Abstract
The use of alcohol by women during pregnancy is a continuing problem. In this review the behavioral effects of prenatal alcohol from animal models are described and related to studies of children and adults with FASD. Studies with monkeys and rodents show that prenatal alcohol exposure adversely affects neonatal orienting, attention and motor maturity, as well as activity level, executive function, response inhibition, and sensory processing later in life. The primate moderate dose behavioral findings fill an important gap between human correlational data and rodent mechanistic research. These animal findings are directly translatable to human findings. Moreover, primate studies that manipulated prenatal alcohol exposure and prenatal stress independently show that prenatal stress exacerbates prenatal alcohol-induced behavioral impairments, underscoring the need to consider stress-induced effects in fetal alcohol research. Studies in rodents and primates show long-term effects of prenatal and developmental alcohol exposure on dopamine system functioning, which could underpin the behavioral effects.
Keywords: Prenatal alcohol exposure, Executive function, Behavior, Sensory processing, Gene × environment, Prenatal stress
Introduction
The influence of alcohol consumption during pregnancy on offspring has received widespread research attention since early reports that prenatal alcohol exposure can have devastating and persistent consequences (Clarren and Smith 1978; Jones and Smith 1973). Alcohol passes freely across the placenta and enters the fetal circulatory system (Waltman and Iniquez 1972). Due to low activity of alcohol dehydrogenase to metabolize the alcohol, the fetus is compromised in its ability to eliminate alcohol, which can persist in amniotic fluid (Brien et al. 1983). Fetal alcohol syndrome (FAS) is the most widely known outcome of heavy drinking during pregnancy. Diagnostic criteria for FAS require symptoms in three areas: distinct craniofacial characteristics, prenatal and/or postnatal growth retardation, and evidence of central nervous system (CNS) dysfunction, including cognitive and behavioral deficits (Jones and Smith 1973). Fetal alcohol spectrum disorders (FASD) is a term used to include children who fail to meet the FAS criteria but show deficits linked to prenatal alcohol exposure (Riley and McGee 2005).
This review focuses on behavioral data from animal models and the relationship of these findings to clinical studies of children and adults with FASD. Rodent findings that parallel those from human epidemiological studies are included as well as experimental studies of prenatal alcohol exposure performed in macaque monkeys. First, an overview of the several important interacting variables that influence how a given individual will respond to prenatal alcohol exposure is presented. Next, the advantages and disadvantages of animal models of FASD are described followed by a review of the early primate studies, foundational work for current studies with rhesus macaques. Finally, a review is presented of prenatal alcohol effects in animal studies on four behavioral domains affected by FASD – neonatal behavior, attention and hyperactivity, sensory processing, and cognitive behavior, all translatable between humans and animal models. Following an examination of these behavioral facets of prenatal alcohol effects, a brief discussion of these results with respect to underlying dopamine brain function will be offered.
Developmental Plasticity and Multiple Risk Factors
Early life experiences such as fetal alcohol exposure do not occur in isolation. Prenatal alcohol exposure can interact with numerous other factors in a complex mutually-influential process to determine individual outcomes. Thus, prenatal alcohol exposure is considered as a risk factor interacting with other variables within the context of the developing organism. This complex bi-directional dynamic process can influence both concurrent and later outcomes.
This viewpoint of development as a bi-directional dynamic process with risk factors considered as probabilistic rather than deterministic is held broadly by developmental psychologists (see Cicchetti and Rogosch 1996; Rutter and Sroufe 2000; Sameroff 2000, for a review). Based on the importance of mutual influence among combined risk factors, other risk factors that can affect the teratogenicity of alcohol and the probability of fetal alcohol-related effects are considered in this paper. Some of the factors shown to be influential in both human and animal studies include dose and timing of exposure, polydrug use, exposure to environmental toxicants, stress-related hormones and certain genetic variants. Maternal age, parity, socioeconomic status, nutrition, and postnatal parental care are also possible interacting risk factors (Abel and Hannigan 1995).
In this paper the potential interactions of prenatal stress and certain genetic polymorphisms with prenatal alcohol exposure are examined. A growing body of animal studies suggests that prenatal stress can exacerbate prenatal alcohol effects (Roberts et al. 2004; Schneider et al. 1997, 2002, 2004, 2008). Fetal alcohol exposed children are often conceived within the context of a stressful environment, given that many women use alcohol to cope with stress. In humans, prenatal stress has been linked to a range of adverse outcomes that are similar to those of fetal alcohol exposure, including lower birth weight, developmental delay, temperamental unadaptability, poor infant stress regulation and later depression, anxiety disorder, ADHD and criminology (Beijers et al. 2010; Wadhwa 2005; Van den Bergh and Marcoen 2004). Animal models of prenatal stress and prenatal alcohol exposure afford the opportunity to manipulate prenatal alcohol exposure and prenatal stress as separate independent variables in an experimental design.
It is also useful to consider genetic variants that might moderate vulnerability to prenatal alcohol effects (Kraemer et al. 2008; Schneider et al. 2009, 2011). Brookes et al. (2006) found evidence that the 10-repeat allele polymorphism in the 3′-untranslated region (UTR) of the dopamine transporter gene (DAT1) mediated the effects of maternal alcohol use during pregnancy in children. Rodent studies have shown differential susceptibility of mouse strains to alcohol effects (Boehm et al. 1997; Chernoff 1980; Giknis and Damjanov 1980). Given the high incidence of mental health issues in individuals with FAS (Streissguth et al. 1996), some studies have focused on a specific genetic variant in both humans and rhesus monkeys, the short (s) allele of the serotonin transporter gene linked polymorphic region (5HTT-LPR) (Caspi et al. 2003). This genetic variant is considered to reflect developmental plasticity, such that individuals with this particular heightened biological sensitivity to context might be more vulnerable to negative events but have a greater capacity to benefit from positive environmental influences, a potentially important consideration for intervention studies (Boyce 2007; Boyce and Ellis 2005). Findings with macaque monkeys have shown that prenatal and genetic conditions work together to induce certain behavioral outcomes, whereas, one factor or the other alone might not lead to the outcome (Kraemer et al. 2008; Schneider et al. 2009, 2011). These types of findings underscore the idea that the number and type of risk factors can be more useful in predicting and understanding offspring outcomes than consideration of either risk factor by itself.
