
Effects of Aroclor 1260 exposure on genes involved in lipid metabolism. Real-time PCR experiments showed the changes in hepatic mRNA expressions caused by Aroclor 1260 exposure for (A) FAS, (B) SCD1, (C) SREBP-1c and (D) CD36. Values are mean ± SEM, p < 0.05, a—Δ due to HFD, b—Δ due to Aroclor 1260 exposure at 20 mg/kg, c—Δ due to Aroclor 1260 exposure at 200 mg/kg. CD—control diet, HFD—high fat diet, Ar—Aroclor 1260.
PPARα is a transcription factor for lipid-catabolizing genes including CPT1A, CPT2 and Cyp4a10. PPARα activation results in hepatic lipid oxidation and may be protective against steatosis. We measured the hepatic expression of PPARα as well as its target genes, Cyp4a10 and CPT1A (Supplemental Fig. 4). The mRNA levels of PPARα and Cyp4a10 did not differ between any of the groups examined. However, CPT1A expression was significantly induced with low dose Aroclor 1260 exposure in the HFD group vs. HFD alone. Therefore, Aroclor 1260 exposure at 20 mg/kg appeared to induce CPT1A in a HFD-setting by mechanisms independent of direct PPARα interaction.
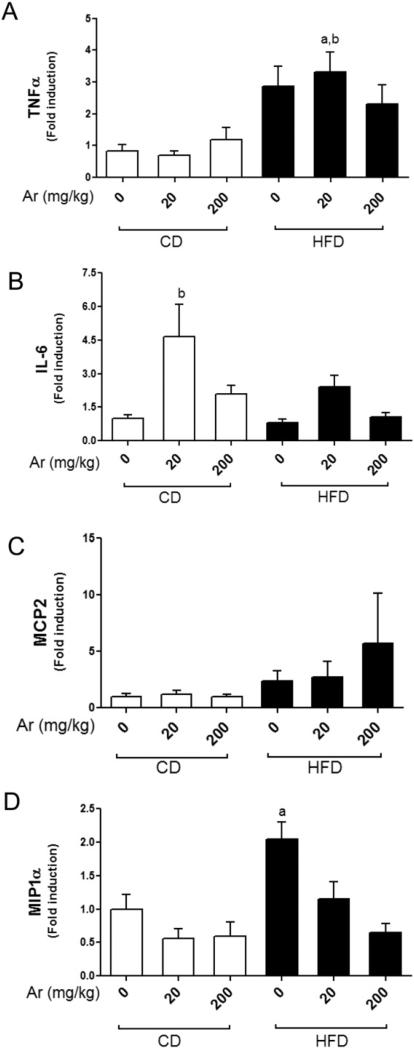
Hepatic expression of TLR-4 target genes
Toll like receptor 4 (TLR-4) activation results in nuclear factor kappa-B (NF-κB) activation which in turn causes upregulation of proinflammatory cytokines (Lu et al., 2008). We hypothesized that Aroclor 1260 may interact directly or indirectly with TLR-4, resulting in monocytic infiltration as observed in liver histology and increased serum cytokine levels. We therefore measured the hepatic expression of TLR-4 target genes namely TNFα and IL-6. HFD alone did not increase TNFα mRNA levels (Fig. 6A). However, exposure to Aroclor 1260 at 20 mg/kg increased TNFα mRNA levels in HFD-fed mice (p < 0.05). Aroclor 1260 exposure at 200 mg/kg did not increase TNFα hepatic expression in either the CD- or HFD-fed mice. Aroclor 1260 exposure at 20 mg/kg in CD fed mice resulted in increased IL-6 mRNA levels vs. CD alone (Fig. 6B). Aroclor 1260 exposure at 200 mg/kg did not increase IL-6 mRNA levels in either the CD- or HFD-fed mice. Clearly, Aroclor 1260 exposure at 20 mg/kg led to increased TNFα and IL-6 hepatic expressions with either HFD or CD respectively. Neither HFD feeding nor Aroclor 1260 exposure had any effect on MCP2 mRNA levels whereas MIP1α mRNA levels was increased only with HFD feeding (Fig. 6C&D). These results appear broadly consistent with the serum cytokine level data, suggesting increased inflammation and possibly sensitization to TNFα-dependent cell death only at the lower dose of Aroclor 1260 (20 mg/kg). Furthermore, the results also suggest that cytokine production may be inhibited at higher concentrations of Aroclor 1260 (200 mg/kg) by other mechanisms.
