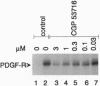
Abstract
The platelet-derived growth factor (PDGF) receptor is a member of the transmembrane growth factor receptor protein family with intrinsic protein-tyrosine kinase activity. We describe a potent protein-tyrosine kinase inhibitor (CGP 53716) that shows selectivity for the PDGF receptor in vitro and in the cell. The compound shows selectivity for inhibition of PDGF-mediated events such as PDGF receptor autophosphorylation, cellular tyrosine phosphorylation, and c-fos mRNA induction in response to PDGF stimulation of intact cells. In contrast, ligand-induced autophosphorylation of the epidermal growth factor (EGF) receptor, insulin receptor, and the insulin-like growth factor I receptor, as well as c-fos mRNA expression induced by EGF, fibroblast growth factor, and phorbol ester, was insensitive to inhibition by CGP 53716. In antiproliferative assays, the compound was approximately 30-fold more potent in inhibiting PDGF-mediated growth of v-sis-transformed BALB/c 3T3 cells relative to inhibition of EGF-dependent BALB/Mk cells, interleukin-3-dependent FDC-P1 cells, and the T24 bladder carcinoma line. When tested in vivo using highly tumorigenic v-sis- and human c-sis-transformed BALB/c 3T3 cells, CGP 53716 showed antitumor activity at well-tolerated doses. In contrast, CGP 53716 did not show antitumor activity against xenografts of the A431 tumor, which overexpresses the EGF receptor. These findings suggest that CGP 53716 may have therapeutic potential for the treatment of diseases involving abnormal cellular proliferation induced by PDGF receptor activation.
Full text
PDF
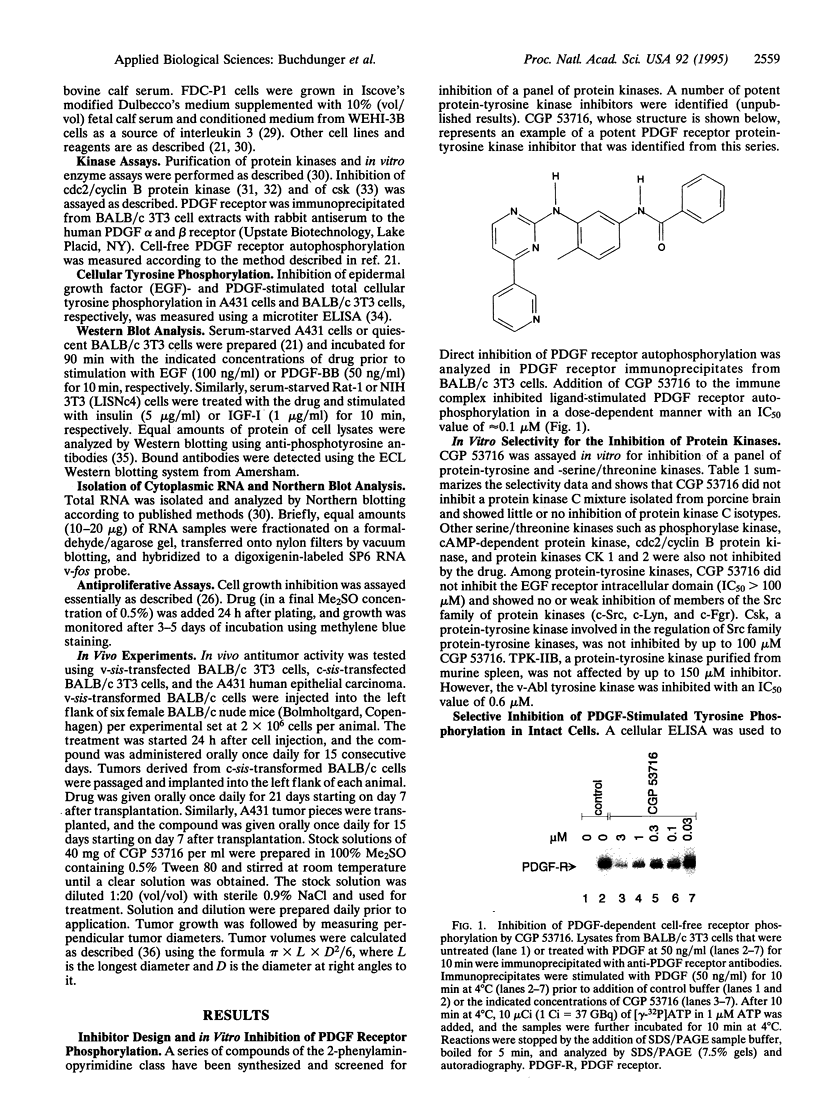
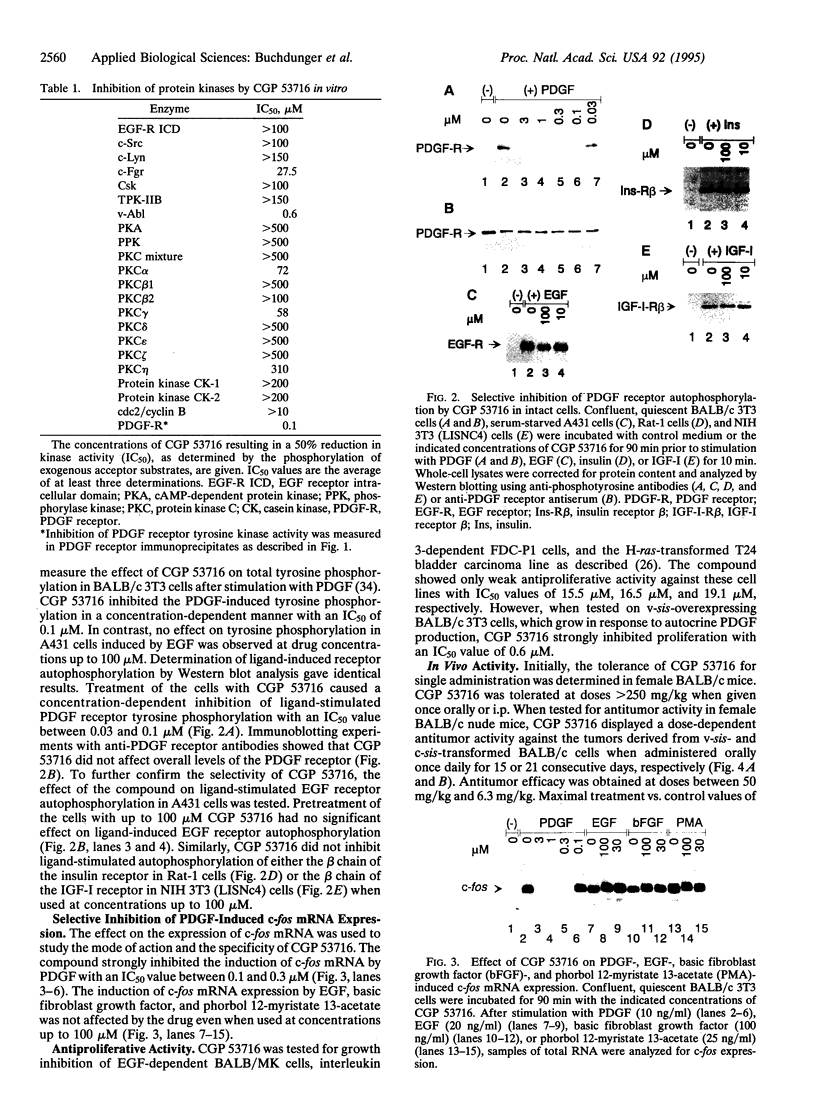
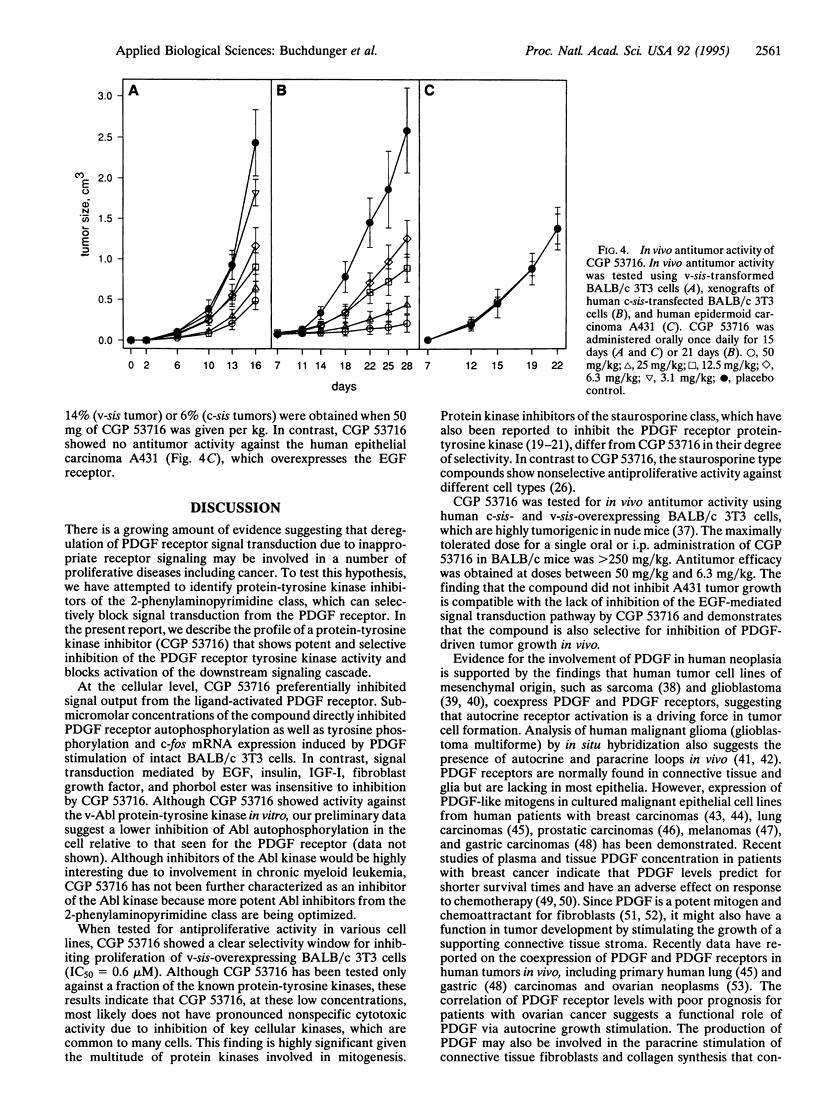

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrejauskas-Buchdunger E., Regenass U. Differential inhibition of the epidermal growth factor-, platelet-derived growth factor-, and protein kinase C-mediated signal transduction pathways by the staurosporine derivative CGP 41251. Cancer Res. 1992 Oct 1;52(19):5353–5358. [PubMed] [Google Scholar]
- Antoniades H. N., Bravo M. A., Avila R. E., Galanopoulos T., Neville-Golden J., Maxwell M., Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990 Oct;86(4):1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Galanopoulos T., Neville-Golden J., O'Hara C. J. Malignant epithelial cells in primary human lung carcinomas coexpress in vivo platelet-derived growth factor (PDGF) and PDGF receptor mRNAs and their protein products. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3942–3946. doi: 10.1073/pnas.89.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariad S., Seymour L., Bezwoda W. R. Platelet-derived growth factor (PDGF) in plasma of breast cancer patients: correlation with stage and rate of progression. Breast Cancer Res Treat. 1991 Dec;20(1):11–17. doi: 10.1007/BF01833352. [DOI] [PubMed] [Google Scholar]
