Abstract
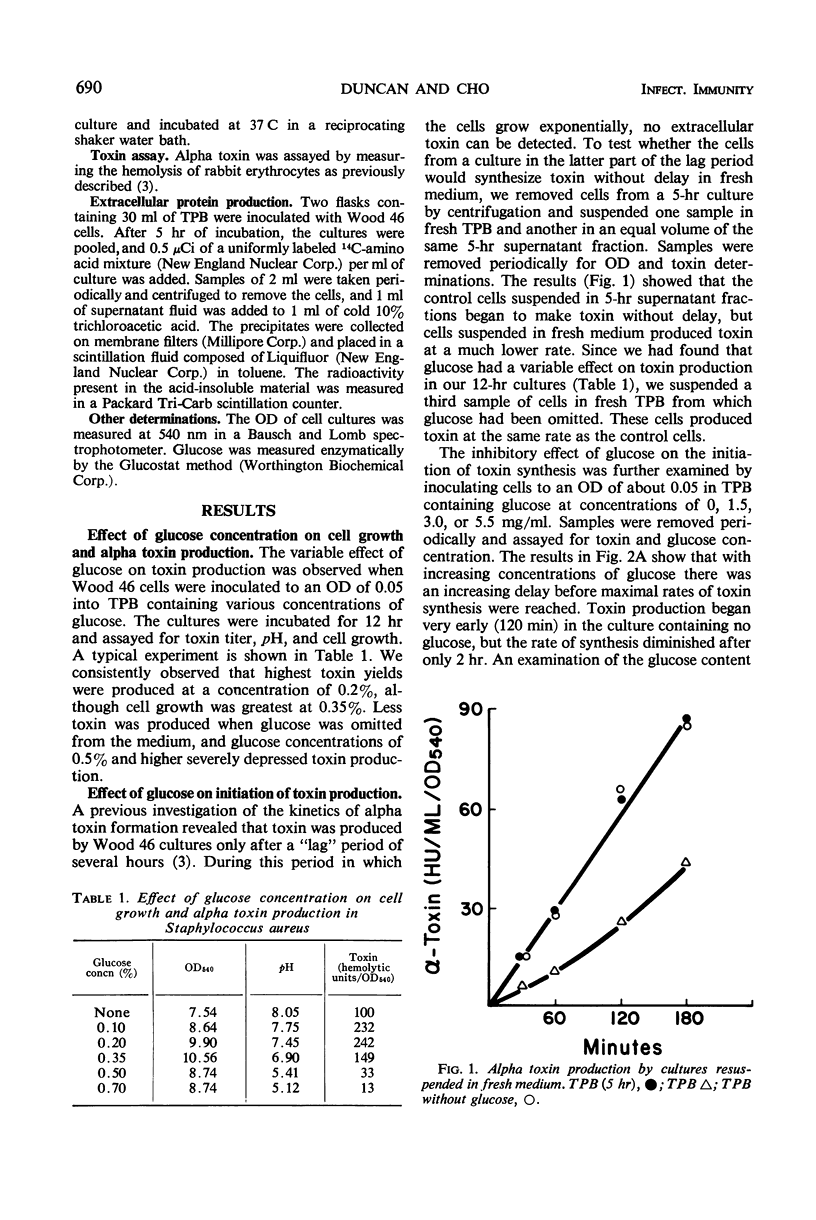
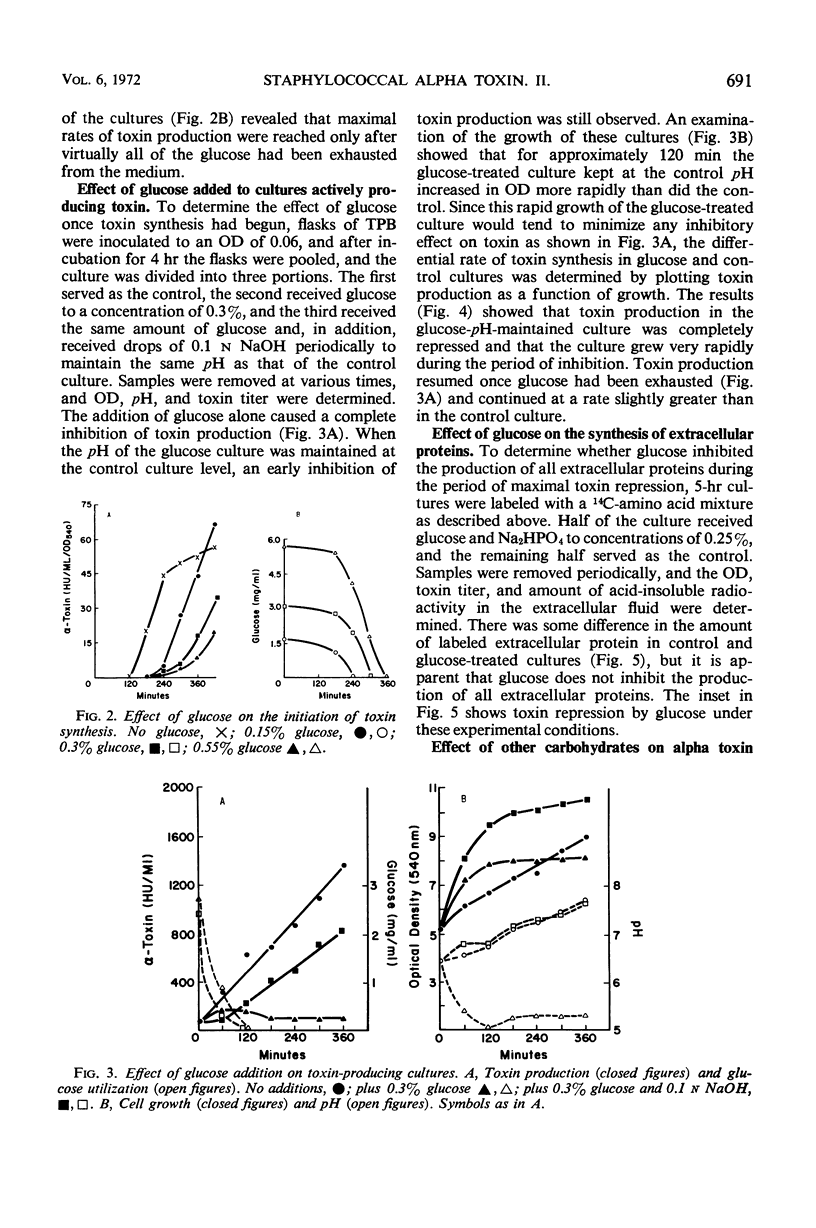
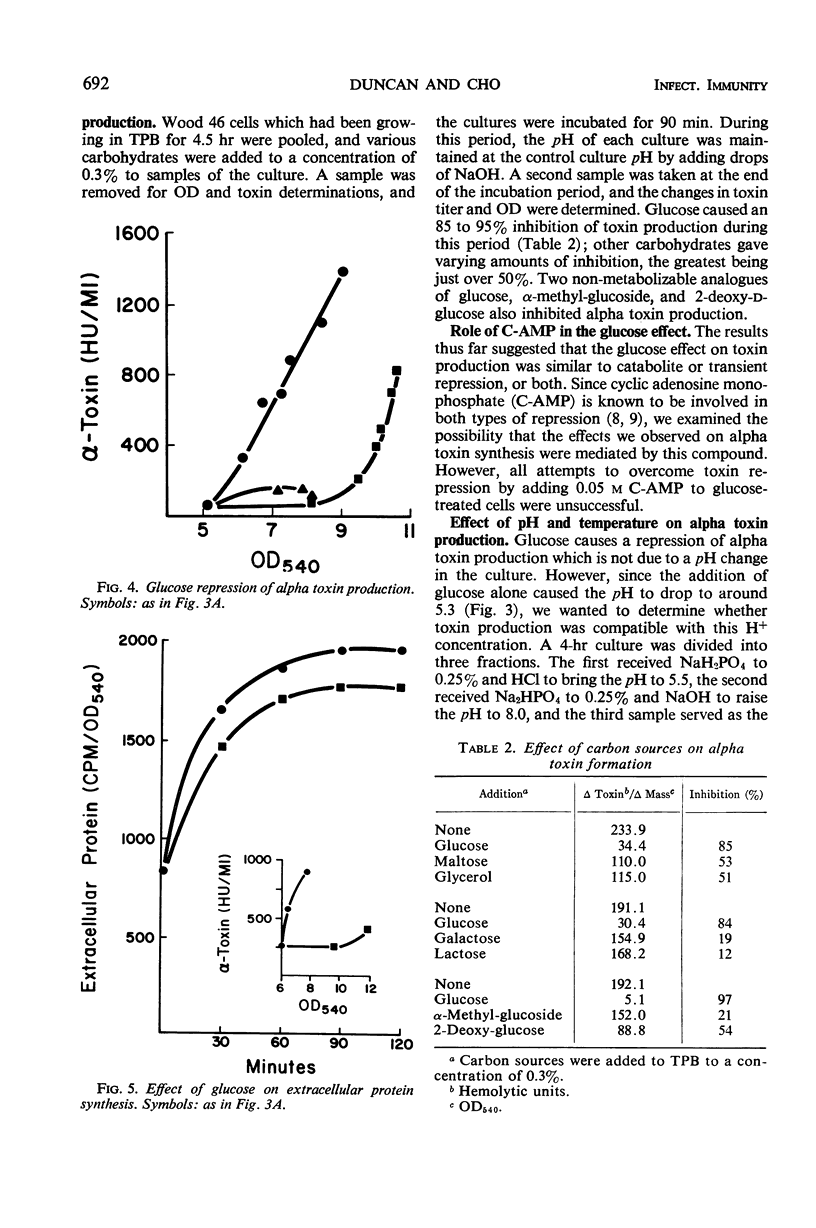
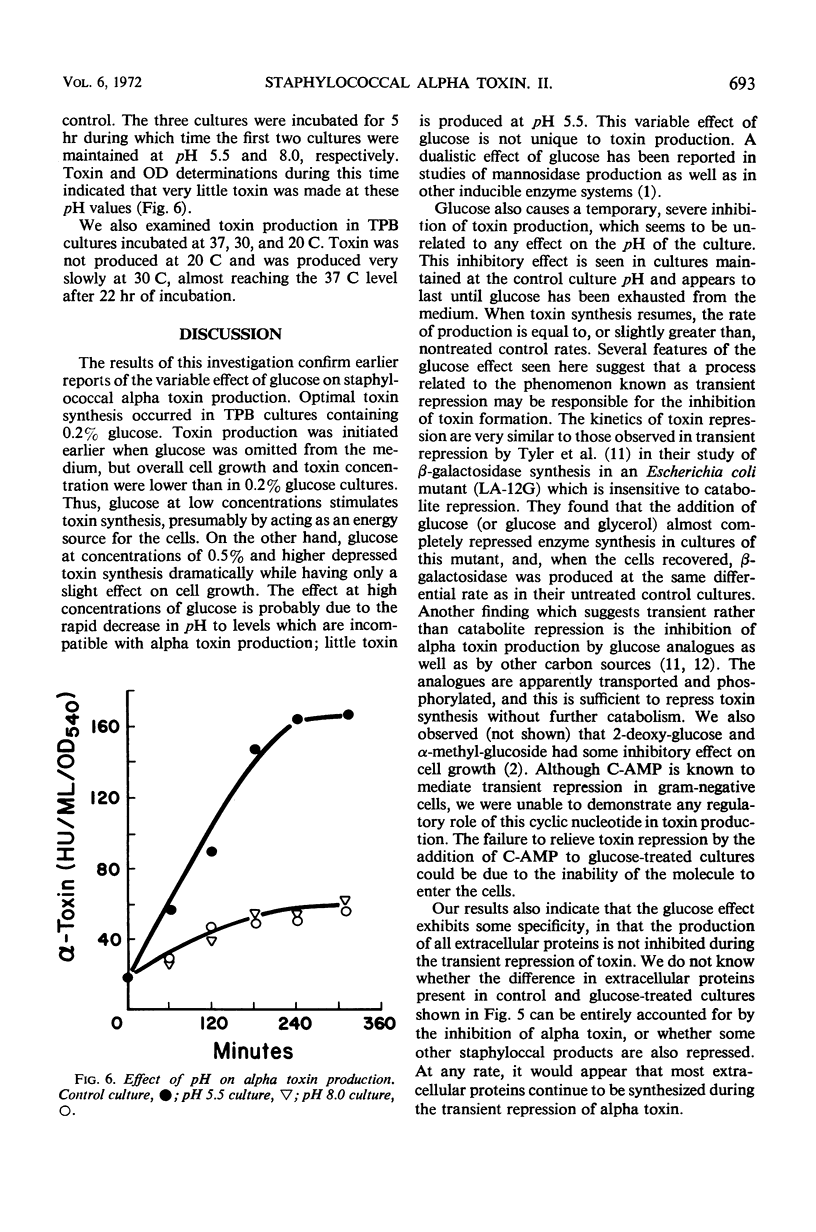
The effect of glucose on alpha toxin production was studied in the Wood 46 strain of Staphylococcus aureus. Optimal toxin production occurred when 0.2% glucose was present in the medium. Omission of glucose gave lower yields of toxin, and concentrations of 0.5% and higher severely depressed toxin formation. Glucose affected the initiation of alpha toxin synthesis in growing cultures. As the glucose concentration increased, the time lag prior to the onset of toxin production also increased, and maximal rates of synthesis were not obtained until essentially all the glucose had been exhausted from