Abstract
Purpose
Fatty liver alters liver transporter expression. Caloric restriction (CR), the recommended therapy to reverse fatty liver, increases Sirtuin1 deacetylase activity in liver. This study evaluated whether CR and CR mimetics reversed obesity-induced transporter expression in liver and hepatocytes.
Methods
mRNA and protein expression was determined in adult lean (lean) and leptin-deficient obese (OB) mice fed ad libitum or placed on 40% (kCal) reduced diet. Hepatocytes were isolated from lean and OB mice, treated with AMP Kinase activators, and gene expression was determined.
Results
CR decreased Oatp1a1, Oatp1b2, and Abcb11 mRNA expression in lean, but not OB mice. CR increased Abcc2 mRNA OB livers, whereas protein expression increased in both genotypes. CR increased Abcc3 protein expression increased in OB livers. CR did not alter Abcc1, 4 and 5 mRNA expression in lean mice but decreased expression in livers of OB mice. CR increased Abcc4 protein in lean, but not OB mice.
Conclusions
CR restriction reversed the expression of some, but not all transporters in livers of OB mice. Overall, these data indicate a potential for CR to restore some hepatic transporter changes in OB mice, but suggest a functional leptin axis is needed for reversal of expression for some transporters.
Keywords: gene expression, liver, nuclear receptor, steatosis, transport
INTRODUCTION
Non-Alcoholic Fatty Liver Disease (NAFLD), or steatosis, is defined as lipid accumulation exceeding 5% by weight in hepatocytes in the absence of substantial alcohol intake, often with increased hepatic triglyceride accumulation (1). Metabolic syndrome (MetS), which is a cluster of risk factors for coronary heart disease (e.g. obesity, inflammation, dyslipidemia, and type-2 diabetes), is considered to be the underlying cause of NAFLD (2). It is estimated that more than 86% of US adults will be overweight or obese, and more than 50% obese by the year 2030 (3). In the United States, prevalence of NAFLD alone, or in combination with increased liver enzymes in serum, was 3.1% and 16.4% among adults, respectively (4). With no intervention, NAFLD can progress to Non-alcoholic steatohepatitis (NASH) and cirrhosis.
Epidemiological and clinical studies demonstrate changed pharmacokinetic and –dynamic parameters of some drugs in obese subjects (5). NAFLD and NASH are associated with altered pharmacokinetics of some drugs (e.g. ezetimibe, acetaminophen), as well as, altered endogenous metabolite levels, such as cholesterol and bilirubin (6,7). Acetaminophen-glucuronide concentration in plasma and urine was higher in children with NAFLD (6), similar to what has been reported in db/db mice that exhibit hepatic steatosis (8). This likely occurs because Phase-I, -II biotransformation enzyme, and drug transporter expression is altered compared to non-steatotic livers (9,10).
Dietary changes, such as caloric restriction (CR) and exercise, are the recommended therapeutic intervention to treat NAFLD and reverse hepatic fat accumulation (11). For example, a regimen of diet and exercise decreased hepatic steatosis and serum lipids in overweight subjects with NAFLD (12). Also, vigorous physical activity was associated with decreased adjusted odds of having NASH in adults with NAFLD (13).
Beneficial effects of CR are attributed to activation of Sirtuin 1 (Sirt1), a deacetylase that activates gluconeogenic and fatty acid oxidation gene expression via deacetylation and upregulation of the transcriptional co-activator, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc-1α) (14). The AMP Kinase (AMPK) secondary messenger pathway is an upstream activator of the Sirt-Pgc1-α cascade during CR. AMPK activates Sirt1 deacetylase in response to changes in redox status (NAD+/NADH ratio, AMP/ATP ratio) of the cell (15). Steatotic livers exhibit decreased AMPK pathway activity in rodents and increased activity can reverse fatty liver (16). CR alters activity and expression of various biotransformation enzymes, such as Sult2a1, Cyp2b10, Ugt1a1, Cyp4a14 in liver (17–19). However, to our knowledge, no studies have shown how CR actually affects drug transporter expression in livers of obese mice, which have hepatic steatosis and could better mimic the population most likely to undergo intervention.
In the present study we hypothesized that CR would reverse the transcription factor (TF) and drug transporter expression changes previously observed in obesity-induced hepatic steatosis (10,20). Constitutive androstane receptor (Car), Farnesoid x receptor (Fxr), Pregnane x receptor (Pxr), and Nuclear factor E2-related factor 2 (Nrf2) are transcription factors previously described to regulate transporter expression in liver (21). Given the well-described association of Nrf2 with Abcc transporter induction (22,23), emphasis was placed on the Nrf2 pathway in the present study, but Car, Fxr, and Pxr were also evaluated. Our data herein illustrate that CR differentially regulates TF, bio-transformation enzyme, and transporter expression in livers from lean and Lepob/ob mice. Furthermore, AMPK and Sirt1 activators differentially modulated transporter and NR expression in lean and steatotic hepatocytes.
MATERIALS AND METHODS
Animals and Treatments
Adult male lean and OB mice were chosen for this study because previous work revealed that transporter expression was markedly altered in livers of OB mice (20). Adult male C57BL/6 (lean, stock no. 000664) and Lepob/ob (OB, B6.V-Lepob/J, stock no. 0000632) mice were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6 mice used in this study as lean controls are the recommended vendor controls (Jackon Laboratories, Bar Harbor, ME). Mice were housed in a temperature-, light-, and humidity-controlled environment in cages with corn-cob bedding, and fed Harlan Teklad LM-485 Mouse/rat sterilizable diet (Harlan Laboratories, Madison, WI) ad libitum. After acclimation for 2 weeks, mice were transferred on to a purified diet (AIN93-G) obtained from Test Diet, IN, USA and allowed to acclimate for a period of 2 weeks. The average caloric consumption was calculated for each mouse over a period of 10 days. At 16 weeks of age, lean (n=7) and OB mice (n=7) were fed ad libitum, or lean (n=10) and OB mice (n=10) were fed a 40% reduced caloric diet for 10 weeks with access to water ad libitum. Body weight and food consumption were monitored at least once per week for the entire study. The study was terminated when weights remained similar for several weeks. Blood glucose measurement and necropsy was performed in the morning between 10–12 am. The study was carried at University of Rhode Island and IACUC approved.
Primary Mouse Hepatocyte Isolation
Primary mouse hepatocytes were obtained from livers of adult C57BL/6 and OB mice using a two-step collagenase perfusion; 1×106 cells/well in 2 mL completed medium (MEM supplied with 10% FBS) were seeded on collagen-coated 6-well plates. After cell attachment (~4 h), they were cultured in serum-free MEM containing 1% ITS supplement (Invitrogen, CA). Approximately 24 h post-plating, hepatocytes were treated with control (media or 0.01% DMSO), AICAR (0.5 mM), NAD+(5 mM) or Metformin (1 mM) for 6 h. Total RNA was isolated from TRIzol reagent (Invitrogen, CA) according to the manufacturer’s instructions.
RNA Isolation and mRNA Quantification
Total RNA was isolated from liver by phenol-chloroform extraction with RNAzol B reagent (Tel-Test Inc., Friends-wood, TX) according to the manufacturer’s instructions. RNA concentration was determined by measuring UV absorbance at 260 nm using NanoDrop™ and integrity was confirmed by formaldehyde gel electrophoresis. The total RNA samples were stored at −80°C until further use for analysis. The total RNA samples were analyzed for mRNA quantification using Branched DNA signal amplification assay (Quantigene 1.0 assay), obtained from Panomics Inc., CA, USA. RNA obtained from hepatocyte treatment was quantified using RT2-PCR methods as described in (10).
Tissue Homogenate, Membrane, and Cytosol Preparation
Liver membrane and cytosol fractions were obtained as previously described for assessing transporter expression (20,24). Briefly, tissues were homogenized 150 mM sucrose in 10 mM Tris–HCl (ST) buffer (1:9 ratio), pH 7.5, using a Potter Elvehjem motorized homogenizer. Homogenates were centrifuged at 100,000×g for 1 h at 4°C. The resulting supernatant (cytosolic fraction) was saved and pellets were re-suspended in ST buffer.
Western Blotting
Fifty microgram protein lysates were solubilized in Laemmli buffer containing β-mercaptoethanol and electrophoretically separated by SDS-PAGE (8% for Abccs and 10% for Oatps, 12% for cytosolic, nuclear and homogenate samples) at 200 V for 50 min and transferred onto a polyvinylidene difluoride membrane at 100 V for 30–45 min or at 75 V for 1.5 h. The membrane was blocked overnight with 5% Non-fat dry milk (NFDM) in phosphate-buffered saline with 0.05% Tween 20 (PBS/T) or 50 mM Tris, 150 mM NaCl, 0.05% Tween 20 (TBS/T). After blocking, the membrane was incubated with primary antibodies diluted in 5% NFDM in PBS/T or TBS/T for 2 h and subsequently with corresponding horseradish peroxidase labeled secondary antibodies also diluted in 5% NFDM in PBS/T as previously described in (8, 10). The blots were incubated in Pierce ECL-Plus western blot detection reagent (Thermo Fisher Scientific, Rockford, IL, USA) and exposed to X-ray film, developed, and visualized. The resulting autoradiography films were quantified using ImageQuant software (Bio-Rad, Hercules, CA). Oatp1a4 and 2b1 western blots were attempted, but not successful. Details of the antibodies used are as previously published (25).