Fig. 6.
Effects of Aroclor 1260 exposure on TLR-4 target genes. Real-time PCR experiments showed the changes in hepatic mRNA expressions caused by Aroclor 1260 exposure for (A) TNFα, (B) IL-6, (C) MCP2 and (D) MIP1α. Values are mean ± SEM, p < 0.05, a—Δ due to HFD, b—Δ due to Aroclor 1260 exposure at 20 mg/kg. CD—control diet, HFD— high fat diet, Ar—Aroclor 1260.
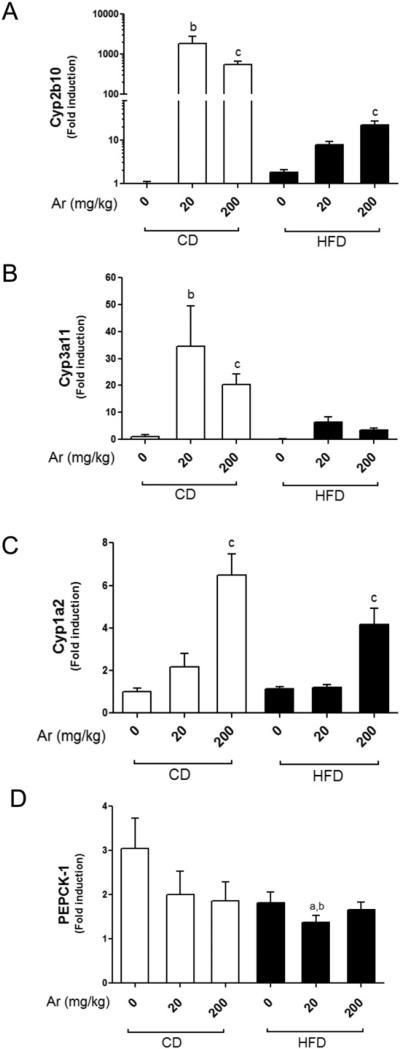
Aroclor 1260 induced hepatic CAR, PXR and AhR target genes
PCBs are known activators of AhR and CAR, which are involved in xenobiotic detoxification (Kopec et al., 2010; National Toxicology Program, 2006a). Recent studies have demonstrated PCBs’ interaction with other nuclear receptors including human PXR and rodent peroxisome-proliferator activated receptor alpha (PPARα) (Al-Salman and Plant, 2012; Robertson et al., 2007; Wahlang et al., 2014). We hypothesized that Aroclor 1260 may interact with these receptors in our animal model. We therefore looked at the hepatic expression levels of these receptors’ target genes in all animal groups.
The mRNA levels of Cyp2b10, a CAR target gene, were significantly up-regulated in all Aroclor 1260-exposed groups (Fig. 7A, p < 0.05). In the CD groups, the fold induction of Cyp2b10 was slightly higher in the lower dose (20 mg/kg) as compared to the higher dose (200 mg/kg) (approximately 1000-fold vs. 500-fold respectively). Feeding a HFD markedly reduced the fold induction of Cyp2b10 with inductions of 4.3-fold and 12-fold being observed at 20 mg/kg and 200 mg/kg exposures, respectively. Thus the reduction in fold induction caused by HFD feeding vs. CD feeding at 20 mg/kg and 200 mg/kg was reduced by approximately 235-fold and 41-fold, respectively. These results appear consistent with previous studies (Ghose et al., 2011; Sugatani et al., 2006), and indicate that the ability of CAR to activate target genes is compromised when animals are fed with HFD.
