Abstract
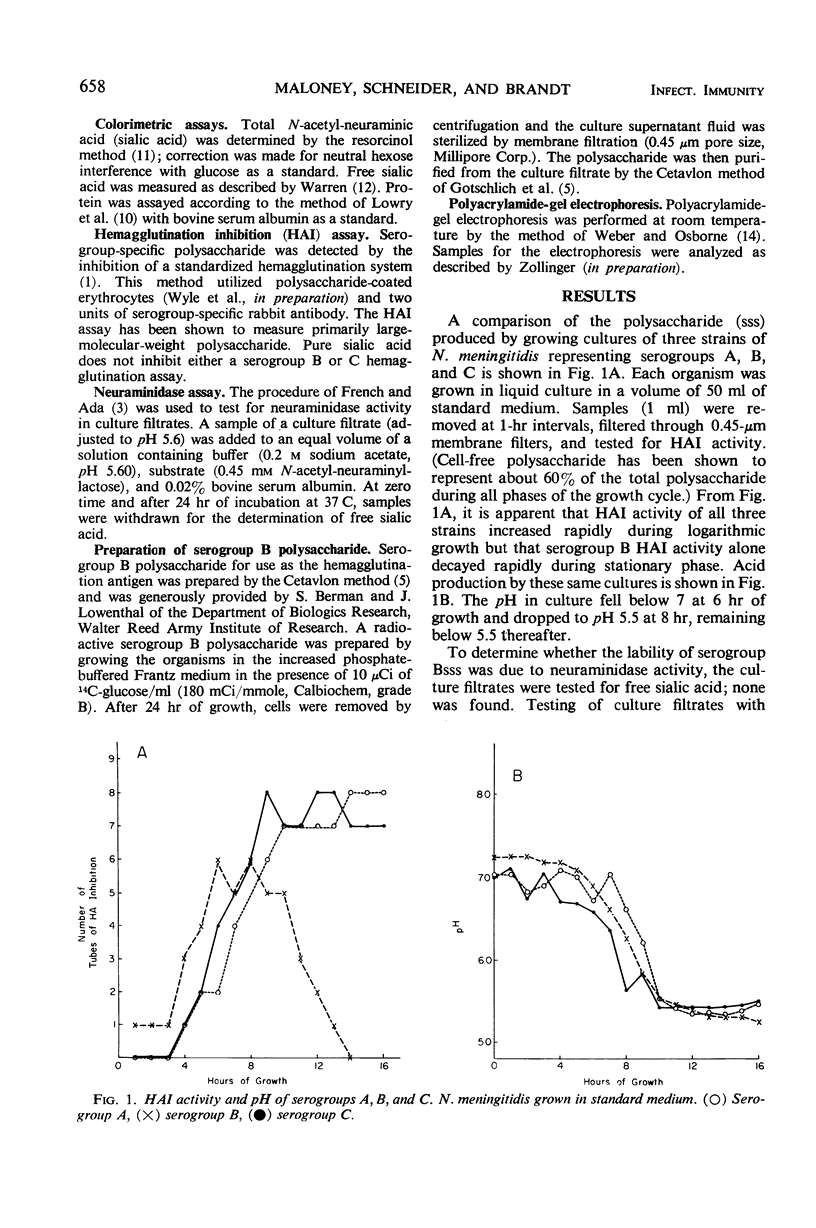
Polysaccharide produced from cultures of serogroups A, B, and C Neisseria meningitidis was assayed by the serogroup-specific hemagglutination inhibition (HAI) test. The polysaccharide produced by all serogroups was found to increase during the exponential phase of growth. For serogroups A and C, the HAI activity was stable during the stationary phase; for serogroup B, however, the HAI decayed rapidly. The degradation of the serogroup B polysaccharide was not caused by enzymatic degradation, but was due to acid accumulation in the culture medium. The large-molecular-size serogroup B polysaccharide was stabilized by increasing the buffering capacity of the medium, which also increased the yield of this antigen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artenstein M. S., Brandt B. L., Tramont E. C., Branche W. C., Jr, Fleet H. D., Cohen R. L. Serologic studies of meningococcal infection and polysaccharide vaccination. J Infect Dis. 1971 Sep;124(3):277–288. doi: 10.1093/infdis/124.3.277. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Branche W. C., Jr, Harkins C. Cutaneous reactions and antibody response to meningococcal group C polysaccharide vaccines in man. J Infect Dis. 1970 Apr;121(4):372–377. doi: 10.1093/infdis/121.4.372. [DOI] [PubMed] [Google Scholar]
- FRENCH E. L., ADA G. L. Stimulation of the production of neuraminidase in Vibrio cholerae cultures by N-acetylneuraminic acid and sialyl-lactose. J Gen Microbiol. 1959 Dec;21:550–560. doi: 10.1099/00221287-21-3-550. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABAT E. A., BEZER A. E. The effect of variation in molecular weight on the antigenicity of dextran in man. Arch Biochem Biophys. 1958 Dec;78(2):306–318. doi: 10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Dunne F. T., Jonssen E. K. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J Biol Chem. 1971 Aug 10;246(15):4703–4712. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Jonssen E. K., Wysocki J. R. Studies on the meningococcal polysaccharides. I. Composition and chemical properties of the group A polysaccharide. J Biol Chem. 1971 May 10;246(9):2849–2858. [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WATSON R. G., SCHERP H. W. The specific hapten of group C (group II alpha) meningococcus. I. Preparation and immunological behavior. J Immunol. 1958 Oct;81(4):331–336. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