Transcription Factor Binding Assay
Nuclear extracts were isolated from livers using a TF Procarta nuclear extraction kit (Panomics Inc, CA, USA) and protein concentrations were measured with BCA protein assay (Pierce, Rockford, IL, USA). The resulting fractions were checked for enrichment of nuclear proteins by western blot with Lamin B1 antibody. Nrf2, AhR, Pxr, Fxr, binding to described consensus sequences was quantified using a Procarta TF custom array (Affymetrix, CA) using 10 µg nuclear extract protein per sample according to manufacturer’s instructions and published work (10). Samples were analyzed using a Luminex Bio-Plex 200 array reader with Luminex 100 xMAP technology, and data were acquired using Bio-Plex Manager software (version 5.0). Data was acquired by a Luminex Bio-plex™ 200 Array reader with Luminex 100 X-MAP technology, and data were acquired using Bio-Plex Data Manager Software Version 5.0 (Bio-Rad, Hercules, CA).
Heatmap and Cluster Analysis
mRNA expression of genes quantified in vivo and in vitro were plotted on a heatmap using the R software environment (R Foundation for Statistical Computing, Vienna, Austria). The genes in lean and OB CR groups were normalized to their respective ad libitum controls and the fold changes relative to control were used for heat map generation. The spectrum of fold changes spans from −14 to +14 fold in reference to control.
Statistics
The statistical significance of differences was determined by a two-way ANOVA followed by a Duncan’s Multiple Range post-hoc test. Groups without a common letter are considered significantly different from each other (p≤0.05).
RESULTS
CR Decreases Body Weight and Hepatic Lipid Content in Lean and OB Mice
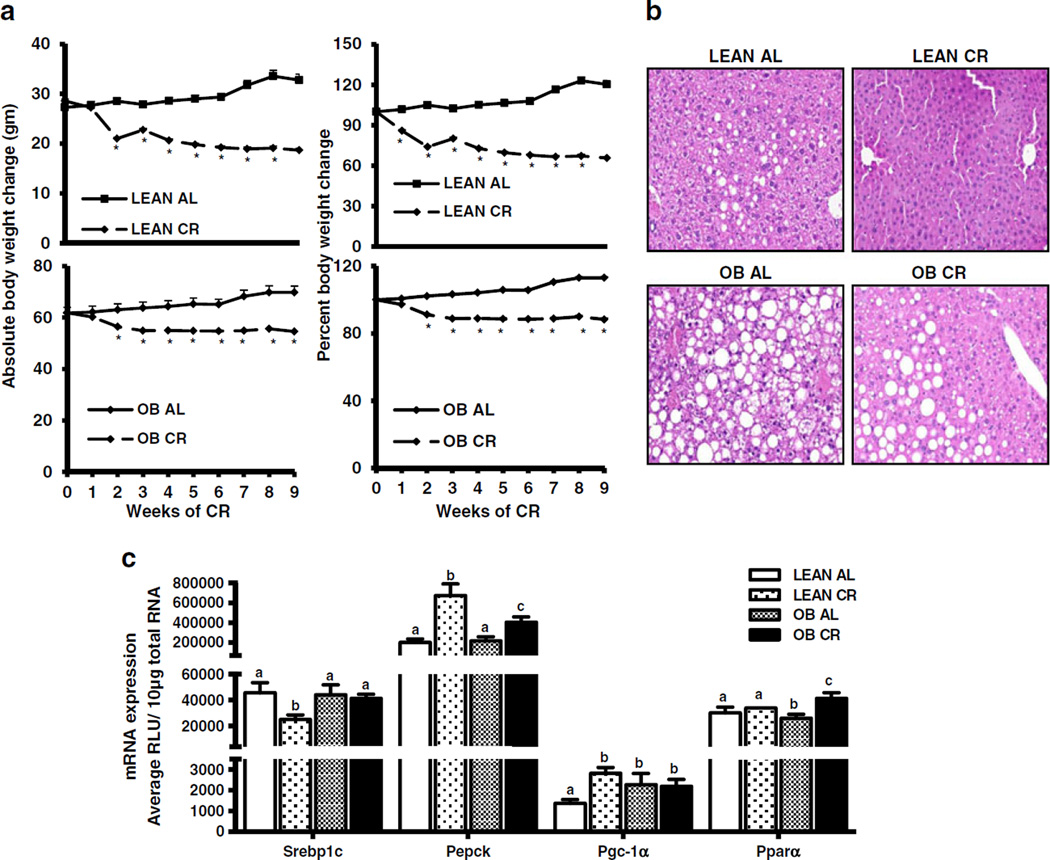
After 10 weeks of CR, average body weight decreased by 30% and 12% in lean and OB mice, respectively (Fig. 1a). CR decreased the average liver and liver-to-body weight ratio in both genotypes, with a greater liver weight decrease in lean mice (Table I). CR also decreased serum glucose (50% in leans), TG (50% in lean and 11% in OB), and increased Non-esterified fatty acid (NEFA) levels (60% in lean and ~100% in OB over the AL fed controls). Additionally, CR decreased micro and macrovesicular vacuolation in both lean and OB mice, but more so in lean mice. Similarly, glucagon expression was increased in OB mice undergoing CR (Table II). CR decreased serum insulin levels in both lean and OB mice (by 80% and 40%, respectively) and decreased leptin. Overall, OB mice were relatively more resistant to CR than lean mice with regard to weight loss and reversal of steatosis, which is consistent with a previous observation (26).
Fig. 1.
Effect of 40% kCAL reduced calorie diet in lean and obese mice. (a) Decreased body weight and percent body weight in C57BL/6 (lean) and OB mice after CR. During 10 weeks of 40% reduced caloric diet (CR), body weights of ad libitum (AL) fed and CR mice were determined weekly. The percent body weight changes were calculated with the body weight at initial time point considered as 100% and each mouse being its own control. (b) Morphological changes in livers of lean and OB mice after CR. Livers sections were stained with hematoxylin and eosin. (c) Induction of Pgc-1α, Pepck, Pparα, Car and downregulation of Srebp-1 mRNA expression in mouse liver after 10 weeks CR. Total RNA was quantified by the Branched DNA signal amplification assay and expressed as relative light units (RLU). The data is represented as average ± SEM (n =7 for AL and n =10 for CR). Groups without a common letter are considered significantly different from each other (p ≤0.05).
Table I.
Effect of CR on Body Weight, Liver-to-Body Weight Ratio, and Serum Biochemistry
| Strain | AL/CR | Average liver wt (g) |
Average liver(g) to body weight (g) ratio |
Serum Glucose (mg/dl) | Serum Triglyceride (mg/dl) |
Serum NEFA(mmol/dl) |
|---|---|---|---|---|---|---|
| LEAN | AL | 1.385±0.05a | 0.042 ±0.0006a | 202.4 ±8.1a | 66.8±6.8a | 0.03 ± 0.002a |
| CR | 0.66 ±0.02b | 0.035 ±0.0009b | 109.8 ±7.45b | 33.9± 1.1b | 0.051 ±0.002b | |
| OB | AL | 5.17±0.4c | 0.0728 ± 0.004c | 184.3 ±8.8c | 71.37±5.6a | 0.028 ±0.0026a |
| CR | 2.28 ±0.13d | 0.0418 ± 0.002a | 222.4 ± 19c | 64.1 ±2.7a | 0.059 ±0.0038c |
Adult lean and OB mice (n=7–10/group) were fed (ad libitum) or put on 40% reduced caloric diet for 9 weeks. At the end of 9 weeks, mice were euthanized. Average liver weights, liver-to-body weight ratios, serum glucose (mg/dl), serum triglycerides (mg/dl) and serum NEFA (mmol/dl) were determined. P <0.05 was considered statistically significant. Groups without a common letter are significantly different
Table II.
Effect of CR on Serum Metabolic Hormones
| Strain | AL/CR | Glucagon | Insulin | Leptin | Resistin |
|---|---|---|---|---|---|
| LEAN | AL | 2121.7±484a | NA | 10486±2376.48a | 14355 ± 1679.5a |
| CR | 388.58 ±57b | NA | 267.22 ±56.78b | 18178 ± 1672b | |
| OB | AL | 17834 ± 3240c | 43.98 ±4.67 a | ND | 20875 ± 1549.5c |
| CR | 6735.8 ±830d | 85.26 ±24b | ND | 25365 ± 2645.83d |
Adult Lean and OB mice (n=7–10/group) were fed (ad libitum) or put on 40% reduced caloric diet for 9 weeks and average serum glucagon, insulin, leptin, and resistin concentrations (pg/ml) were determined. P <0.05 was considered statistically significant. Groups without a common letter are significantly different. ND, not detectable
CR Decreases Lipogenic Gene and Increases Gluconeogenic Gene Expression
CR induces glucagon secretion to increase mitochondrial biogenesis and up regulate glucose production. This well characterized response results in the induction of genes needed to increase gluconeogenesis (27). Therefore, genes known to be regulated by CR were measured to further confirm response to the food restriction. mRNA expression of the lipogenic master regulator, Srebp-1c, was decreased in lean mouse livers but remained unchanged in OB mouse liver after CR (Fig. 1c). CR increased expression of gluconeogenic genes Pgc-1α and Pepck in lean mouse livers (2 and 3.5 fold, respectively), however this increase was decreased in OB mouse livers (Fig. 1c), which has been previously described (14). CR did not increase Ppar-α mRNA expression in leans, but did in OB mice.