Importance of Animal Models
Animal models provide randomization to prenatal conditions, rigorous experimental control over numerous potentially confounding variables as well as control of the dose and timing of the alcohol exposure. Animal models have the advantage of un-confounding multiple factors by manipulating them independently. By contrast, causal conclusions are difficult to reach in human studies due to the clustering of negative events. For example, alcohol consumption and smoking tobacco often go hand-in-hand, and alcohol use usually affects nutrition intake in people. However, in order to relate animal models to human development the relative states of brain development at times of alcohol exposure need to be carefully considered. Mammalian brains have a growth spurt of neurogenesis, followed by a period of neuronal migration and synaptogenesis. During neurogenesis, neurons are generated which subsequently migrate to specific sites, after which they grow axons and dendrites forming networks and structures. Hence the timing of alcohol exposure in relation to the developing neural system can profoundly affect the behavioral outcomes.
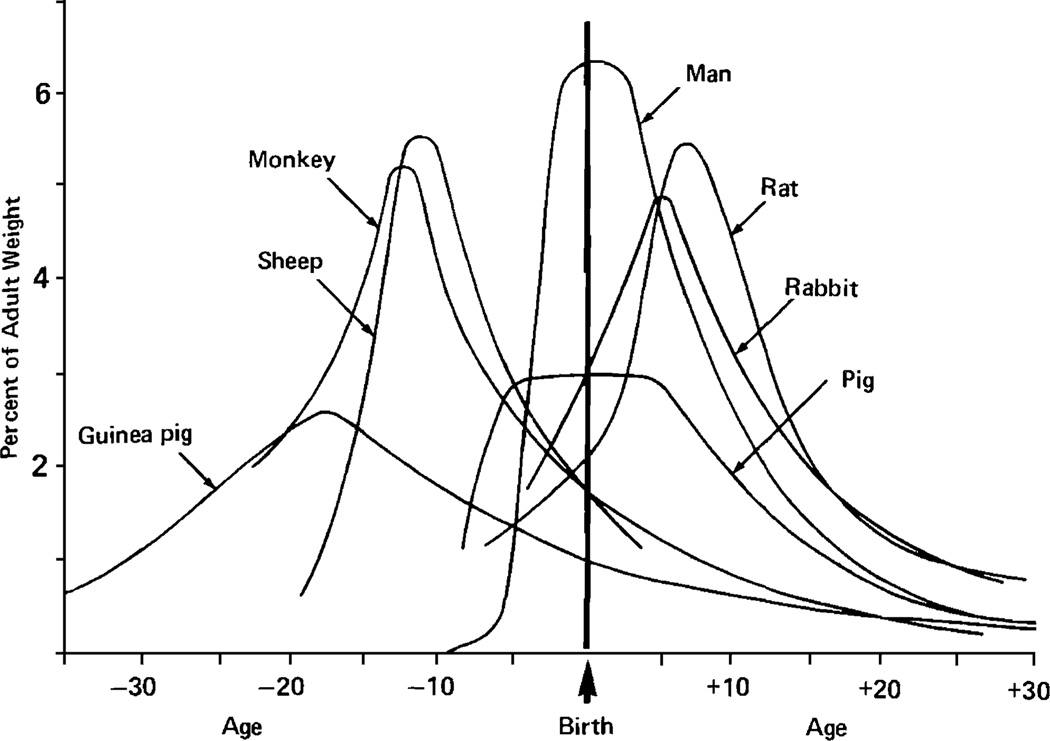
Most animal models of gestational alcohol effects have been conducted in rodents because of economy and short periods of maturation. Alcohol is often administered to the neonatal rat to model the third trimester of human pregnancy, in terms of brain development. In humans, the third trimester is characterized by rapid neuronal growth and cell proliferation, and is thought to be a sensitive period for alcohol-related effects on the CNS. In rats, the “brain growth” spurt extends into early postnatal period (Dobbing and Sands 1979) occurring approximately between one and ten days of age (West 1993) (see Fig. 1). Thus, neuronal systems present at birth in the human develop postnatally in the rat. Accordingly, exposure to alcohol during the early neonatal period in the rodent is used to model alcohol exposure during the third trimester brain growth spurt in humans (Slawecki et al. 2004).
Fig. 1.
The brain growth spurts of seven mammalian species expressed as first-order velocity curves of the increase in weight with age. The units of time for each species are as follows: guinea pig [3]: days; rhesus monkey [1]: 4 days; sheep [9]: 5 days; pig [2]: weeks; man [5]: months; rabbit [8]: 2 days; rat [4]: days. Rates are expressed as weight gain as a percentage of adult weight for each unit of time. From “Comparative aspects of the brain growth spurt,” J. Dobbing and J. Sands, 1979, Early Human Development, 3, p. 81. Copyright Elsevier 1979. Reprinted with permission
Primate studies have the benefit of gestational characteristics and early development that are more similar to humans than are rodents, including slow fetal growth, long gestation (23–24 weeks), single birth, and slow postnatal growth rate. Rakic (1988) carefully mapped the three broad phases of brain development in rhesus monkeys: generation of neurons, neuronal migration, and synaptogensis. Rakic suggested that, in both humans and rhesus monkeys, cortical neurons are generated near the surface of the cerebral ventricle during early gestation (0 – gestation day 40). After their last division, postmitotic cells produced within the proliferation zones migrate along radial glial fascicles and enter the developing cortical plate, forming ontogenetic columns (gestation days 40–70/100) (Rakic 1995). By gestation day 112, the developing cortex has its full complement of neurons. The phase of rapid synaptogenesis, which occurs synchronously in the somatosensory, motor, and association areas (Zecevic and Rakic 1991) begins at gestation day 112 and continues to the third month postnatally (Bourgeois and Rakic 1993). Hence prenatal alcohol timing can be studied in rhesus monkeys in a way that allows it to be closely homologous to the human.
