Abstract
Reconstitution of a damaged or exhausted immune system by injection of genetically compatible immunocompetent cells (immunologic rejuvenation) is a promising approach for restoration of immune activity. By using this model, spleen cells from young-adult mice, previously immunized with Salmonella typhimurium, were transferred to either young-adult or old, syngeneic recipients before or after storage at −196 C. The susceptibility of recipient mice was then determined by challenging them at increasing time intervals after reconstitution with lethal doses of the virulent organisms. The findings, although preliminary in nature, demonstrate that (i) immunological rejuvenation of mice is possible with immunocompetent cells from specifically immunized donors; (ii) prolonged “takes” of these cells can occur even in nonirradiated recipient mice, and (iii) storage at −196 C does not impair their protective capacity.
Full text
PDF

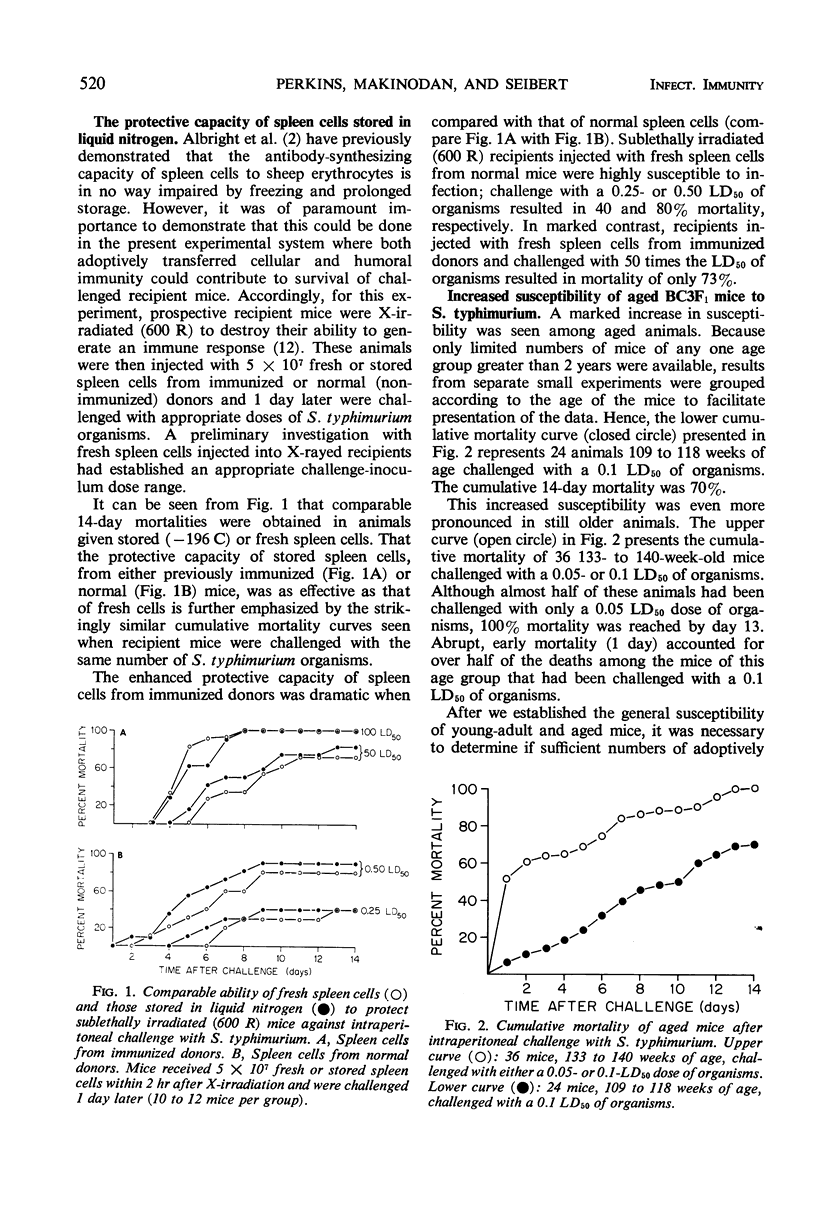


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRIGHT J. F., MAKINODAN T., MAZUR P. PRESERVATION OF ANTIBODY-PRODUCING CELLS AT LOW TEMPERATURE: A METHOD OF STORAGE THAT ALLOWS COMPLETE RECOVERY OF ACTIVITY. Proc Soc Exp Biol Med. 1963 Nov;114:489–493. doi: 10.3181/00379727-114-28711. [DOI] [PubMed] [Google Scholar]
- Albright J. F., Makinodan T., Deitchman J. W. Presence of life-shortening factors in spleens of aged mice of long lifespan and extension of life expectancy by splenectomy. Exp Gerontol. 1969 Dec;4(4):267–276. doi: 10.1016/0531-5565(69)90015-1. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. An immunological approach to ageing. Lancet. 1970 Aug 15;2(7668):358–360. doi: 10.1016/s0140-6736(70)92886-2. [DOI] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966 Jul 1;124(1):1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino F., Makinodan T., Lever W. E., Peterson W. J. The immune systems of mice reared in clean and in dirty conventional laboratory farms. I. Life expectancy and pathology of mice with long life-spans. J Gerontol. 1971 Oct;26(4):497–507. doi: 10.1093/geronj/26.4.497. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in nonvaccinated mice challenged by various routes. J Bacteriol. 