CR Alters mRNA Expression of Various Drug Biotransformation Enzymes, Transporters and Transcription Regulators in Livers of Lean and OB Mice
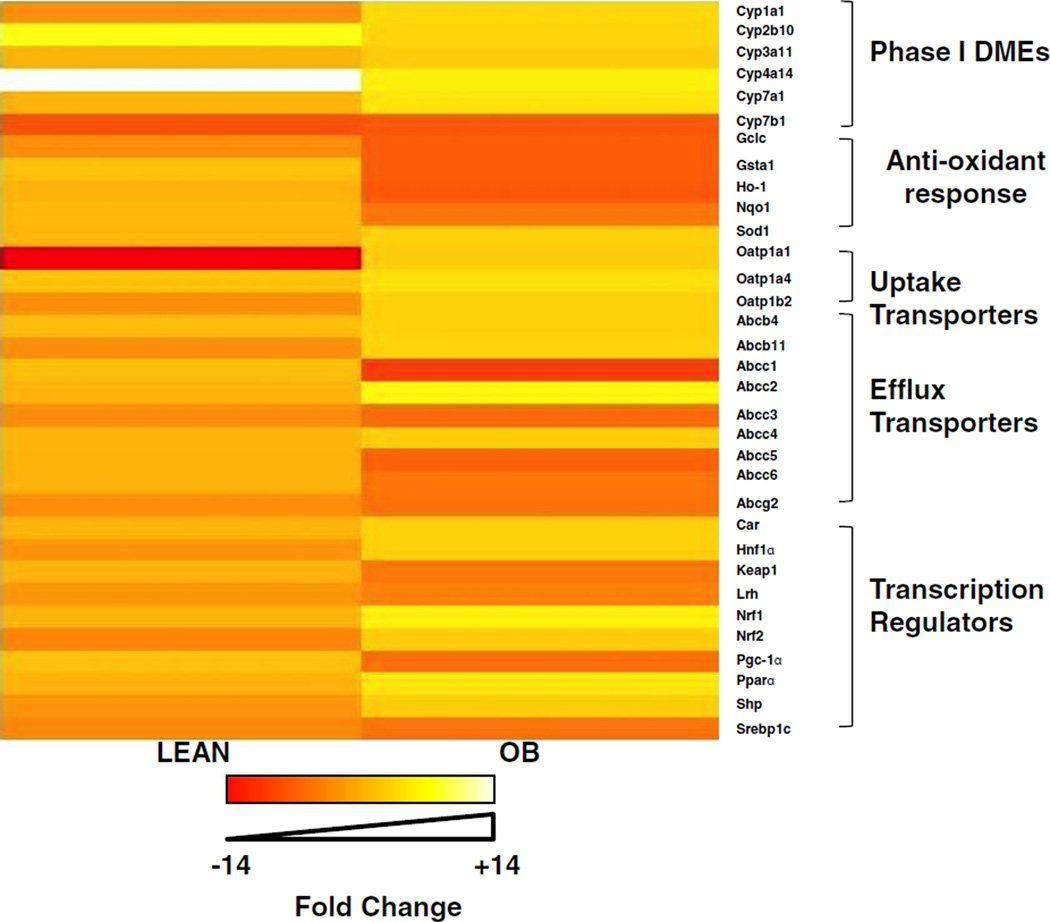
The effect of CR on mRNA expression of various Phase I drug biotransformation enzymes, Phase II conjugation enzymes, antioxidant enzymes, drug transporters, and transcriptional regulators in livers from lean and OB was determined and heat maps were generated using R-software. As seen in Fig. 2 and Table III CR affected the gene expression differently in lean and OB livers. Overall, there was little relative change in lean mice after CR, whereas multiple genes examined had decreased expression in livers of OB mice after CR.
Fig. 2.
Heatmap analysis of biotransformation enzyme and transporter expression patterns in lean and obese mouse livers after CR. Total RNA was isolated from lean and OB mouse livers after CR and Phase I, Phase II biotransformation enzyme, and transporter expression was determined using the the Branched DNA signal amplification assay. Heat maps represent the spectrum of fold change compared to ad libitum fed mice, spanning from −14 to +14 fold.
Table III.
Summary of Gene Expression Changes in Lean and Obese Mice Following Caloric Restriction
| Transcript | Lean CR | OB CR |
|---|---|---|
| Transcription factors and metabolic regulators | ||
| Car | 1.15 | 1.15 |
| Hnf1alpha | −1.12 | 1.09 |
| Keap1 | 1.08 | 1.00 |
| Lrh | −1.06 | −1.31 |
| Nrf1 | 1.17 | 1.83 |
| Nrf2 | −2.22 | 1.03 |
| Pepck | 3.32 | 1.85 |
| Pgc-1 alpha | 2.03 | −1.18 |
| Ppar alpha | 1.11 | 1.58 |
| Shp | −1.06 | 1.03 |
| Srebp1c | −1.83 | −1.07 |
| Phase-I biotransformation enzymes | ||
| Cyp1a1 | −1.5 | 1.31 |
| Cyp2b10 | 6.97 | 1.23 |
| Cyp3a11 | 1.12 | 1.00 |
| Cyp4a14 | 13.77 | 1.78 |
| Cyp7b1 | −6.27 | −1.75 |
| Cyp7a1 | 1.05 | 1.56 |
| Antioxidant enzymes | ||
| Gclc | −1.41 | −1.64 |
| Gsta1 | 2.09 | −1.65 |
| Ho1 | 1.03 | −1.75 |
| Nqo1 | 1.51 | −1.01 |
| Sod1 | 1.44 | 1.12 |
| Uptake Transporters | ||
| Oatp1a1 | −14.91 | 1.02 |
| Oatp1a4 | 2.26 | 1.43 |
| Oatp1b2 | −1.46 | 1.11 |
| Efflux transporters (ABC family) | ||
| Abcb4 | 1.79 | 1.08 |
| Abcb11 | −1.54 | 1.22 |
| Abcc1 | 1.28 | −2.48 |
| Abcc2 | 1.19 | 1.98 |
| Abcc3 | −1.59 | −1.31 |
| Abcc4 | 1.29 | 1.07 |
| Abcc5 | 1.11 | −1.51 |
| Abcc6 | 1.16 | −1.08 |
| Abcg2 | −1.45 | −1.14 |
The following table represents fold changes (increase or decrease) in expression of various drug metabolizing and transporter genes in livers of lean and OB mice upon 40% CR. The data is represented as fold change in gene expression over the respective ad libitum fed controls
Effect of CR on Phase I Biotransformation Enzyme Expression in Livers of Lean and OB Mice
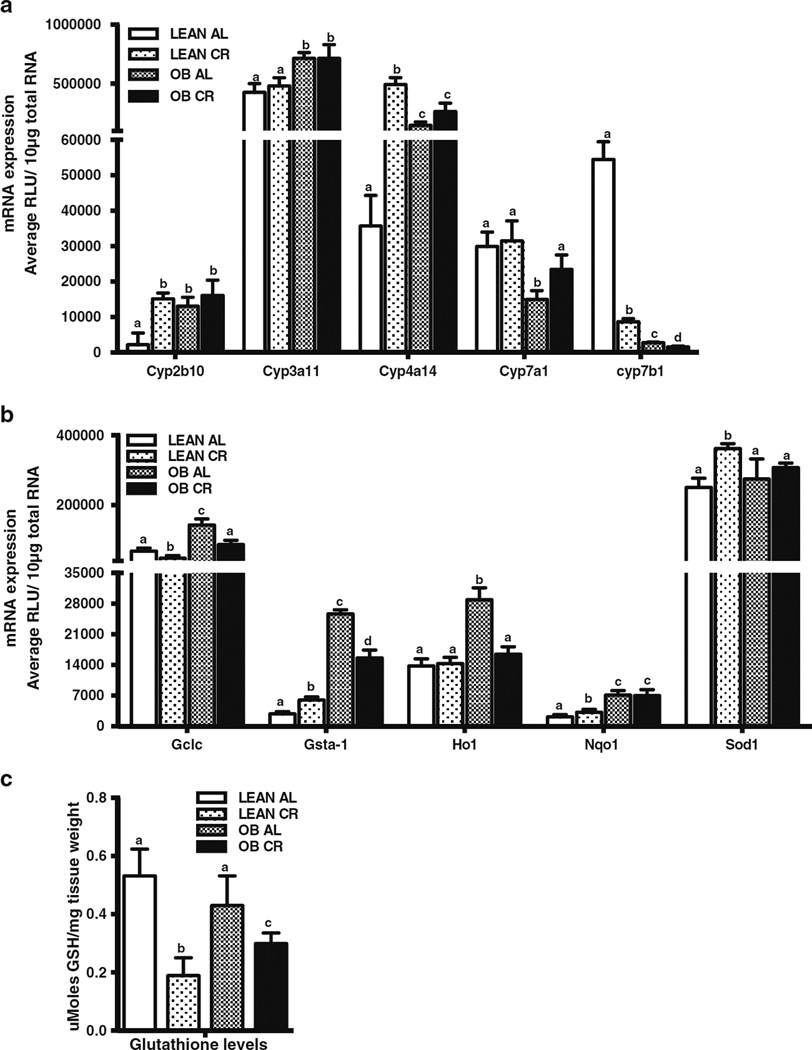
Phase I biotransformation enzymes are typical downstream target genes for NRs (28,29) and are often measured as indirect markers of NR activation/repression. Therefore, several Cyps described to be regulated via NR activation were measured in lean and OB mice fed AL or after CR. Consistent with previous observations (20), Cyp2b10, Cyp3a11 and Cyp4a14 basal expression was higher in OB mice compared to lean mice (Fig. 3a). In contrast, Cyp7a1 and Cyp7b1 basal expression was lower in OB mouse livers as compared to lean mice (Fig. 3a). CR increased expression of Cyp2b10 and Cyp4a14 mRNA expression in lean, but not in OB mouse livers. Cyp3a11 mRNA expression was similar between AL and CR mice. In contrast, CR significantly decreased expression of Cyp7b1 in both lean and OB mouse livers (Fig. 3a). CR did not affect Cyp7a1 mRNA expression in lean mice, but increased Cyp7a1 mRNA expression in livers from OB mice (Fig. 3a).