Fig. 7.
Aroclor 1260 exposure altered hepatic expression of CAR, PXR and AhR target genes. Real-time PCR experiments showed the changes in hepatic mRNA expressions caused by Aroclor 1260 exposure for (A) Cyp2b10 (CAR target gene), (B) Cyp3a11 (PXR target gene), (C) Cyp1a2 (AhR target gene) and (D) PEPCK-1 (an indirect target of CAR and PXR). Values are mean ± SEM, p < 0.05, a—Δ due to HFD, b—Δ due to Aroclor 1260 exposure at 20 mg/kg, c—Δ due to Aroclor 1260 exposure at 200 mg/kg. CD—control diet, HFD—high fat diet, Ar—Aroclor 1260.
Hepatic Cyp3a11 (PXR target gene) expression levels were also significantly up-regulated in all Aroclor 1260-exposed groups (Fig. 7B, p < 0.05). The Cyp3a11 fold induction caused by Aroclor 1260 exposure was significantly lower compared to Cyp2b10 (approximately 30- to 34-fold for the 20 mg/kg exposure and 20- to 25-fold for the 200 mg/kg exposure). Contrary to Cyp2b10 expression, HFD did not affect the fold inducibility of this particular gene. However, HFD feeding decreased the basal expression level of Cyp3a11 by approximately 8-fold.
The mRNA levels of Cyp1a2 (AhR target gene) were also measured in all the groups to determine Aroclor 1260 activation of the AhR. Hepatic Cyp1a2 expression was up-regulated in both dietary groups exposed to Aroclor 1260 only at 200 mg/kg (Fig. 7C, p < 0.05) but not at 20 mg/kg. Feeding with a HFD had no effect on either fold inducibility or basal level expression of this gene. These results suggest that with Aroclor 1260, the levels of congeners that activate CAR/PXR are present in much higher concentrations than those that activate AhR and the receptor based-effects of Aroclor 1260 at the lower dose are likely to be mediated primarily through CAR/PXR activation.
In addition to direct targets, both CAR and PXR are capable of binding, potentially sequestering and altering the transcriptional activity of FOXO1 (Kodama et al., 2004). FOXO1 is an important transcription factor controlling the expression of a wide range of gluconeogenic and lipogenic genes. To examine if FOXO1 mediated gene transcription was being affected, we examined the effects of Aroclor exposure on PEPCK-1, a prototypical FOXO1 target gene and the rate limiting step in gluconeogenesis. HFD feeding did not affect PEPCK-1 mRNA levels alone but Aroclor 1260 exposure at 20 mg/kg significantly reduced it in the HFD group (Fig. 7D).
Discussion
Although several PCB mixtures were commercially produced and used widely (e.g. Aroclor(s) 1260, 1254, 1248, 1242 and 1016), Aroclor 1260 was selected for this study because of the similarity in its congener composition pattern to that in human fat (McFarland and Clarke, 1989). The major congeners in Aroclor 1260 are the high molecular weight PCBs that have either 5-, 6-, 7- or 8- chlorine substituents, which in turn results in di-ortho substitution, and hence are non-coplanar in structure. These high molecular weight PCBs are not well metabolized and therefore bioaccumulate in humans (Beyer and Biziuk, 2009). PCB toxicity has been associated with cancer, endocrine disruption, and impaired cognitive development, but recent epidemiologic studies have shown that PCB exposures can also result in metabolic disorders associated with NAFLD, including obesity, insulin resistance/diabetes, and the metabolic syndrome (Ruzzin et al., 2010; Silverstone et al., 2012; Smith et al., 1982). Occupational exposure to PCB mixtures has also been associated with elevated plasma levels of liver enzymes (Cave et al., 2010a). Chronic exposures to these chlorinated compounds appear to disrupt both lipid and glucose homeostasis and consequently lead to diabetes and associated metabolic disorders. The current study investigated the effects of environmental pollutant–nutrient interactions, which is clinically relevant because all humans are exposed to PCBs and over 75% of the US adult population is considered to be either overweight or obese. Exposure to toxicants such as PCBs may act as a ‘second hit’ that eventually drives this population to steatohepatitis and the metabolic syndrome.