1969 Feb;97(2):667–675. doi: 10.1128/jb.97.2.667-675.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J., Leuchars E., Wallis V. Chimaerism after introduction of lymphocytes into normal mice. Nature. 1970 Sep 26;227(5265):1352–1354. doi: 10.1038/2271352a0. [DOI] [PubMed] [Google Scholar]
- MAKINODAN T., KASTENBAUM M. A., PETERSON W. J. Radiosensitivity of spleen cells from normal and preimmunized mice and its significance to intact animals. J Immunol. 1962 Jan;88:31–37. [PubMed] [Google Scholar]
- Makinodan T., Chino F., Lever W. E., Brewen B. S. The immune systems of mice reared in clean and in dirty conventional laboratory farms. II. Primary antibody-forming activity of young and old mice with long life-spans. J Gerontol. 1971 Oct;26(4):508–514. doi: 10.1093/geronj/26.4.508. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Perkins E. H., Chen M. G. Immunologicc activity of the aged. Adv Gerontol Res. 1971;3:171–198. [PubMed] [Google Scholar]
- Metcalf D., Moulds R., Pike B. Influence of the spleen and thymus on immune responses in ageing mice. Clin Exp Immunol. 1967 Jan;2(1):109–120. [PMC free article] [PubMed] [Google Scholar]
- Micklem H. S., Clarke C. M., Evans E. P., Ford C. E. Fate of chromosome-marked mouse bone marrow cells tranfused into normal syngeneic recipients. Transplantation. 1968 Mar;6(2):299–302. [PubMed] [Google Scholar]
- Ram J. S. Aging and immunological phenomena--a review. J Gerontol. 1967 Jan;22(1):92–107. [PubMed] [Google Scholar]
- Rowley D., Turner K. J. Number of molecules of antibody required to promote phagocytosis of one bacterium. Nature. 1966 Apr 30;210(5035):496–498. doi: 10.1038/210496a0. [DOI] [PubMed] [Google Scholar]
- Teague P. O., Friou G. J. Antinuclear antibodies in mice. II. Transmission with spleen cells; inhibition or prevention with thymus or spleen cells. Immunology. 1969 Nov;17(5):665–675. [PMC free article] [PubMed] [Google Scholar]
- Walford R. L. Antibody diversity, histocompatibility systems, disease states, and ageing. Lancet. 1970 Dec 12;2(7685):1226–1229. doi: 10.1016/s0140-6736(70)92184-7. [DOI] [PubMed] [Google Scholar]