Fig. 3.
Effect of CR on expression of Phase-I and-II biotransformation enzymes and antioxidant genes in lean and obese mouse livers. mRNA expression was quantified by the Branched DNA signal amplification assay. (a) Expression of Phase-I biotransformation enzymes in lean and OB mouse livers after CR. (b) Changes in expression of Phase-II biotransformation antioxidant enzymes in lean and OB mouse livers after CR. The data is represented as average ± SEM (n =7 for AL and n =10 for CR). Means with the same letter are not significantly different from each other (P <0.05). (c) Effect of CR on hepatic glutathione (GSH) levels in lean and OB mice. The data is represented as average ± SEM moles GSH/mg tissue (n =7 for AL and n =10 for CR). Groups without a common letter are considered significantly different from each other (p ≤0.05).
Effect of CR on Antioxidant and Phase II Biotransformation Enzyme Expression in Livers of Lean and OB Mice
Nuclear factor-E2-related factor 2 (Nrf2) is a transcription factor, which upregulates antioxidant enzymes (e.g. Super-oxide dismutase, Sod-1; Glutamate cysteine ligase catalytic subunit, Gclc), biotransformation enzymes (e.g. Glutathi-one S-transferase a1, Gsta1; Heme oxygenase 1, Ho-1; Nad(p)h:oxidoreductase 1, Nqo1) and ATP Binding Cassette transporters (Multidrug resistance-associated proteins) (30). Multiple studies have shown an association between Nrf2 activation and ATP Binding Cassette (Abcc) transporter induction (22) . Moreover, Nrf2 binding and target genes are increased in steatotic livers (10,20,31). Nrf2 and Nrf2 target gene expression is increased in livers of OB mice (10,31). Compared to lean, OB mouse livers had increased Gclc, Gsta1, Nqo1, and Ho-1 mRNA expression (Fig. 3b). CR significantly increased Gsta-1, Nqo1, and Sod1 in livers of lean mice. In livers of OB mice, CR decreased Gsta-1 expression, but not Nqo1 and Sod1 expression (Fig. 3b). Ho-1 expression was similar in livers of lean mice fed AL or placed on CR, whereas CR decreased Ho-1 by about 50% in livers of OB mice. Consistent with the data in Fig. 3b, CR decreased hepatic glutathione (GSH) by 60% in lean and 30% in OB mice (Fig. 3c).
Effect of CR on Oatp Expression in Livers of Lean and OB Mice
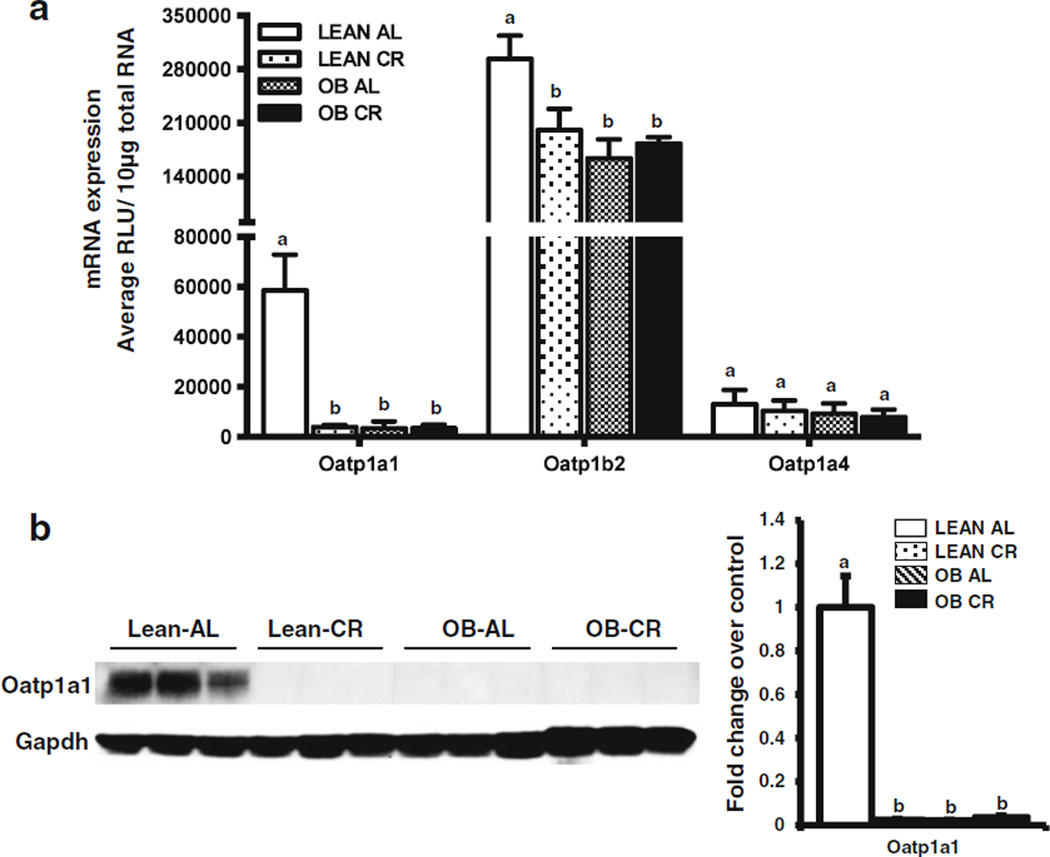
Consistent with previous observations (20) Oatp1a1 and 1b2 mRNA expression was lower in livers of OB than lean mice and Oatp1a4 expression was similar between the two genotypes (Fig. 4a). CR decreased Oatp1a1 and 1b2 mRNA, but not 1a4 mRNA expression in livers of lean mice (Fig. 4a) (19). Oatp1a1 and 1b2 expression remained low in livers OB mice even after CR, perhaps indicating importance for leptin-associated regulation. Lastly, Oatp1a1 protein expression was relatively undetectable in livers from OB mice, consistent with Cheng et al., 2008. Oatp1a1 protein expression was undetectable in livers from lean and OB mice after CR (Fig. 4b).
Fig. 4.
Effect of CR on liver uptake transporter expression in lean and obese mice. (a) Relative mRNA expression was quantified by the Branched DNA signal amplification assay. The data is represented as mean relative light units (RLU)/ 10 µg total RNA ± SEM (n =7 for AL and n =10 for CR) and groups without a common letter are considered significantly different from each other (p ≤0.05). (b) Membrane proteins were isolated from livers of AL and CR lean and OB mice. Protein expression of Oatp1a1 was quantified by western blot. The data is representative of three individual protein quantifications.