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder G. E., Krawiec J. A., McVety K., Gazit A., Gilon C., Lyall R., Zilberstein A., Levitzki A., Perrone M. H., Schreiber A. B. Tyrphostins inhibit PDGF-induced DNA synthesis and associated early events in smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C721–C730. doi: 10.1152/ajpcell.1991.260.4.C721. [DOI] [PubMed] [Google Scholar]
- Bryckaert M. C., Eldor A., Fontenay M., Gazit A., Osherov N., Gilon C., Levitzki A., Tobelem G. Inhibition of platelet-derived growth factor-induced mitogenesis and tyrosine kinase activity in cultured bone marrow fibroblasts by tyrphostins. Exp Cell Res. 1992 Apr;199(2):255–261. doi: 10.1016/0014-4827(92)90432-8. [DOI] [PubMed] [Google Scholar]
- Buchdunger E., Trinks U., Mett H., Regenass U., Müller M., Meyer T., McGlynn E., Pinna L. A., Traxler P., Lydon N. B. 4,5-Dianilinophthalimide: a protein-tyrosine kinase inhibitor with selectivity for the epidermal growth factor receptor signal transduction pathway and potent in vivo antitumor activity. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2334–2338. doi: 10.1073/pnas.91.6.2334. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chung C. K., Antoniades H. N. Expression of c-sis/platelet-derived growth factor B, insulin-like growth factor I, and transforming growth factor alpha messenger RNAs and their respective receptor messenger RNAs in primary human gastric carcinomas: in vivo studies with in situ hybridization and immunocytochemistry. Cancer Res. 1992 Jun 15;52(12):3453–3459. [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Druker B. J., Mamon H. J., Roberts T. M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989 Nov 16;321(20):1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Barr P. J., Williams L. T. Role of tyrosine kinase and membrane-spanning domains in signal transduction by the platelet-derived growth factor receptor. Mol Cell Biol. 1988 Dec;8(12):5126–5131. doi: 10.1128/mcb.8.12.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B. D., Smith I. E., Shorthouse A. J., Millar J. L. A comparison of the response of human lung carcinoma xenografts to vindesine and vincristine. Br J Cancer. 