PCBs bioaccumulate in the liver and adipose tissue due to their hydrophobicity, thus, making these sites principal targets for PCB toxicity. Lipid-adjusted serum PCB levels were measured in NHANES participants and in the PCB exposed Anniston cohort with the highest reported levels ranged from 75 to 170 ng/g (Cave et al., 2010a; Goncharov et al., 2011). Additionally, the National Toxicology Program (NTP) studies measured PCB levels in a 2-year gavage study in rats. Interestingly, PCB liver levels were at least 10-fold higher and adipose levels were at least 200-fold higher than lipid-adjusted serum levels irrespective of the dose administered or treatment time (National Toxicology Program, 2006b). Although Aroclor 1260 levels were not measured in our study, we speculate that the distribution will be similar to other PCBs used in NTP studies. In those studies, a 20 mg/kg cumulative dose yielded the following tissue levels: serum—176 ng/g, liver—3663 ng/g and adipose—92,840 ng/g while a 200 mg/kg cumulative dose yielded levels: serum—1788 ng/g, liver—34,010 ng/g and adipose—1,118,300 ng/g. Thus the 20 mg/kg dose employed is expected to produce serum levels similar to the maximum levels reported for the Anniston cohort (170.4 ng/g). The 200 mg/kg dose is similar to that used in the NTP TR 530 for cancer studies. In the present study, we exposed mice to PCBs using gavage which was designed to mimic human exposure routes. A potential caveat is that these mice received either a single exposure or four separate exposures rather than the intermittent exposures that humans encounter from eating PCB-contaminated food. We also attempted to have several weeks between exposure to PCBs and the measurement of study endpoints to maximize the effects from bioaccumulated PCBs rather than the metabolized congeners.
The main finding in this study is the Aroclor 1260-mediated transition of steatosis to steatohepatitis in the DIO model. Paradoxically, NASH was associated with decreased % fat composition and increased lean body mass at the low dose exposure. Additionally, CD groups showed a decrease in body weight gain which may be attributed to stress experienced due to oral gavage. None of the CD groups exposed to Aroclor 1260 manifested hepatitis (H&E staining). Contrarily, Aroclor 1260 exposure in HFD groups worsened liver necro-inflammation. Co-exposure to HFD and Aroclor 1260 (20 mg/kg) resulted in elevated serum ALT, IL-6 and tPAI-1 and upregulated hepatic TNFα expression. However, Aroclor 1260 at 200 mg/kg did not induce systemic inflammation, despite histologic signs of liver injury.
The results from this study differ markedly from our earlier work on a single congener, PCB 153, where HFD + PCB 153 co-exposure worsened steatosis and obesity in male C57Bl/6J mice without causing inflammation (Wahlang et al., 2013). We documented PCB 153-mediated adipokine dysregulation; a phenomenon which was absent in the current study. Furthermore, HFD + PCB 153 co-exposure altered hepatic expression of genes involved in fatty acid metabolism including increased FAS and decreased CPT1A mRNA expression. Although PCB 153 is present in Aroclor 1260, other congeners are present in this PCB mixture that may contribute to steatohepatitis and yet have no effect on obesity. Thus, exposing animals to a mixture can yield outcomes that are entirely different from that of a single congener.