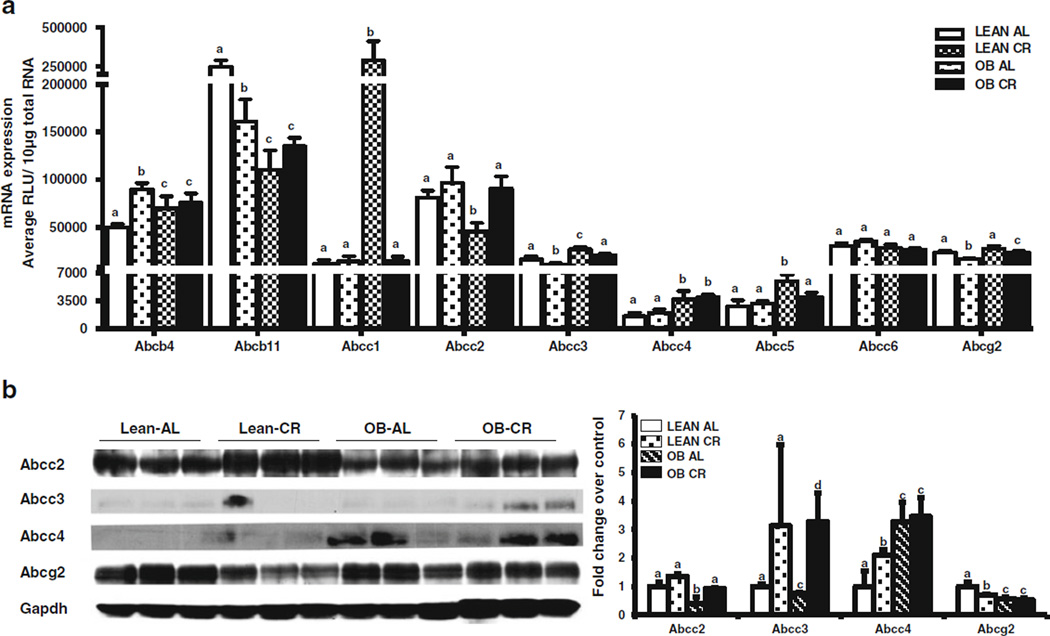
Effect of CR on Abc Transporter Expression in Livers of Lean and OB Mice
OB mice fed ad libitum had higher liver expression of several Abc transporters (e.g. Abcb4, Abcc1, Abcc3–5) and lower expression of others (e.g. Abcb11, Abcc2) (Fig. 5a) compared to AL fed lean mice. Abcc4 protein expression increased, whereas Abcc2 and 3, Abcg2 protein expression was decreased in livers of OB AL fed mice compared to lean AL fed mice (Fig. 5b).
Fig. 5.
Effect of CR on liver efflux transporter mRNA and protein expression in lean and obese mice. (a) Abcb4, Abcb11, Abcc1–6, Abcg2 mRNA was quantified by the Branched DNA signal amplification assay. The data is represented as mean relative light units (RLU)/10 µg total RNA ± SEM (n =7 for AL and n =10 for CR). (b) Relative Abcc2–4 and Abcg2 protein expression quantified by western blot. The data is representative of three individual protein quantifications. Groups without a common letter are considered significantly different from each other (p ≤0.05).
Figure 5a and b depict CR effects on Abc mRNA and protein expression in liver. CR induced Abcb4 in livers of lean mice, but not OB mice. CR decreased Abcb11 mRNA expression in lean, but not OB mice. CR did not change Abcc1 expression in leans, but decreased it in OB mice. Abcc2 mRNA and did not change after CR in lean mice, but increased in OB mice (Fig. 5a & b). CR decreased Abcc3 mRNA expression in lean and OB mice while protein expression increased in Lean CR livers, but this was not observed at the protein level (Fig. 5a & b). CR did not affect Abcc4 mRNA expression in lean or OB mice, but did increased protein expression in livers of lean mice. Abcc5 mRNA expression was similar between fed and CR lean mice, but decreased in OB mice after CR. Abcc6 expression was similar between all groups. CR decreased Abcg2 mRNA expression in lean and OB mice, an also Abcg2 protein expression in lean mice (Fig. 5b).
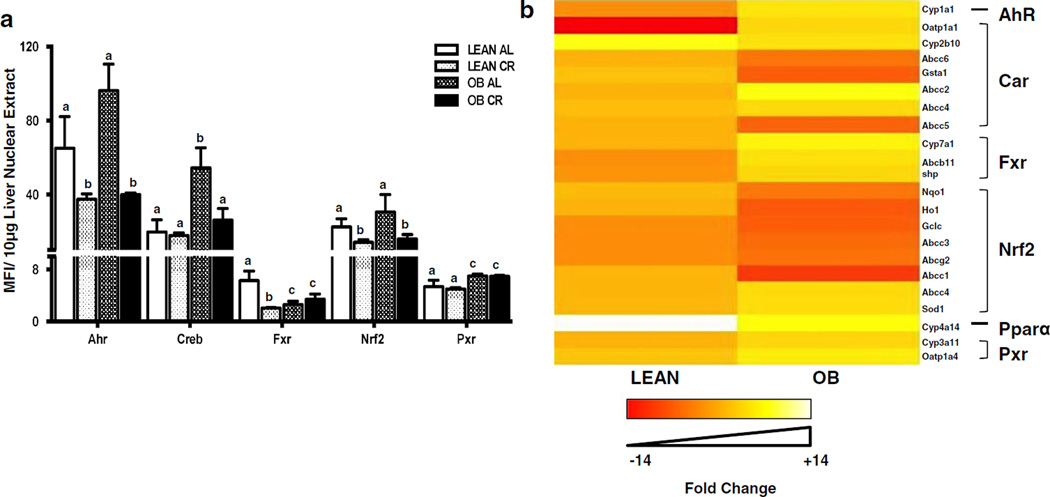
Transcription Factor Binding Activity in Lean and OB Mouse Livers After CR
Transcription factor binding to prototypical consensus sequences was also determined in nuclear fractions isolated from liver (Fig. 6a). Creb, and Pxr binding was increased in liver nuclear fractions from OB mice compared to lean mice, with a trend for increased Ahr binding (p<0.07). In lean mice, CR decreased Ahr, Fxr, and Nrf2 binding, but did not affect Creb or Pxr binding. In OB mice, CR decreased Ahr, Creb, and Nrf2 binding.
Fig. 6.
Effect of CR on transcription factor activity and relative expression patterns of prototypical target genes of transcription factors in livers of lean and obese mice. (a) CR changes the transcription factor binding activity in livers of lean and obese (OB) mice. Ahr, Creb, Fxr, Nrf2 and Pxr binding activity in nuclear fractions was determined using a Procarta TF binding assay. The data is represented as average mean fluorescence intensity (MFI)/10 µg protein ± SEM (n =7 for AL and n =10 for CR) fold change over the fed controls. Groups without a common letter are considered significantly different from each other (p ≤0.05). (b) CR differentially affects expression of prototypical target genes of transcription factors in lean and OB mouse livers. Heat maps were created for visual pattern analysis using R statistical software. Gene expression is represented as fold change of lean and OB CR mice over respective AL groups.
To determine whether prototypical targets of these transcription factors were affected similarly by CR, a heat map was created to better visualize differences how CR affects transcriptional pathways in lean and OB mice (Fig. 6b). For the most part, prototypical Ahr, Car, Fxr, Pxr, and Nrf2 target genes were regulated similarly in lean livers. However, in the OB livers, the CR effect was not consistent among the transcriptional pathways. For example, CR induced some Car target gene expression, but not others. In contrast, CR predominantly decreased the expression of most Nrf2 target genes.