1982 Mar;45(3):466–468. doi: 10.1038/bjc.1982.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Gordon D., Schwartz S. M. Replication of arterial smooth muscle cells in hypertension and atherosclerosis. Am J Cardiol. 1987 Jan 23;59(2):44A–48A. doi: 10.1016/0002-9149(87)90175-5. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Ernlund A., Rorsman C., Rönnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989 May 25;264(15):8905–8912. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990 Jul;1(8):555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen R., Funa K., Wilander E., Bäckström T., Ridderheim M., Oberg K. Expression and prognostic significance of platelet-derived growth factor and its receptors in epithelial ovarian neoplasms. Cancer Res. 1993 Oct 1;53(19):4550–4554. [PubMed] [Google Scholar]
- Hermanson M., Funa K., Hartman M., Claesson-Welsh L., Heldin C. H., Westermark B., Nistér M. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992 Jun 1;52(11):3213–3219. [PubMed] [Google Scholar]
- Hermansson M., Nistér M., Betsholtz C., Heldin C. H., Westermark B., Funa K. Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7748–7752. doi: 10.1073/pnas.85.20.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Hinohara T., Selmon M. R., Braden L. J., Simpson J. B. Primary peripheral arterial stenoses and restenoses excised by transluminal atherectomy: a histopathologic study. J Am Coll Cardiol. 1990 Feb;15(2):419–425. doi: 10.1016/s0735-1097(10)80071-3. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., Heldin C. H., Westermark B. Antibodies against platelet-derived growth factor inhibit acute transformation by simian sarcoma virus. Nature. 1985 Oct 3;317(6036):438–440. doi: 10.1038/317438a0. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochem Biophys Res Commun. 1982 Jan 15;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Rutter W. J., Miller A. D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990 Feb;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. D., Haldeman B. A., Grant F. J., Murray M. J., Seifert R. A., Bowen-Pope D. F., Cooper J. A., Kazlauskas A. Platelet-derived growth factor (PDGF) stimulates PDGF receptor subunit dimerization and intersubunit trans-phosphorylation. J Biol Chem. 1991 May 15;266(14):8987–8992. [PubMed] [Google Scholar]
- Lee J. C., Hapel A. J., Ihle J. N. Constitutive production of a unique lymphokine (IL 3) by the WEHI-3 cell line. J Immunol. 1982 Jun;128(6):2393–2398. [PubMed] [Google Scholar]