Hepatic P450s, including Cyp3a11 (PXR target) and Cyp2b10 (CAR target), were induced by Aroclor 1260 exposure in both CD and HFD-fed mice. PXR and CAR are critically involved in xenobiotic metabolism and drug disposition, but recent studies demonstrated the importance of CAR and PXR regulation on physiological processes such as glucose and lipid metabolism, and this could impact NASH (Chai et al., 2013; Gao and Xie, 2010; Konno et al., 2008). Gao et al. demonstrated CAR as an anti-obesity receptor whose activation was protective against DIO and insulin resistance (Gao et al., 2009). On the contrary, the role of PXR in obesity remains controversial with studies reporting either anti-obesity or obesity promoting effects (He et al., 2013; Ma and Liu, 2012; Nakamura et al., 2007). Our RT-PCR data strongly suggest CAR activation by Aroclor 1260 as indicated by the ~1000- to 500-fold induction of Cyp2b10 at both the low and high exposure levels. Cyp3a11 induction is a hallmark of PXR activation, but activated CAR can also bind to the Cyp3a11 response element and drive its expression, albeit, at lower levels. Although Cyp3a11 was induced by ~30-fold, this induction may be mediated by CAR rather than PXR. These results indicate that Aroclor 1260 exposure activated CAR/PXR, hence the possible obesity-protective effects seen in the HFD-fed mice exposed to Aroclor 1260.
Furthermore, CAR/PXR activation suppresses FoxO1-insulin response sequence (IRS) binding activity (Kodama et al., 2004) resulting in decreased gluconeogenesis and hence the lowered fasting blood glucose levels seen in CD + Aroclor 1260 groups. Additionally, FoxO1, is a negative regulator of SREBP1 transcription and its sequestration by activated CAR/PXR could result in increased SREBP1 gene expression (Deng et al., 2012; Kodama et al., 2004). Notably in our study, hepatic SREBP-1c expression was upregulated in HFD-fed mice exposed to Aroclor 1260. However, activated CAR and PXR can transcriptionally activate the anti-lipogenic gene Insig-1, consequently leading to reduced SREBP1 activity and decreased SREBP1 target gene expression such as FAS (Roth et al., 2008). Hence, the decrease in FAS expression observed in this study could possibly be due to loss of SREBP1 activity even though the gene was induced. Additionally, CD36, a lipid scavenger receptor and target gene shared by AhR, PXR and LXR was also induced by Aroclor 1260. Other novel findings in this study included induction of CPT1A and decreased hepatic FAS expression with Aroclor 1260 exposure in HFD-fed mice. In concert, the effects of Aroclor 1260 on lipogenesis appeared complex, and interactions with CAR/PXR could contribute to the observed decrease in % fat composition in HFD +Aroclor 1260 groups. Moreover, glucose metabolism was abnormal in HFD-fed mice exposed to Aroclor 1260 (200 mg/kg), because, while HOMA-IR improved, glucose tolerance failed to improve. It appears that HOMA-IR and glucose tolerance test may be insufficient to evaluate glucose metabolism in PCB studies, given the partially divergent effects of PCB exposure in the fed and fasted states. However, these observations need to be pursued further to elucidate the mechanisms involved.
Regardless of Aroclor 1260 exposure, it appeared that HFD consumption reduced the inducibility of CAR/PXR target genes as compared to CD consumption and it was only HFD-fed mice exposed to Aroclor 1260 that exhibited liver injury. Therefore, it is plausible to say that activation of xenobiotic receptors such as CAR/PXR protect against PCB toxicity in a low fat diet setting. Therefore HFD consumption interferes with CAR/PXR activation by PCBs, and therefore attenuates the protective effects of these receptors against PCB toxicity with the net result being increased liver injury only in Aroclor 1260 + HFD co-exposed animals. The progression of NAFLD from simple steatosis to steatohepatitis requires both hepatic fat accumulation and inflammation. In this study, it was observed that in steatotic mice exposed to low dose Aroclor 1260, inflammation and liver injury were aggravated, while at the high dose, inflammation was suppressed and liver injury was attenuated. Our results suggest that it is the inflammatory dysfunction that PCBs induce rather than the degree of steatosis observed that may dictate appearance of steatohepatitis.