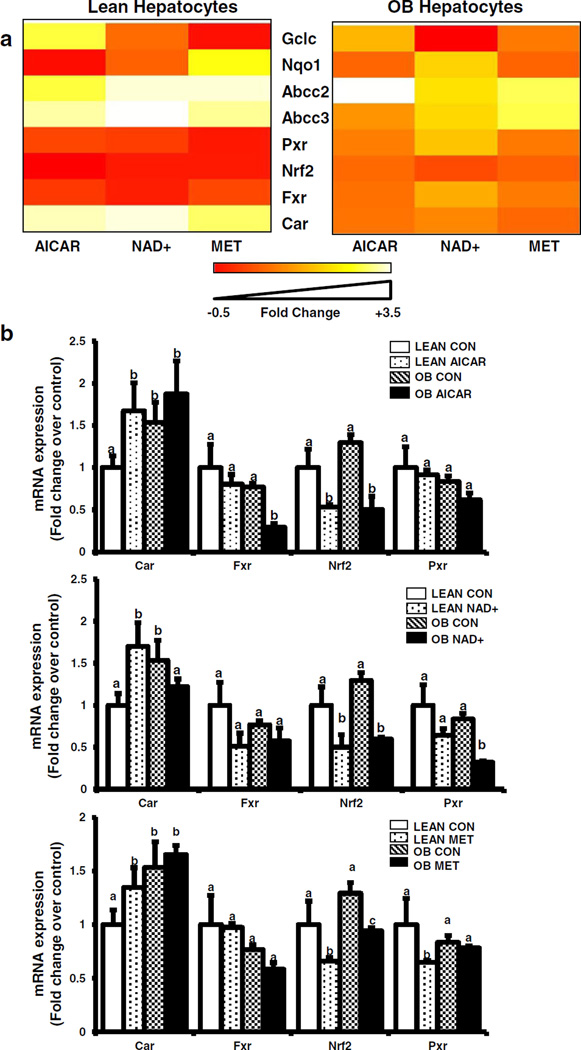
Effect of AMPK and Sirt1 Activators on Abcc and NR Induction in Hepatocytes Obtained From Lean and OB Mice
The AMPK pathway is an important CR signal transduction pathway upstream of transcription factor activation. Hence, primary mouse hepatocytes were isolated from 11-week old lean and OB mice and treated with pharmacological AMPK (AICAR, Metformin) and Sirt1 (NAD+) activators (Fig. 7). Heat maps illustrate the effect of AICAR, NAD+, and Metformin on transcription factor, Abcc, and Nrf2 target gene expression. Overall, the treatments downregulated expression of many genes in hepatocytes from lean mice, but less so in hepatocytes from OB mice. Abcc2 and 3 mRNA expression was increased about 3 fold in hepatocytes isolated from OB mice compared to lean mice (Fig. 7a and Supplementary Fig. 1), which is similar to mRNA expression observed in intact livers from lean and OB mice of the same age (20). Treatment with AMPK activators (AICAR, NAD+) significantly increased mRNA expression of Abcc2 and Abcc3 in lean mouse hepatocytes (Fig. 7a and Supplementary Fig. 1). As opposed to lean hepatocytes, AICAR and NAD+ treatment decreased Abcc2 and 3 mRNA expression in steatotic hepatocytes, thus reversing their expression back to normal levels (Supplementary Fig. 1). The effect of AICAR, Metformin, and NAD+ treatment on NR expression was also evaluated (Fig. 7b). Car mRNA expression was increased about 50% above control in hepatocytes isolated from OB mice compared to lean mice (Fig. 7a), which is similar to what was observed in intact livers from lean and OB mice of the same age (10). Fxr, Nrf2, and Pxr expression was equivocal between lean and OB mice, which is different from what was observed in intact livers from lean and OB mice of the same age (10). AICAR, Metformin, NAD+ treatment increased CAR, decreased Nrf2, and did not change Fxr expression in lean hepatocytes. In lean hepatocytes, Pxr expression slightly decreased with Metformin treatment, and did not change with AICAR or NAD+ treatment. In OB hepatocytes, Car expression was not affected by AICAR or NAD+ treatment, but slightly decreased by Metformin treatment. AICAR decreased Fxr, but Metformin and NAD+ did not in OB hepatocytes. AICAR, Metformin, and NAD+ treatment decreased Nrf2 mRNA expression in OB hepatocytes. In OB hepatocytes, Pxr expression was decreased by NAD+ treatment, but not AICAR or Metformin treatment.
Fig. 7.
Effect of AMPK pathway activation on transcription factor expression in hepatocytes from lean and obese mice. (a) AMPK activators differentially transcription factor expression in lean and OB mice. Heat maps represent as fold change of lean and OB CR mice over respective AL groups. (b) mRNA quantification of transcription factor expression. Primary hepatocytes were obtained from lean and obese (OB) mouse livers and treated with media alone, AICAR (0.5 mM), NAD+(5 mM), or Metformin (1 mM) for 6 h. Total RNA was isolated 6 h after treatment and Car, Fxr, Nrf2, and Pxr mRNA expression was quantified using quantitative PCR. The data is represented as fold change over the fed controls and target gene expression was normalized to 18S rRNA. Groups without a common letter are considered significantly different from each other (p ≤0.05).
DISCUSSION
The study herein demonstrates that CR affects the expression of some drug transporters in conjunction with alterations in NR binding and NR-target gene expression. As CR is known to activate AMPK and Sirt1 activity, the work herein demonstrates that CR mimetics, such as AICAR, Metformin, and NAD+ can modulate transporter and NR expression in hepatocytes from lean and OB mice. Treatment with AICAR, Metformin, and NAD+ indicated that AMPK and Sirt1 are potential upstream regulators of NR and Abcc transporter expression in hepatocytes. Lastly, our work illustrates that CR produced different gene expression patterns in the lean or obese condition in vivo, and that leptin-associated regulation for some transporters (e.g. Oatp1a1) was present, but not for others (e.g. Abcc2)
In lean mice, CR increased Cyp2b10 and Cyp4a14 mRNA expression, which is similar to previously published observations (19,32). Consistent with induction of Cyp2b10, Car mRNA expression was increased in lean mice, and induced in hepatocytes from lean mice treated with AICAR and NAD+, consistent with a previous report (33). Cyp7a1 mRNA expression was unchanged in livers from lean mice after CR, which corresponded to minimal or no changes observed in Fxr mRNA expression of hepatocytes treated with AICAR, NAD+, or Metformin. In lean mice, mRNA expression of Phase-II and antioxidant enzymes, such as Gsta-1, Nqo1, and Sod1 increased after CR, whereas Gclc expression decreased and Ho-1 mRNA expression remained unchanged. Induction of Nqo1 and (Gsta-1) in livers of lean mice is consistent with a previous report (34). CR decreased mRNA expression of Oatp1a1, Oapt1a4 and Oatp1b2 in lean mice, with protein expression following a similar trend in general. These observations, in part, agree with previously published data by (19). mRNA expression of efflux transporters of the ABC family did not follow a consistent pattern. For example, in lean mice, Abcb4 and Abcc4 mRNA expression increased after CR, whereas Abcb11 and Abcg2 expression decreased and Abcc1, 5 and 6 expression remained unchanged. On the other hand, Abcc2 and 4 protein expression in livers from lean calorically restricted mice increased. Abcg2 protein expression was similar to the observed mRNA expression, being decreased in expression after CR.
The effect of CR on transporter expression in liver differed in OB mice compared to leans. In livers of OB mice, CR did not cause marked changes in mRNA expression of Phase-I enzymes or some transporters as compared to AL livers. For example, Cyp2b10, Cyp3a11, Cyp4a14, Oatp1a1, Oatp1a4 and Oatp1b2, Abcb4, Abcb11, Abcc4 and Abcc6 mRNA expression remained unchanged in livers of OB mice after CR. In addition, CR decreased the expression of multiple Nrf2 regulated genes, such as Gsta-1, Gclc, Ho-1, Abcc1, 3, and 5 in livers of OB mice.
The Nrf2-ARE pathway is an upstream regulator of various Phase-II biotransformation enzymes, as well as, Abc transporters along with a battery of genes responsive to oxidative stress. It has been previously reported that CR restriction induces Nqo1 expression via an Nrf2 dependent mechanism (34). The present study had similar observations in lean mice that underwent CR, which were consistent with the latter study – induction of Nqo1 in liver. However, the present observations point to decreased Nrf2 target gene expression and Nrf2 binding in livers of OB mice after CR. First, Ob/ob mice exhibit increased Nrf2 gene expression and markers of oxidative stress, which can be decreased by treatment with antioxidant compounds (20,35). CR decreases markers of lipid oxidation in obese mice (36) Therefore, a likely reason in our study is that CR decreased fat content and inflammation-induced oxidative stress, which in turn, decreased Nrf2 activation. Second, AMPK and Sirt1 can potentially modulate Nrf2 binding activity. For example, Sirt1 has been described to decrease Nrf2 binding and anti-oxidant response element activation (37), whereas P300 activators increase Nrf2 target gene activation (38). This could be through acetylation sites present on the Nrf2 protein (38). So, perhaps, CR induced Sirtuin activity, which resulted in decreased Nrf2 acetylation along with Nrf2 binding to various antioxidant response elements. The effect of CR on Abcc3, Abcg2, Gclc was similar between OB and lean mouse livers, despite lack of Pgc-1α induction in OB mice. Third, decreased recruitment of Pgc-1α to the Nrf2 transcriptional complex could result in decreased Nrf2 binding. Additionally, recruitment of different cofactors within the transcriptional complex of Nrf2 at the promoters of target transporters such as GCN5 (39), p300/cbp (38) in the OB livers, could affect activation of Pgc-1α and thus the target gene transcription. Last, as significant cross-talk between the AhR and Nrf2 transcriptional pathways has been decribed (40), it should be considered whether decreased AhR binding could result in decreased Nrf2 target gene expression or vice versa. As increased AhR activity has been implicated in steatosis (41), thus the effect of CR mimetics on AhR activity should be considered.