- Maguire M. P., Sheets K. R., McVety K., Spada A. P., Zilberstein A. A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J Med Chem. 1994 Jul 8;37(14):2129–2137. doi: 10.1021/jm00040a003. [DOI] [PubMed] [Google Scholar]
- Meijer L., Arion D., Golsteyn R., Pines J., Brizuela L., Hunt T., Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989 Aug;8(8):2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Regenass U., Fabbro D., Alteri E., Rösel J., Müller M., Caravatti G., Matter A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int J Cancer. 1989 May 15;43(5):851–856. doi: 10.1002/ijc.2910430519. [DOI] [PubMed] [Google Scholar]
- Nistér M., Claesson-Welsh L., Eriksson A., Heldin C. H., Westermark B. Differential expression of platelet-derived growth factor receptors in human malignant glioma cell lines. J Biol Chem. 1991 Sep 5;266(25):16755–16763. [PubMed] [Google Scholar]
- Nistér M., Libermann T. A., Betsholtz C., Pettersson M., Claesson-Welsh L., Heldin C. H., Schlessinger J., Westermark B. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988 Jul 15;48(14):3910–3918. [PubMed] [Google Scholar]
- Olsen R., Melder D., Seewald M., Abraham R., Powis G. Staurosporine inhibition of intracellular free Ca2+ transients in mitogen-stimulated Swiss 3T3 fibroblasts. Biochem Pharmacol. 1990 Mar 1;39(5):968–972. doi: 10.1016/0006-2952(90)90216-8. [DOI] [PubMed] [Google Scholar]
- Partanen J., Armstrong E., Bergman M., Mäkelä T. P., Hirvonen H., Huebner K., Alitalo K. cyl encodes a putative cytoplasmic tyrosine kinase lacking the conserved tyrosine autophosphorylation site (Y416src). Oncogene. 1991 Nov;6(11):2013–2018. [PubMed] [Google Scholar]
- Pech M., Gazit A., Arnstein P., Aaronson S. A. Generation of fibrosarcomas in vivo by a retrovirus that expresses the normal B chain of platelet-derived growth factor and mimics the alternative splice pattern of the v-sis oncogene. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2693–2697. doi: 10.1073/pnas.86.8.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres R., Betsholtz C., Westermark B., Heldin C. H. Frequent expression of growth factors for mesenchymal cells in human mammary carcinoma cell lines. Cancer Res. 1987 Jul 1;47(13):3425–3429. [PubMed] [Google Scholar]
- Ross R. Platelet-derived growth factor. Lancet. 1989 May 27;1(8648):1179–1182. doi: 10.1016/s0140-6736(89)92760-8. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J., Taylor-Papadimitriou J. Production of PDGF-like growth factor by breast cancer cell lines. Int J Cancer. 1985 Aug 15;36(2):247–252. doi: 10.1002/ijc.2910360218. [DOI] [PubMed] [Google Scholar]