Hepatic expression of Cyp1a2 (AhR target gene) was up-regulated only in groups receiving Aroclor 1260 at the highest dose tested, suggesting dose-dependent activation of this receptor. AhR activation by PCBs is well documented with coplanar (‘dioxin-like’) PCBs including PCB 126 being good rodent AhR activators. A wasting syndrome and chloracne are characteristic features of AhR activation by its classic ligand TCDD (Pohjanvirta and Tuomisto, 1994). AhR activation is also associated with immune suppression via AhR interference with NF-κB signaling (Kimura et al., 2009). Consistent with these results, animals exposed to Aroclor 1260 (200 mg/kg) displayed lower body weight and a suppression of serum pro-inflammatory cytokines and resistin levels. Resistin, also known as the adipocyte secretory factor, is secreted by the adipose tissue and appears to participate in inflammatory processes as well (Menezes-Garcia et al., 2014). While reduced body weight has frequently been reported with animals exposed to AhR ligands, in murine and human models, steatosis increases, presumably due to a redistribution of dietary fat (Angrish et al., 2012). Thus activation of the AhR may lead to increased steatosis but decreased steatohepatitis, as a function of its immunosuppressive effects. The activation of AhR by Aroclor 1260 is likely due to the presence of coplanar congeners such as PCB 126 but these compounds exist in relatively low percentages in this mixture (<1%), and hence a higher Aroclor 1260 exposure level is required to observe the ‘dioxin-like’ effects. Therefore, absence of inflammation at the higher dose may be due to the immune-suppressive properties of activated AhR.
Thus, we identified both CAR/PXR and AhR activation as potential mode(s) of action of this PCB mixture in NASH. Nonetheless, rodent and human receptors may have differences pertaining to ligand binding activity and target gene battery. Off target effects are also possible mechanisms in PCB-driven NASH, but these were not evaluated in this study. Moreover, the current study failed to distinguish between CAR and PXR activation which is a potential drawback since the observed Aroclor 1260 effects may be based solely on CAR activation. Thus further investigation using PXR/CAR knockout models is required in this direction. The study also failed to assess overall metabolism and employing metabolic chambers would have been useful in this regard. Furthermore, another drawback in this study was using serum ALT/AST as a NASH biomarker based on low sensitivity, and evaluating other bio-markers is a possibility in future studies (Cave et al., 2010b). In addition, our studies were performed using male mice; hence it is pertinent to note that the observed effects may vary with gender and species.
In conclusion, Aroclor 1260 exposure caused toxicant-associated steatohepatitis in animals fed with HFD. In contrast to our previous study wherein a single congener (PCB 153) was used, this PCB mixture neither increased the body weight/visceral adiposity nor worsened insulin resistance/DIO. There was a significant difference between the low and high exposure doses in terms of hepatic/systemic inflammation, which could potentially be due to AhR activation. Our additional findings demonstrate that Aroclor 1260 activated CAR and PXR and to a lesser extent AhR, suggesting congener composition and exposure levels to be critical in determining a mixture's mode(s) of actions. Lastly, CAR and PXR activation could be protective against PCB-mediated toxicity but HFD consumption may blunt this protection. More studies are needed on the role of PCB-nuclear receptor interactions in steatohepatitis.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) [Grant 1RO1ES021375 and T35ES14559] and the National Institutes of Health (NIH) [Grant K23AA018399]. This research was also supported [in part] by the Division of the National Toxicology Program of the NIH, NIEHS.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ALT
alanine transaminase
- AST
aspar-tate transaminase
- AUC
area under the curve
- CAE
chloroacetate esterase
- CAR
constitutive androstane receptor
- CPT1A
carnitine palmitoyl transferase 1A
- Cyp
cytochrome P450
- DIO
diet-induced obesity
- FAS
fatty acid synthase
- FOX01
forkhead transcription factor 01
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HDL
high density lipoproteins
- H&E
hematoxylin–eosin
- HFD
high fat diet
- HOMA-IR
homeostasis model assessment of insulin resistance
- IL-6
interleukin-6
- LDL
low density lipoproteins
- LXR
liver-X-receptor
- NAFLD
non-alcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NHANES
national health and nutrition examination survey
- NTP
National Toxicology Program
- PCBs
polychlorinated biphenyls
- PCR
polymerase chain reaction
- PEPCK-1
phosphoenolpyruvate carboxy kinase
- POPs
persistent organic pollutants
- PPAR
peroxisome proliferator-activated receptor
- PXR
pregnane-xenobiotic receptor
- Scd1
stearoyl coenzyme A desaturase1
- SEM
standard error mean
- SREBP-1c
sterol regulatory element binding protein 1c
- TASH
toxicant associated steatohepatitis
- TCDD
tetrachlorodibenzo-p-dioxin
- TLR-4
toll-like receptor 4
- TNFα
tumor necrosis factor alpha
- tPAI-1
tissue plasminogen activator inhibitor 1
Footnotes
Conflict of interest statement
This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS) or the National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of the NIEHS, NIH or the United States government.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.taap.2014.06.019.