The shorter life span of monkeys, compared with humans, makes longitudinal studies easier to conduct with monkeys than with humans but more difficult than with rodents. Primate models afford greater generalization to humans than rodents with regard to complex behavior and social development as well as the brain architecture and function that underlies higher cognitive processes (Suomi and Higley 1991). Primate models also afford the opportunity to assess prenatal alcohol effects on complex behaviors while examining neural underpinnings of the behavioral deficits that parallel those found in human clinical studies. At the same time rodent models are most appropriate when exploration of physiological mechanisms is the goal.
Early Primate Studies
Prenatal alcohol exposure has received limited study in nonhuman primates, despite the advantages of primate models. Most early studies used small samples and employed wide variations in dose, method of dosing (voluntary consumption or other) and gestational timing of alcohol administration. In addition, the assessments of infant outcome were not extensive but rather focused mainly on infant viability.
In a pioneering study, Elton and Wilson (1977) offered an orange-flavored alcohol solution to four pig-tailed macaques (Macaca nemestrina) before conception and throughout pregnancy. Three monkeys drank little and produced apparently normal infants while the fourth monkey consumed a high dose of alcohol throughout pregnancy and produced a hyperactive and tremulous infant. Jacobson and colleagues (1980) administered alcohol orally to two crab-eating macaque (Macaca fascicularis) monkeys. One animal consumed 5.4 g/kg/day for the last 40 days of pregnancy, producing a small infant that was hypotonic (low muscle tonus) and slower in motor performance than control monkeys. The second monkey consumed alcohol during the first 60 days of pregnancy and produced an infant that was normal in size but showed delays in visual pursuit. Altshuler and Shippenberg (1981) administered alcohol to pregnant rhesus macaques by indwelling intragastric cannulae. In two pregnant females blood alcohol concentrations (BAC) was maintained at 200 mg/dL and both aborted in the first trimester. Lower alcohol exposure, <150 mg/dL, appeared to allow viable pregnancies.
Clarren and colleagues (1987) were the first to use a range of doses in a large sample. They administered alcohol orally by gavage once weekly to 54 adult female pig-tailed macaques in doses of 0.3, 0.6, 1.2, 1.8, 2.5, 3.3, or 4.1 g/kg as a model of binge drinking. Blood alcohol concentrations varied from 24±6 mg/dL at the 0.3 g/kg dose (comparable to human light social drinking) to 549±71 at the 4.1 g/kg dose (comparable to the human very heavy binge consumption). An increased rate of spontaneous abortion was found at alcohol dose at or above 1.8 g/kg (mean BAC for the dose was 205 mg/dL). Five of six animals (83%) had pregnancy failure at doses above 2.5 g/kg (mean BAC for the dose was 250 mg/dL) compared with 50% pregnancy failure rate in the colony, while 100% of the pregnancies failed at 4.1 g/kg/dose (BAC over 500 mg/dL).
Scott and Fradkin (1984) administered high dose alcohol daily (maximum dosage of 4 or 5 g/kg in divided doses at 8 am and 5 pm) by gavage to 16 female crab-eating macaques yielding 33 pregnancies. Fourteen female controls were untreated or were given a sucrose solution equal in calories. There were high rates of fetal loss in this study: 38% for animals administered 4 g/kg/day and 67% for those administered 5 g/kg/day. Interestingly, controls showed 29% fetal loss, compared with the authors’ expectations of approximately 10% in their laboratory. They proposed that the increased fetal loss in controls was due to stress from the gavage treatment by unfamiliar personnel on weekends. While there was no effect of the prenatal alcohol treatment on the gestation length of viable pregnancies, reductions in birth weights, head circumferences and crown-rump length were reported in prenatal alcohol-exposed offspring. This study also included behavioral observations of the animals in their home cages and when separated from their mothers. The authors reported that the general well-being, alertness, and locomotor activity of the alcohol-exposed infants were indistinguishable from controls. Because this study did not employ rigorous behavioral assessments, the lack of behavioral findings is not informative. Moreover, the apparent lack of behavioral findings in this study in which mothers were administered high dose alcohol under stressful conditions (gavage) yielding reduced birth weights and head circumferences and increased fetal loss in control subjects (compared to the colony at large) underscored the need at that time for more rigorous measures of early behavior and for experiments in which alcohol exposure can be separated from prenatal stress exposure.
Clarren and Bowden (1982) developed an intermittent binge model of prenatal alcohol exposure in which precise behavioral assessments were conducted that parallel those used with human infants. In their first study, 4 pig-tailed macaques were given 2.5 g/kg weekly doses by gavage from 30–40 days after conception through delivery. Three animals were given “moderate” dosage (2.5 g/kg, comparable to six drinks) weekly and one animal was given a “high” dosage (4.1 g/kg, comparable to ten drinks) weekly. The moderate-dose infants were normal in facial morphology while the high-dose animal showed a flattened philtrum, wide nose and retrusive maxilla, similar to the human FAS facial anomalies. To assess neonatal behavioral integrity the infants were separated from their mothers after birth and infant reflexes were tested in a way analogous to tests of human infants. The moderate dose (2.5 g/kg/week) alcohol-exposed infants had normal neonatal assessments at day 10 and typical motor development. The high dose (4.1 g/kg/week) alcohol-exposed animal had limb and oral reflex delays and retardation of audiovisual response and delayed motor development. Moderate dosed monkeys were mildly delayed on an object permanence test, while the high dose infant was profoundly impaired on the task, performing like a 22-day old infant at 116 days of life (Clarren and Bowden 1982).