- Secrist J. P., Sehgal I., Powis G., Abraham R. T. Preferential inhibition of the platelet-derived growth factor receptor tyrosine kinase by staurosporine. J Biol Chem. 1990 Nov 25;265(33):20394–20400. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour L., Dajee D., Bezwoda W. R. Tissue platelet derived-growth factor (PDGF) predicts for shortened survival and treatment failure in advanced breast cancer. Breast Cancer Res Treat. 1993;26(3):247–252. doi: 10.1007/BF00665802. [DOI] [PubMed] [Google Scholar]
- Shamah S. M., Stiles C. D., Guha A. Dominant-negative mutants of platelet-derived growth factor revert the transformed phenotype of human astrocytoma cells. Mol Cell Biol. 1993 Dec;13(12):7203–7212. doi: 10.1128/mcb.13.12.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaras N. M., Sariban E., Bravo M., Pantazis P., Antoniades H. N. Constitutive production of platelet-derived growth factor-like proteins by human prostate carcinoma cell lines. Cancer Res. 1988 Apr 1;48(7):1930–1935. [PubMed] [Google Scholar]
- Smits A., Funa K., Vassbotn F. S., Beausang-Linder M., af Ekenstam F., Heldin C. H., Westermark B., Nistér M. Expression of platelet-derived growth factor and its receptors in proliferative disorders of fibroblastic origin. Am J Pathol. 1992 Mar;140(3):639–648. [PMC free article] [PubMed] [Google Scholar]
- Sonobe M. H., Bravo R., Armelin M. S. Imbalanced expression of cellular nuclear oncogenes caused by v-sis/PDGF-2. Oncogene. 1991 Sep;6(9):1531–1537. [PubMed] [Google Scholar]
- Thies R. S., Webster N. J., McClain D. A. A domain of the insulin receptor required for endocytosis in rat fibroblasts. J Biol Chem. 1990 Jun 15;265(17):10132–10137. [PubMed] [Google Scholar]
- Trinks U., Buchdunger E., Furet P., Kump W., Mett H., Meyer T., Müller M., Regenass U., Rihs G., Lydon N. Dianilinophthalimides: potent and selective, ATP-competitive inhibitors of the EGF-receptor protein tyrosine kinase. J Med Chem. 1994 Apr 1;37(7):1015–1027. doi: 10.1021/jm00033a019. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Platelet-derived growth factor in autocrine transformation. Cancer Res. 1991 Oct 1;51(19):5087–5092. [PubMed] [Google Scholar]
- Westermark B., Johnsson A., Paulsson Y., Betsholtz C., Heldin C. H., Herlyn M., Rodeck U., Koprowski H. Human melanoma cell lines of primary and metastatic origin express the genes encoding the chains of platelet-derived growth factor (PDGF) and produce a PDGF-like growth factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7197–7200. doi: 10.1073/pnas.83.19.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]