the medium. The addition of glucose to toxin-producing cultures caused a temporary, almost complete repression of toxin formation which was not due to pH changes in the culture. The synthesis of most extracellular proteins was not inhibited during the period of repression. After recovery, toxin was produced at rates equal to those of untreated control cultures. The kinetics of toxin repression and the observation that the glucose analogues, 2-deoxy-d-glucose and α-methyl-glucoside, as well as other carbon sources, inhibit toxin production suggest that transient repression is responsible for the inhibition of toxin formation. No evidence for a regulatory role of adenosine 3′, 5′-cyclc monophosphate in alpha toxin production was obtained.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demain A. L., Inamine E. Biochemistry and regulation of streptomycin and mannosidostreptomycinase (alpha-D-mannosidase) formation. Bacteriol Rev. 1970 Mar;34(1):1–19. doi: 10.1128/br.34.1.1-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Cho G. J. Production of staphylococcal alpha toxin. I. Relationship between cell growth and toxin formation. Infect Immun. 1971 Oct;4(4):456–461. doi: 10.1128/iai.4.4.456-461.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Mah R. A., Dobrogosz W. J. Regulation of staphylococcal enterotoxin B. J Bacteriol. 1969 Apr;98(1):4–9. doi: 10.1128/jb.98.1.4-9.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., De Crombrugghe B., Pastan I. Cyclic AMP regulates catabolite and transient repression in E. coli. Nature. 1969 Aug 23;223(5208):810–812. doi: 10.1038/223810a0. [DOI] [PubMed] [Google Scholar]
- Tyler B., Loomis W. F., Jr, Magasanik B. Transient repression of the lac operon. J Bacteriol. 1967 Dec;94(6):2001–2011. doi: 10.1128/jb.94.6.2001-2011.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Physiological basis of transient repression of catabolic enzymes in Escherichia coli. J Bacteriol. 1970 May;102(2):411–422. doi: 10.1128/jb.102.2.411-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny J., Mathieu L. G. A comparison of use of carbohydrate, cell structure, and toxin formation in naturally occurring and thymine-requiring Staphylococcus aureus strains. Can J Microbiol. 1971 Nov;17(11):1343–1350. doi: 10.1139/m71-215. [DOI] [PubMed] [Google Scholar]