In a follow-up study, animals were given alcohol once weekly (1.8 g/kg) by gavage during the first 3 or 6 weeks or for the entire 24 weeks of pregnancy. Eighty-nine breedings in 27 adult female pig-tailed macaques (with no unusual history of negative pregnancy outcomes) yielded 35 pregnancies (Clarren and Astley 1992). Seven of 28 (25%) of the pregnancies exposed to alcohol aborted, whereas none of the seven confirmed pregnancies in the control group resulted in abortion. Viable infants were assessed for behavioral and cognitive function. While animals in the 3-week exposure group did not differ from controls, the small sample size in this study precludes conclusions that these animals were unaffected. Moreover, one subject in this group showed numerous impairments (poor cognitive and motor performance and distractibility).
Those in both 6-week and 24-week exposure groups showed delays in a battery of memory and learning tasks administered in the Wisconsin General Test Apparatus (Harlow 1959). The 24-week monkeys required significantly more trials to reach criteria on the cognitive tasks. Both the 6- and 24-week exposure groups also failed to show a preference for novel over familiar objects on a visual recognition memory assessment sensitive to risks for cognitive deficits (Gunderson et al. 1989). Typically developing macaques show robust novelty preference by one month of age (Brickson and Bachevalier 1984). Uncoordinated walking and difficulty climbing a ramp were seen in alcohol exposure for 6- and 24-weeks compared with controls. Moreover, the 6- and 24-week exposed monkeys appeared to be equally likely to show negative outcomes, suggesting that prenatal alcohol-induced damage persisted after later abstinence (Clarren et al. 1992).
Summary
Despite wide variations in dose, timing, and methods of administration of alcohol in early primate studies of fetal alcohol effects, it is clear that high dose alcohol during pregnancy produces significant behavioral impairments in primate offspring. These impairments include hyperactivity, hypotonicity, delayed visual pursuits and retardation of audiovisual responses, slow motor development and impaired cognitive performance. Even moderate dose alcohol exposure administered via gavage, produced high fetal loss and significant behavioral impairments. The latter finding provides support for the notion that prenatal stress could exaggerate prenatal alcohol-induced effects. Moreover, these early studies showed the need for rigorous measures of behavior that are translatable between humans and animal models.
Domains Affected by Prenatal Alcohol Exposure
Neonatal Behavior
Human Studies
Neonatal behavior, characterized by rapid neuromotor development, increased state regulation, and emerging attentional capabilities is linked to the maturation of neural pathways and is considered a direct reflection of CNS functioning and integrity (Als 1986). The best-known and most widely used research tool for assessing human neonates is the Brazelton Newborn Behavioral Assessment Scale (NBAS) (Brazelton 1973). Fetal alcohol exposed human infants were lower than non-exposed infants on arousal on the NBAS, showed lower peaks of excitement and state lability, and demonstrated less of the full range of awake to crying states (Jacobson et al. 1984; Landesman-Dwyer et al. 1978; Oberlander et al. 2010; Streissguth et al. 1983). Coles et al. (1985) found sub-optimal reflexes in human neonates at 3 days of age who had been exposed to alcohol during the second and third trimesters. Infants exposed through the third trimester were less able to maintain an alert and attentive state during testing and showed more negative reactions to interactions with the examiner. Infants exposed at any time during pregnancy scored lower on motor maturity and had higher activity levels in comparison to non-exposed neonates. Human neonates from alcohol-exposed pregnancies were also found to show abnormal suckling, and this was demonstrated even after in utero exposure to only moderate level self-reported doses of alcohol, or social drinking. Infants were also found to take longer to initiate suckling on a nonnutritive nipple, and their suckling pressure was reduced (Martin et al. 1979).
Using the NBAS Streissguth et al. (1983) found that fetal alcohol exposed neonates showed deficits in habituation, or reduced responses to repetitive or redundant stimulation. Habituation is considered to be a protective mechanism given the multitude of stimuli in the environment and lack of habituation later in infancy is a strong predictor of cognitive delay (Fagan and Singer 1983). Poor habituation to environmental stimulation is also consistent with the Coles et al. (1985) finding of reduced ability to cope with stimulation by the examiner. Lack of habituation may reflect abnormalities in the maturation of neural pathways modulating or gating sensory stimulation. Finally, Oberlander et al. (2010) found that fetal alcohol-exposed neonates showed lower heart rate variability and reduced cortisol response to an acute noxious event (heel lance for medical blood sample) suggesting blunted behavioral and physiological responses to noxious stimuli. Similarly, Fifer et al. (2009) found lower heart rate variability in active sleep in fetal alcohol exposed neonates. Lower heart rate variability is associated with poor attention, greater CNS dysfunction and worse developmental outcomes (Fox and Porges 1985).
Primate Studies
More recent primate studies of alcohol exposure and neonatal behavior have used a standardized version of the Brazelton Newborn Assessment Scale adapted for use with nonhuman primates (see Schneider et al. 2006, for a review), This assessment combines items into subscales that parallel the factors in the human NBAS (Coe et al. 2010; Schneider et al. 1991). The test assesses a variety of attention, neuromotor and temperamental responses and characteristics that mature across the first month of life in rhesus macaques as are assessed in the NBAS for humans (Schneider and Suomi 1992). For example, orientation items consist of ratings of orienting to visual and auditory stimuli as well as duration of looking behavior. Motor functions assess coordination, response speed and reflex items. Ratings of irritability and consolability are based on behavior noted during the administration of orienting and neuromotor items, as in the human NBAS.