Activation of metabolic pathways during CR is mediated physiologically by hormones, such as glucagon, insulin, and leptin. CR is known to increase serum glucagon, decrease insulin, as well as, reverse increased leptin levels present in obesity (42). These conditions of low glucose, increased pyruvate, NAD+/NADH ratio, and glucagon levels during CR lead to activation of Sirt1/Pgc-1α cascade which in turn activates the expression of fatty acid oxidation regulators such as Pparα along with gluconeogenic genes such as Pepck (43,44). The AMPK pathway is regarded as one of the major secondary pathways delegating intracellular signals of CR, inhibiting gluconeogenic pathway activity and increasing fatty acid oxidation, as well, as glucose uptake into cells. Sirt1/Pgc-1α cascade and AMPK pathway can regulate activation of one another by phosphorylation and acetylation dependent mechanisms as shown by (45–47). Pparα and γ regulate pathways responsible for fatty acid oxidation and synthesis respectively (48,49). PPARα is activated in models of CR via activation by Pgc-1 α via Protein Kinase A and AMPK secondary messenger pathways (43). In the study herein, CR likely activated Pparα, as observed by increased mRNA expression of Pparα target gene Cyp4a14 in lean mouse liver along with an important coactivator Pgc-1α (18,32). With leptin signaling being non-functional in OB mice, the signal transduction is hampered and hence Cyp4a14 and Pgc-1α (Figs. 1c and 3a) were induced in OB livers, as previously described (50). Consistent with these observations, CR increased Abcc4 protein expression in livers of wild type mice, which could suggest Pparα as an upstream regulator of Abcc4 expression during CR. Previous studies have described Pparα mediated induction of Abcc4 protein expression in mouse liver (51). As endogenous metabolites, such as cAMP and cGMP, are substrates Abcc4 (52), the increased Abcc4 protein expression could be in response to increased liver concentrations of these substrates during CR (53). In OB mice, CR does not increase Cyp4a14 levels, which is likely due to lack of a functional leptin axis. The leptin axis is needed for proper liver responses to nutrient deprivation, as previously described (50). Lack of Abcc4 induction in OB mice after CR is consistent with lack of Cyp4a14 and Pgc1a induction, suggesting that a functional leptin axis is needed for Abcc4 induction. Biotransformation enzymes and transporters are regulated by various nuclear receptors and transcription factors such as Ahr, Car, Fxr, Pxr, and (29). Apart from metabolic pathways, activation of Pgc-1α during CR is likely a critical factor in also regulating expression biotransformation enzymes. Pgc-1α is a known co-activator of Car, Fxr, Hnf4α, Pxr pathways (32). In lean mice, CR induced mRNA expression of Car, Cyp2b10. Car is known to activate Abcc2 by certain xenobiotic compounds (21). As observed in Fig. 5a, Abcc2 expression follows a pattern similar to Car and Cyp2b10 (Fig. 3).
The heatmap analyses illustrate that the CR elicits a different gene expression pattern in livers of lean and OB mice. As leptin deficient OB mice, were used to model obesity and steatosis, the differential expression could be due to the influence of leptin on the overall regulation of NR activity, expression, and regulation of the downstream targets. The data suggest an importance of the leptin axis in regulating downstream effects of CR on expression of transcription factors and drug processing genes. Serum levels of leptin are known to correlate with the severity of steatosis (54,55). Hepatic and central leptin resistance is known to cause insulin resistance and obesity (56). CR and exercise being AASLD recommended regimen to reverse NAFLD, the observations with leptin deficient models demonstrates a potential difference in the steatotic human population response to drug biotransformation enzyme and transporter changes after CR. Liver fat content markedly decreased in the steatotic liver after CR, but the biotransformational pathways are not completely reversed in these models, suggesting that a functional leptin axis is needed for reversal to occur. This could potentially explain why differences in effects on gene expression were observed between in vivo experiments compared to hepatocytes treated with AMPK activators.
Mimicking CR in vitro is deemed difficult to mimic due to the complex nature of signaling pathways involved in the effect. Studies have demonstrated that sera obtained from animals undergoing CR, mimic CR induced resistance to oxidative stress in hepatocytes (57). AMPK activators have been used to mimic AMPK activation and AMPK dependent Sirt1 activation during CR and their convergent beneficial downstream effects (44). Differences in NR and transporter expression were not entirely consistent between CR data from the in vivo study and in hepatocytes treated with caloric restriction mimetic compounds. When comparing both sets of experiments, CR induced Cyp2B10 and Car in livers of lean mice with virtually no expression change observed in livers of OB mice, which was consistent with increased Car expression in the hepatocytes treated with AMPK activators. However, in general, CR did not markedly affect the expression of many biotransformation, antioxidant enzyme or transporter genes in lean mice (e.g. expression change was less than 1 fold), whereas treatment of hepatocytes with AMPK or Sirt1 activators resulted in some decreased expression of Fxr, Nrf2 and Pxr expression. A potential reason for this observation could be the presence of high levels of glucose in the media diminishing the responsiveness of the hepatocytes to AMPK activation. In vivo, multiple hormone and signaling pathways elicited by CR converge to have an effect, and many have to be considered, whereas in vitro, additional of a single chemical activator may better reflect a potential regulation by AMPK or Sirt1. Overall, it is appreciated that modeling the CR effect in hepatocytes from lean and obese mice is complex.
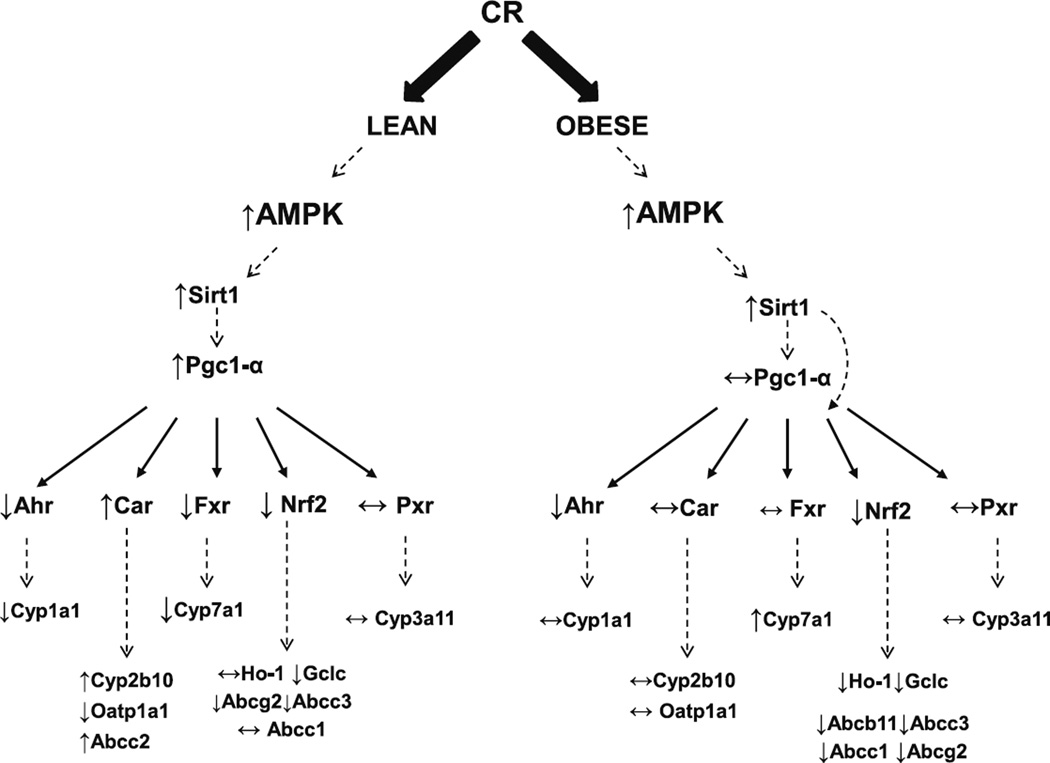
Figure 8 depicts the observed changes in binding, gene expression and the potential mechanism by which the regulation might be occur in livers of lean and obese mice after CR in vivo. First, CR is known to increase AMP/ATP ratios, resulting in increased AMPK activity. In lean mice, CR decreased Ahr, Fxr and Nrf2 binding activity to known consensus sequences, with no observed change in Pxr binding activity, and increased Car pathway activity. It has been previously reported that although altered, the AMPK-Sirt1 pathway is functional in OB mice and can be targeted for therapeutic interventions (58,59). However, CR induces Pgc-1α expression in livers of lean mice, but not in OB mice. Fasting mediated Pgc-1α induction has been described to be dependent upon the leptin receptor (60). In OB mice, decreased Nrf2 and Ahr activity with no marked change in Car, Pxr, or Fxr activity. CR decreased Nrf2 binding activity in livers OB mice, which was associated with decreased Ho-1, Gclc, Abcb11, Abcc1, Abcc3, Abcg2.
Fig. 8.
CR-mediated biotransformation enzyme and transporter expression differs in liver differs for lean and obese mice. The observed effects of caloric restriction (CR) on liver gene expression differed between lean and obese (OB) mice. The figure depicts potential regulation based on nuclear receptor binding and mRNA expression data from the in vivo study. CR is described to activate the AMPK pathway and induce Sirtuin 1 (Sirt1) activation. CR induced Pgc-1α mRNA expression in livers of lean mice, consistent with previous reports, but did not do so in OB mice. Based on previous reports, Pgc-1 and Sirt1 are known to regulate some NRs at the level of mRNA expression and binding to responsive elements. In general, receptor binding and downstream gene expression differed in the lean and OB mice after CR, suggesting that perhaps Sirt1, leptin, and perhaps Pgc-1α, are upstream modulators of biotransformation and excretion processes.
Understanding how these results translate to differential human exposure to medications or chemicals through occupation or environmental is challenging because few studies address weight loss and chemical clearance. There are limited studies, some of which evaluate nutritional status and hepatic clearance, but some exist that use dyes to measure hepatic clearance. For example, indocyanine green (ICG) is a dye and organic anion used to evaluate hepatic clearance, and most likely a substrate for OATPs (61) and ABCC2 because it undergoes biliary excretion via Mrp2-mediated transport (62,63). ICG hepatic clearance was decreased in swine that were fasted to have weight loss for 20% of their body weight (64). Our results also indicated that CR increased MRP2 expression, which is also consistent with observations that ICG clearance is increased humans and rats after fasting (65).