References
- Al-Salman F, Plant N. Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol. Appl. Pharmacol. 2012;263:7–13. doi: 10.1016/j.taap.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Angrish MM, Mets BD, Jones AD, Zacharewski TR. Dietary fat is a lipid source in 2,3,7,8-tetrachlorodibenzo-rho-dioxin (TCDD)-elicited hepatic steatosis in C57BL/6 mice. Toxicol. Sci. 2012;128:377–386. doi: 10.1093/toxsci/kfs155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A, Biziuk M. Environmental fate and global distribution of polychlorinated biphenyls. Rev. Environ. Contam. Toxicol. 2009;201:137–158. doi: 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners—a mass balance approach. 1. Global production and consumption. Sci. Total Environ. 2002;290:181–198. doi: 10.1016/s0048-9697(01)01075-0. [DOI] [PubMed] [Google Scholar]
- Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ. Health Perspect. 2010a;118:1735–1742. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M, Falkner KC, Ray M, Joshi-Barve S, Brock G, Khan R, Bon Homme M, McClain CJ. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 2010b;51:474–481. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Zeng S, Xie W. Nuclear receptors PXR and CAR: implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opin. Drug Metab. Toxicol. 2013;9:253–266. doi: 10.1517/17425255.2013.754010. [DOI] [PubMed] [Google Scholar]
- Deng X, Zhang W, O-Sullivan I, Williams JB, Dong Q, Park EA, Raghow R, Unterman TG, Elam MB. FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J. Biol. Chem. 2012;287:20132–20143. doi: 10.1074/jbc.M112.347211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Xie W. Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab. Dispos. 2010;38:2091–2095. doi: 10.1124/dmd.110.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, He J, Zhai Y, Wada T, Xie W. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J. Biol. Chem. 2009;284:25984–25992. doi: 10.1074/jbc.M109.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose R, Omoluabi O, Gandhi A, Shah P, Strohacker K, Carpenter KC, McFarlin B, Guo T. Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci. 2011;89:57–64. doi: 10.1016/j.lfs.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ. Health Perspect. 2011;119:319–325. doi: 10.1289/ehp.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Gao J, Xu M, Ren S, Stefanovic-Racic M, O'Doherty RM, Xie W. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes. 2013;62:1876–1887. doi: 10.2337/db12-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno Y, Negishi M, Kodama S. The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab. Pharmacokinet. 2008;23:8–13. doi: 10.2133/dmpk.23.8. [DOI] [PubMed] [Google Scholar]
- Kopec AK, Burgoon LD, Ibrahim-Aibo D, Mets BD, Tashiro C, Potter D, Sharratt B, Harkema JR, Zacharewski TR. PCB153-elicited hepatic responses in the immature, ovariectomized C57BL/6 mice: comparative toxicogenomic effects of dioxin and non-dioxin-like ligands. Toxicol. Appl. Pharmacol. 2010;243:359–371. doi: 10.1016/j.taap.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Cabello E, Garcia-Mediavilla MV, Miquilena-Colina ME, Vargas-Castrillon J, Lozano-Rodriguez T, Fernandez-Bermejo M, Olcoz JL, Gonzalez-Gallego J, Garcia-Monzon C, Sanchez-Campos S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin. Sci. (Lond.) 2011;120:239–250. doi: 10.1042/CS20100387. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Ma Y, Liu D. Activation of pregnane X receptor by pregnenolone 16 alpha-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS One. 2012;7:e38734. doi: 10.1371/journal.pone.0038734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congenerspecific analysis. Environ. Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Garcia Z, Oliveira MC, Lima RL, Soriani FM, Cisalpino D, Botion LM, Teixeira MM, Souza DG, Ferreira AV. Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion. Obesity (Silver Spring) 2014;22:663–672. doi: 10.1002/oby.20142. [DOI] [PubMed] [Google Scholar]