Schneider and colleagues (1997) exposed rhesus macaque monkeys to moderate dose prenatal alcohol and used the standardized primate version of the NBAS. Schneider et al. (1997) defined moderate dose alcohol exposure in these studies in accord with Dawson et al. (1995), who classified moderate drinkers as those who consumed 4–14 drinks per week. The female breeders in these studies voluntarily drank a dose (0.6 g/kg/day) comparable to one or two drinks per day for an average-sized woman, yielding blood alcohol concentrations of 20–50 mg/dL. Voluntary alcohol consumption un-confounds the potential stress of gavage from prenatal alcohol exposure. Female breeders in the colony were pre-screened for willingness to consistently consume a 0.6 g/kg/day artificially sweetened alcohol solution and then were randomly assigned to prenatal conditions: (1) alcohol-exposed (0.6 g/kg/day) throughout gestation (from breeding to birth), (2) prenatal stress-exposed (10-minute removal from home cage and exposure to three short, <1-second noise bursts randomly administered over a 10-minute period, 5 days per week during gestation days 90–145), (3) prenatal stress in combination with prenatal alcohol exposure, as described above, and (4) a control group that consumed a sucrose sweetened solution with the same caloric content as the alcohol solution throughout gestation (Schneider et al. 1997).
Macaque monkey infants exposed to moderate prenatal alcohol during pregnancy showed reduced orientation (visual orienting and attention) and lower motor maturity (muscle tone and coordination) in comparison to controls across four assessment time-points (Schneider et al. 1997). This occurred even though the infants were normal in birth weight, gestation length, and apparent facial dimensions. These findings provide a striking parallel to the human effects from prenatal alcohol exposure on neonatal behavior. But because of random assignment and elimination of confounding variables, the primate model adds force to causal interpretations of the effects of alcohol exposure in humans.
The effects of prenatal stress alone and in conjunction with prenatal alcohol exposure help inform interpretation of the human work. The results showed that males exposed to the combination of prenatal alcohol and prenatal stress condition had reduced birth weights, and both males and females from the prenatal alcohol in combination with prenatal stress condition showed slower response speed and worse coordination than controls. Thus prenatal stress was found to exacerbate alcohol-induced motor impairments. Interestingly, although alcohol-consuming and control females all produced viable offspring, alcohol accompanied by prenatal stress resulted in 23% fetal losses versus 5% fetal loss in the laboratory (Schneider et al. 1997).
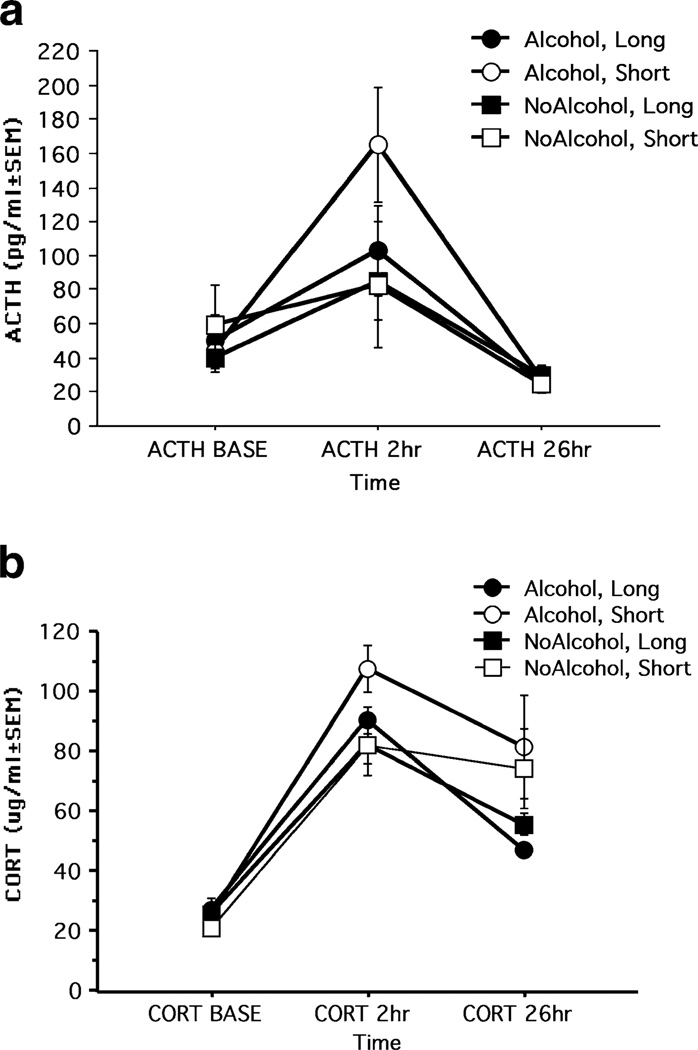
Not all monkeys exposed to alcohol prenatally showed impairments, of course. A genotype-based reanalysis of neonatal behavioral data asked whether prenatal alcohol exposed monkeys carrying the short length polymorphism in the serotonin transporter gene promoter (rh-5HTT-LPR) region would differ from prenatal alcohol exposed monkeys carrying two copies of the long polymorphism (Kraemer et al. 2008). In humans and rhesus monkeys, the cellular effects of having the s/s or s/l combination of alleles of the 5HTT-LPR gene, by comparison with l/l, include reduced gene transcription (Heils et al. 1997). In mice, it is known that the 5HTT-LPR genotypes influence the early patterning of the brain through effects on neurite growth, migration, and synaptogenesis (Lesch et al. 1997). A significant interaction between genotype and prenatal alcohol exposure on neonatal irritability was found such that prenatal alcohol-exposed monkeys carrying the short rh-5HTT-LPR alleles were more irritable and difficult to console during the infant testing. These animals also showed a higher surge of adrenocorticotrophic hormone (ACTH) in response to the stress of weaning at 6 months of age (see Fig. 2). Scores on neonatal irritability predicted hormone measures of stress responsiveness (ACTH) at 6 months of age, showing that the behavioral and neuroendocrine effects of prenatal alcohol exposure lasted beyond infancy (Kraemer et al. 2008).