All the above observations indicate that activation of CR pathways can only partly reverse the changes in drug bio-transformation enzyme and transporter expression that occurs with obesity induced by leptin deficiency. Overall, leptin, AMPK, and Sirt1 can modulate biotransformation enzyme and transporter expression.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants to from the National Institute of Health [4R01ES016042 and 5K22ES013782 to ALS], and also supported, in part, by Rhode Island IDeA Network of Biomedical Research Excellence [Award # P20RR016457] from the National Center for Research Resources, National Institute of Health.
ABBREVIATIONS
- Abcc
ATP-Binding Cassette, sub-family C
- Ahr
Aryl hydrocarbon receptor
- AL
ad libitum
- AMPK
AMP Kinase
- Car
Constitutive androstane receptor
- CR
caloric restriction
- Creb
cAMP response element binding protein
- Cyp
Cytochrome P450
- Fxr
Farnesoid x receptor
- Gclc
Glutamate-cysteine ligase, catalytic subunit
- Gsta1
Glutathione S-transferase a1
- Ho-1
Heme oxygenase 1
- Nqo1
NADPH:quinone oxidoreductase
- NR
nuclear receptor
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- Oatp
Organic anion transporting polypeptide (Oatp)
- Pepck
Phosphoenolpyruvate carboxykinase
- PGC1α
Peroxisome proliferator-activated receptor-γ coactivator-1α
- PPAR
Peroxisome proliferator-activated receptor
- PXR
Pregnane X receptor
- RXR
Retinoid X receptor
- Sod1
Superoxide dismutase 1
- Srebp-1c
Sterol regulatory element binding protein 1c
- TF
transcription factor
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11095-013-1140-2) contains supplementary material, which is available to authorized users.
This work was presented, in part, at the annual Society of Toxicology (SOT) meeting, March 7–11, 2010, Salt Lake City, Utah
REFERENCES
- 1.Edmisonand J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007;11:75–104. doi: 10.1016/j.cld.2007.02.011. ix. [DOI] [PubMed] [Google Scholar]
- 2.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Hepatology. Vol. 40. Baltimore, Md: 2004. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity; pp. 1387–1395. [DOI] [PubMed] [Google Scholar]
- 5.Abernethyand DR, Greenblatt DJ. Drug disposition in obese humans An update. Clin Pharmacokinet. 1986;11:199–213. doi: 10.2165/00003088-198611030-00002. [DOI] [PubMed] [Google Scholar]
- 6.Barshop NJ, Capparelli EV, Sirlin CB, Schwimmer JB, Lavine JE. Acetaminophen pharmacokinetics in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2011;52:198–202. doi: 10.1097/MPG.0b013e3181f9b3a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrieber SJ, Hawke RL, Wen Z, Smith PC, Reddy KR, Wahed AS, et al. Differences in the disposition of silymarin between patients with nonalcoholic fatty liver disease and chronic hepatitis C. Drug Metab Dispos. 2011;39:2182–2190. doi: 10.1124/dmd.111.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.More VR, Wen X, Thomas PE, Aleksunes LM, Slitt AL. Severe diabetes and leptin resistance cause differential hepatic and renal transporter expression in mice. Comp Hepatol. 2012;11:1. doi: 10.1186/1476-5926-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37:2087–2094. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Kulkarni SR, Li L, Slitt AL. UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug Metab Dispos: Biol Fate Chem. 2012;40:259–266. doi: 10.1124/dmd.111.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, et al. Obesity. Vol. 16. Silver Spring; 2008. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function; pp. 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460–468. doi: 10.1038/ajg.2010.488. quiz 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudharyand N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care. 2009;12:431–437. doi: 10.1097/MCO.0b013e32832cdaae. [DOI] [PubMed] [Google Scholar]
- 15.Fulcoand M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha SK, Kim J, Chae C. Role of AMP-activated protein kinase and adiponectin during development of hepatic steatosis in high-fat diet-induced obesity in rats. J Comp Pathol. 2011;145:88–94. doi: 10.1016/j.jcpa.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Corton JC, Apte U, Anderson SP, Limaye P, Yoon L, Latendresse J, et al. Mimetics of caloric restriction include agonists of lipid-activated nuclear receptors. J Biol Chem. 2004;279:46204–46212. doi: 10.1074/jbc.M406739200. [DOI] [PubMed] [Google Scholar]
- 18.Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 2004;279:19832–19838. doi: 10.1074/jbc.M313601200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YK, Saupe KW, Klaassen CD. Energy restriction does not compensate for the reduced expression of hepatic drug-processing genes in mice with aging. Drug Metab Dispos. 2010;38:1122–11231. doi: 10.1124/dmd.110.032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, et al. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm. 2008;5:77–91. doi: 10.1021/mp700114j. [DOI] [PubMed] [Google Scholar]
- 21.Aleksunesand LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab Dispos. 2012;40:1366–1379. doi: 10.1124/dmd.112.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 23.Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos: Biol Fate Chem. 2005;33:956–962. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- 24.Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci. 2006;89:370–379. doi: 10.1093/toxsci/kfi332. [DOI] [PubMed] [Google Scholar]
- 25.Moreand VR, Slitt AL. Alteration of hepatic but not renal transporter expression in diet-induced obese mice. Drug Metab Dispos. 2011;39:992–999. doi: 10.1124/dmd.110.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloan C, Tuinei J, Nemetz K, Frandsen J, Soto J, Wride N, et al. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes. 2011;60:1424–1434. doi: 10.2337/db10-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bordoneand L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 28.Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 29.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 30.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 31.Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2010;38:2293–2301. doi: 10.1124/dmd.110.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortonand JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- 33.Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, et al. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- 34.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, et al. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J Nutr Biochem. 2011;22:393–400. doi: 10.1016/j.jnutbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediat Inflamm. 2012;2012:984643. doi: 10.1155/2012/984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the anti-oxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, et al. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, Wada T, Febbraio M, He J, Matsubara T, Lee MJ, et al. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010;139:653–663. doi: 10.1053/j.gastro.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takemori K, Kimura T, Shirasaka N, Inoue T, Masuno K, Ito H. Food restriction improves glucose and lipid metabolism through Sirt1 expression: a study using a new rat model with obesity and severe hypertension. Life Sci. 2011;88:1088–1094. doi: 10.1016/j.lfs.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantoand C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1 Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tontonozand P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 49.Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010;8:e002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 51.Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE, et al. Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol Sci. 2008;106:319–328. doi: 10.1093/toxsci/kfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sassi Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, Cohen Aubart F, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29:4617–4624. doi: 10.1038/onc.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayonrinde OT, Olynyk JK, Beilin LJ, Mori TA, Pennell CE, de Klerk N, et al. Hepatology. Vol. 53. Baltimore, Md: 2011. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease; pp. 800–809. [DOI] [PubMed] [Google Scholar]
- 55.Lebensztejn DM, Wojtkowska M, Skiba E, Werpachowska I, Tobolczyk J, Kaczmarski M. Serum concentration of adiponectin, leptin and resistin in obese children with non-alcoholic fatty liver disease. Adv Med Sci. 2009;54:177–182. doi: 10.2478/v10039-009-0047-y. [DOI] [PubMed] [Google Scholar]
- 56.Scarpaceand PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Exp Gerontol. 2003;38:631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 60.Kakuma T, Wang ZW, Pan W, Unger RH, Zhou YT. Role of leptin in peroxisome proliferator-activated receptor gamma coactivator-1 expression. Endocrinology. 2000;141:4576–4582. doi: 10.1210/endo.141.12.7804. [DOI] [PubMed] [Google Scholar]
- 61.Cui Y, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- 62.Sathirakul K, Suzuki H, Yasuda K, Hanano M, Tagaya O, Horie T, et al. Kinetic analysis of hepatobiliary transport of organic anions in Eisai hyperbilirubinemic mutant rats. J Pharmacol Exp Ther. 1993;265:1301–1012. [PubMed] [Google Scholar]
- 63.Hosokawa S, Tagaya O, Mikami T, Nozaki Y, Kawaguchi A, Yamatsu K, et al. A new rat mutant with chronic conjugated hyperbilirubinemia and renal glomerular lesions. Lab Anim Sci. 1992;42:27–34. [PubMed] [Google Scholar]
- 64.Kudsk KA, Kisor DF, Waters B, Mirtallo JM, Campbell 3rd AJ, Wooding-Scott RA. Effect of nutritional status on organic anion clearance by the swine liver. Surgery. 1992;111:188–194. [PubMed] [Google Scholar]
- 65.Ohkubo H, Musha H, Okuda K. Effects of caloric restriction on the kinetics of indocyanine green in patients with liver diseases and in the rat. Am J Dig Dis. 1978;23:1017–1024. doi: 10.1007/BF01263102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.