- Merrell MD, Cherrington NJ. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab. Rev. 2011;43:317–334. doi: 10.3109/03602532.2011.577781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya M, Gomez-Lechon MJ, Castell JV, Jover R. Enhanced steatosis by nuclear receptor ligands: a study in cultured human hepatocytes and hepatoma cells with a characterized nuclear receptor expression profile. Chem. Biol. Interact. 2010;184:376–387. doi: 10.1016/j.cbi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, Sueyoshi T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J. Biol. Chem. 2007;282:9768–9776. doi: 10.1074/jbc.M610072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program NTP toxicology and carcinogenesis studies of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in female Harlan Sprague–Dawley rats (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2006a:4–246. [PubMed] [Google Scholar]
- National Toxicology Program Toxicology and carcinogenesis studies of a binary mixture of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) and 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2006b:1–258. [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-pdioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol. Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- Robertson LW, Berberian I, Borges T, Chen LC, Chow CK, Glauert HP, Filser JG, Thomas H. Suppression of peroxisomal enzyme activities and cytochrome P450 4A isozyme expression by congeneric polybrominated and polychlorinated biphenyls. PPAR Res. 2007;2007:15481. doi: 10.1155/2007/15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Looser R, Kaufmann M, Blattler SM, Rencurel F, Huang W, Moore DD, Meyer UA. Regulatory cross-talk between drug metabolism and lipid homeostasis: constitutive androstane receptor and pregnane X receptor increase Insig-1 expression. Mol. Pharmacol. 2008;73:1282–1289. doi: 10.1124/mol.107.041012. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ. Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ. Health Perspect. 2010;118:796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RK, Kumar A, Das S, Kadiiska MB, Michelotti G, Diehl AM, Chatterjee S. Environmental toxin-linked nonalcoholic steatohepatitis and hepatic metabolic reprogramming in obese mice. Toxicol. Sci. 2013;134:291–303. doi: 10.1093/toxsci/kft104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ. Health Perspect. 2012;120:727–732. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Schloemer J, Lowry LK, Smallwood AW, Ligo RN, Tanaka S, Stringer W, Jones M, Hervin R, Glueck CJ. Metabolic and health consequences of occupational exposure to polychlorinated biphenyls. Br. J. Ind. Med. 1982;39:361–369. doi: 10.1136/oem.39.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani J, Wada T, Osabe M, Yamakawa K, Yoshinari K, Miwa M. Dietary inulin alleviates hepatic steatosis and xenobiotics-induced liver injury in rats fed a high-fat and high-sucrose diet: association with the suppression of hepatic cyto-chrome P450 and hepatocyte nuclear factor 4alpha expression. Drug Metab. Dispos. 2006;34:1677–1687. doi: 10.1124/dmd.106.010645. [DOI] [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, Cave M. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J. Nutr. Biochem. 2013;24:1587–1595. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Clair HB, Al-Eryani L, Prough RA, States JC, Coslo DM, Omiecinski CJ, Cave MC. Human receptor activation by Aroclor 1260, a polychlorinated biphenyl mixture. Toxicol. Sci. 2014 doi: 10.1093/toxsci/kfu083. (Epub ahead of print, pii: kfu083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, Silverstein RL, Xie W. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.