Fig. 2.
a ACTH (top panel) and b CORT (bottom panel) levels before and after mother-infant separation in Alcohol and NoAlcohol monkeys carrying the s allele (Short) or l/l (Long) genotype. Time postseparation represents baseline samples (BASE) and samples collected at 2 hours and 26 h after separation. Subjects per group: n (Alcohol, Long)=10; n (Alcohol, Short)=9; n (NoAlcohol, Long)=11; n (NoAlcohol, Short)=8. a ACTH levels were highest in the Alcohol, Short group 2 hours postseparation by comparison with all other groups [F(1,36)=4.90; p=0.03)]. b CORT was higher overall in monkeys carrying the short versus long allele at 26 h postseparation [F (1,36)=5.60; p=0.02]. ACTH, adrenocorticotropic hormone; BASE, baseline samples; CORT, cortisol. From “Moderate level fetal alcohol exposure and serotonin transporter gene promoter polymorphism affect neonatal temperament and limbic-hypothalamic-pituitary-adrenal axis regulation in monkeys,” G. W. Kraemer, C. F. Moore, T. K. Newman, C. S. Barr, and M. L. Schneider, 2008, Biological Psychiatry, 63, p. 320. Copyright 2008. Reprinted with permission
Reduced neonatal orientation and motor maturity replicated in a second primate study of moderate dose alcohol consumption (Schneider et al. 2001a). In this study, female breeders were randomly assigned to: (1) early alcohol exposure, in which mothers voluntarily consumed the alcohol solution (0.6 g/kg/day) on gestation days 0–50; (2) mid-to-late gestation exposure, in which mothers consumed the alcohol solution gestation days 50–135; (3) continuous alcohol exposure, in which mothers consumed the alcohol solution gestation days 0–135; and (4) controls, in which mothers consumed an isocaloric control solution during pregnancy. This study showed that early gestation alcohol exposure was as deleterious to neonatal behavior as late gestation or continuous exposure. Interestingly, scores on the primate neonatal behavioral assessment were a more sensitive marker of early gestation alcohol exposure than were the growth parameters of birth weight or crown-torump length. The finding that early gestation alcohol exposure had adverse effects on neurodevelopment raises the possibility that subtle neurodevelopmental effects might be induced even before pregnancy is detected in social drinkers.
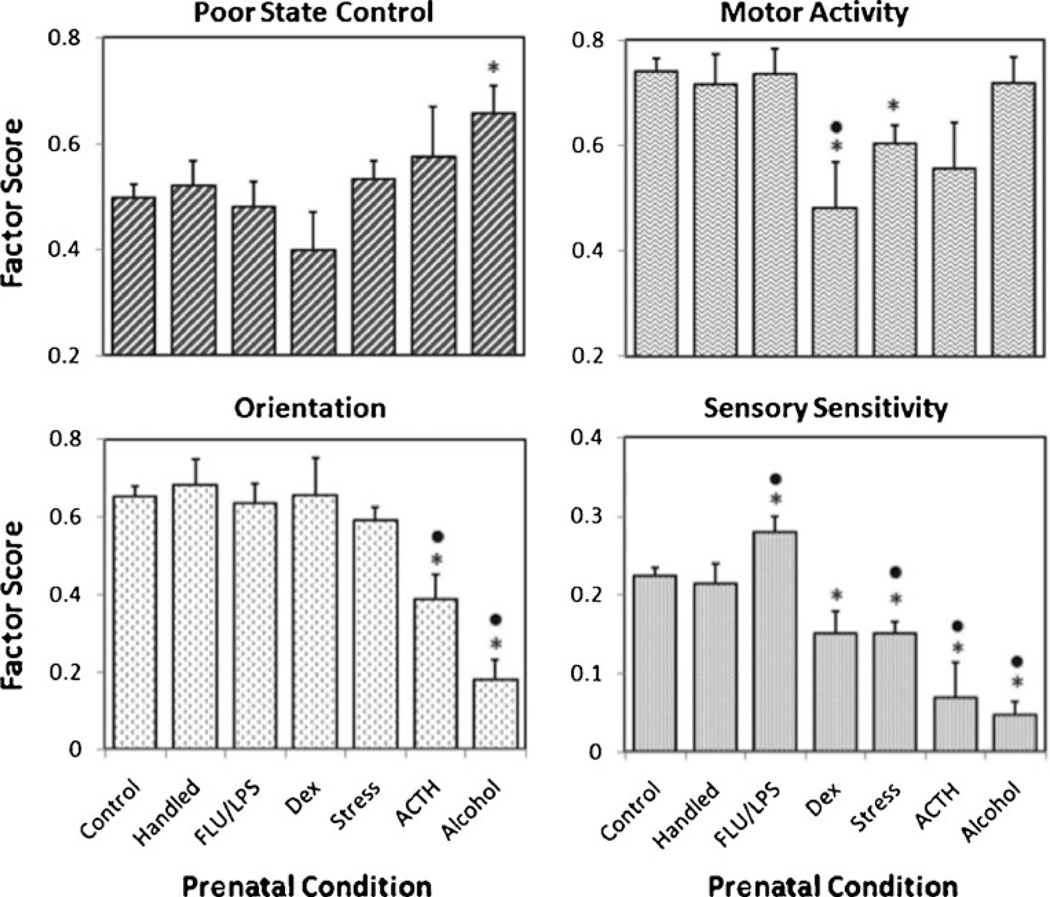
At the University of Wisconsin-Madison Harlow Primate Laboratory the standardized neonatal assessment has been used in a variety of research projects for approximately 20 years. A laboratory-wide comparison of neonatal scores showed that the largest impairments overall were seen in the monkeys from prenatal alcohol exposure compared with the other prenatal conditions (Coe et al. 2010) (see Fig. 3). More specifically, neonatal test scores for 413 rhesus monkey neonates from 7 different types of pregnancies were compared: 177 undisturbed controls, 35 handled controls, 16 dexamethasone- or betamethasone-treated, 33 influenza virus-treated, 100 prenatally stressed, 13 ACTH-treated and 39 prenatal alcohol-exposed. In all conditions the animals were reared by their biological mothers. Prenatal alcohol exposed infants had the worst state control (increased irritability and emotional reactivity during testing) and lowest orientation (low visual orient and attention) across the seven pregnancy conditions. Prenatal alcohol-exposed monkeys also showed reduced sensory scores (indicating high aversion to tactile stimuli) compared with controls and other conditions.
Fig. 3.
Influence of the seven pregnancy conditions on the four IBAS factors. Panel A: Poor State Control (upper left) was most evident in infants from the prenatal alcohol condition. Panel B: Low scores for Motor Activity were evinced by infants in the Dex-treatment, ACTH-stimulation, and Prenatal Stress conditions (upper right). Panel C: Orientation ratings were most impaired by Alcohol-exposure and ACTH-stimulation (lower left). Panel D: Similarly, these two groups had the most aberrant Sensory Sensitivity scores, although infants from the Dex-treatment and Prenatal Stress conditions were also impacted (lower right). In contrast, the prenatal infection paradigm resulted in trend for scores to be shifted in the opposite direction. Asterisk (*) indicates significant difference from Undisturbed Controls; dot (·) indicates a difference from Handing Disturbance control pregnancies. From “Challenges to maternal wellbeing during pregnancy impact temperament, attention, and neuromotor responses in the infant rhesus monkey,” C. L. Coe, G. R. Lubach, H. R. Crispen, E. A. Shirtcliff, and M. L. Schneider, 2010, Developmental Psychobiology, 52, p. 631. Copyright 2010. Reprinted with permission
Primate neonatal behavior has the most relevance to humans. But it is still very important to consider rodent neonatal behavior, in part because there are many more rodent studies in the literature. Also, if analogous results can be established across mammalian species from human to nonhuman primates to rodents, then the rodent work can be interpreted more confidently as having relevance to humans.
Rodent Studies
Data from rodent models has shown that directly after birth animals prenatally exposed to ethanol have longer latencies to attach to the nipple of an anesthesized female dam (Chen et al. 1982; Riley and Rockwood 1984). Also, chronic neonatal alcohol exposure increased latency to attach and decreased time on the nipple of an anesthetized female (Barron et al. 1991). After being separated from their dam and conspecifics, neonatal alcohol-exposed rodents took longer to vocalize and displayed fewer isolation-induced ultrasonic vocalizations (Barron et al. 2000). Since vocalizations elicit maternal attention and retrieval, a reduction of vocalization could have important implications for the pups in terms of social development and nutritional intake. Furthermore, vocalizations play an important role in social behaviors, such as aggression, play, maternal-infant behavior, and social communication, other behaviors also adversely affected by prenatal and/or neonatal alcohol exposure (Brudzynski 2005).
Summary
The most pronounced neonatal findings from the animal studies include lower orienting, attention, arousal and motor maturity in primate studies and reduced suckling and increased latency to attach to the nipple and to vocalize in rodent studies. These animal findings are directly translatable to findings, which include low arousal, attention, motor maturity and abnormal suckling in human neonates. The animal findings, because they are experiments, add strength to causal interpretation of fetal alcohol effects in humans. The primate studies showed that early gestation alcohol exposure was as deleterious to the neonatal behavioral outcome as late or continuous gestation exposure. Also, more severe behavior effects were shown in primates exposed to both prenatal stress and prenatal alcohol, underscoring the importance of consideration of multiple risk factors in fetal alcohol research.
Hyperactivity and Attention
Human Studies
Attention deficits and hyperactivity are considered hallmark features of FASD in children (Brown et al. 1991; Crocker et al. 2011; Fried et al. 1992; Kooistra et al. 2010; Lee et al. 2004; Mattson and Riley 1998; Nanson and Hiscock 1990; O’Malley and Nanson 2002; Streissguth et al. 1986). Activity and attention can be measured by standardized parent report, as a clinical diagnosis of ADHD, and in laboratory tasks that require attention and inhibitory control (executive control). Surprisingly, some studies found little evidence of hyperactivity and impulsivity in prenatal alcohol-exposed children (Coles et al. 1997, 2002). For example, Burden and colleagues (2005) found no association between prenatal alcohol exposure and focused or sustained attention. Instead, working memory was found to be the most important aspect of attention adversely affected by prenatal alcohol exposure. Moreover, these negative effects on working memory were more severe for children of mothers aged 30 or older. Kooistra and colleagues (2010) tested inhibitory control in children with FASD compared with ADHD children. Most (29/30) children with FASD also met criteria for ADHD, however, data on alcohol use during pregnancy were not available from mothers of children from the ADHD or control groups. Both FASD and ADHD children had sustained attention deficits on the Continuous Performance Test. The Go/No-Go task, however, differentiated the groups in that children with ADHD had problems in slow-paced sustained attention tasks (large inter-stimulus intervals) while children with FASD struggled in fast-paced tasks. These data suggest that children with FASD may have problems with speed of processing, overstimulation or activation regulation.
Primate Studies
Studies with primates have measured activity by laboratory observations during cognitive tasks requiring attention and inhibition of motor activity. Overall, studies in primates parallel findings with humans: prenatal alcohol exposure produces hyperactive and distractible offspring. For example, Elton and Wilson (1977) reported hyperactivity in a pig-tailed macaque from a high dose voluntary drinking experiment. Clarren and colleagues (1992) reported increased distractibility as a result of weekly “binge” alcohol administration in macaque monkeys compared with non-exposed monkeys. Schneider and colleagues (1997, 2001a, b) reported that monkeys exposed to moderate level alcohol during pregnancy showed reduced attention as neonates and monkeys exposed to alcohol in combination with prenatal stress were rated as higher on activity level than non-exposed monkeys during cognitive testing. Higher scores on irritability, activity, stereotypies and impulsivity were correlated with more trials to reach criterion on a non-match-to-sample task cognitive task (Schneider et al. 2001b).
Rodent Studies
In rodents, there are several ways of measuring behavior thought to be related to the higher activity levels, attention and executive control problems seen in children. For example, rodent activity can be measured by the open field test in which movements are detected by photobeams and automatically recorded. Exploratory activity is measured with an apparatus with holes in the floor where an animal can dip its head (Abel and Berman 1994). Most, but not all rodent studies have demonstrated increased activity (Abel and Berman 1994; Bond 1981; Meyer and Riley 1986; Ulug and Riley 1983), with results depending on the age at testing, method of testing, and pattern of alcohol exposure. For example, Downing et al. (2009) failed to find effects from prenatal alcohol exposure (3 g/kg) administered on gestational days 7 through 18. Others found that neonatal exposure, corresponding to third trimester exposure in human brain development, was the time of greatest vulnerability to alcohol-induced overactivity (Dursun et al. 2006). Moreover, the strain of the rat may play an important role in developmental outcome. Exposure to binge-like alcohol during early gestation produced high activity in rats bred for high alcohol sensitivity but not for those bred for low alcohol sensitivity (Thomas et al. 1996). Furthermore, age at testing matters. When tested at weaning age, early neonatal alcohol exposure induced greater hyperactivity in rats bred for alcohol preference compared with non-alcohol-preferring rats (Riley et al. 1993), but effects on the two groups were equal when tested in adulthood (Melcer et al. 1995).
Summary
Attention deficits and hyperactivity are hallmark features of FASD in children, and many children with FASD meet criteria for ADHD. In animal models, alcohol-induced hyperactivity and distractibility depend upon the pattern and timing of alcohol exposure, method of testing and age of the animal at testing. These findings echo the theme of multiple risk factors associated with prenatal alcohol exposure, including temporal vulnerability, dose and patterns of alcohol exposure, and age of the mother.
Sensory Processing Disorders and Habituation
Human Studies
Adolescents with facial dysmorphology typical of FAS showed less efficient processing of visual information compared with auditory processing (Coles et al. 2002; Mattson and Roebuck 2002). Moreover, fetal alcohol exposed infants showed slower registration of auditory and visual stimuli, as measured by the cardiac orienting reflex and slower neurophysiological responses implicated in encoding environmental stimuli and initiating attention as well as higher levels of behavioral arousal compared with non-exposed infants (Jacobson et al. 1984; Kable and Coles 2004). Taken together, these studies provide substantive evidence that children exposed to alcohol during pregnancy compared with non-exposed children have less efficient sensory processing based on parent rating scales and behavioral observations.
Using the standardized parent rating scale (Dunn 1999), children exposed to alcohol during pregnancy compared with typically developing peers were three times more likely to be classified as having clinically significant sensory processing problems (Jirikowic et al. 2008). Sensory processing disorders are regulatory disorders characterized by atypical responses to non-noxious sensory stimulation (Ayres 1972). A core deficit in sensory processing disorders is difficulty suppressing irrelevant sensory stimuli and showing inappropriately high aversive behavioral responses to those stimuli, including lack of habituation (Baranek and Berkson 1994; Davies et al. 2009; McIntosh et al. 1999; Miller et al. 1999a; Parush et al. 2007). In children, sensory processing disorders are measured by parent report on standardized scales (Dunn 1999), by standardized clinical assessments such as the Miller Assessment for Preschoolers and the Sensory Integration and Praxis Tests (Ayres 1989; Miller 1988) and by laboratory procedures involving exposure to repeated sensory stimulation while the child is engaged in a task.
Primate Studies
Primate studies have employed an assessment of tactile sensory responsivity for monkeys, using a procedure adapted from human studies of sensory processing disorder (Baranek and Berkson 1994; McIntosh et al. 1999). Human laboratory studies of sensory processing disorder administer repeated trials of tactile stimuli to the face and rate touch-aversive behaviors. In an analogous primate assessment, prenatal alcohol-exposed monkeys showed increased or exaggerated responses to sensory stimuli (Schneider et al. 2008, 2009), paralleling studies with children with fetal alcohol spectrum disorder (Jirikowic et al. 2008). Specifically, monkeys exposed to alcohol throughout pregnancy, in comparison to non-alcohol-exposed monkeys, showed an exaggerated withdrawal response to repeated tactile stimuli when tested in adulthood (Schneider et al. 2008).
Rodent Studies
In rodent studies, habituation and sensory responsiveness can be measured by respiratory responses and cardiac orienting to repeated trials of a novel odor. Prenatal alcohol-exposed neonatal rats showed a tendency for requiring more trials to habituate to olfactory stimuli than controls (Barron and Riley 1992). Neonatally alcohol-exposed rats also showed reduced habituation of the cardiac orienting reflex to a novel olfactory stimulus (Hunt and Phillips 2004). Rats exposed to alcohol both prenatally and neonatally showed alcohol-related alterations in somatosensory cortex activity, measured with C-Fos immuno-reactivity (Lawrence et al. 2008) and decreased cellular density in the somatosensory cortex (Miller 1997; Popova 2004). Finally, postnatal alcohol exposure induced hyperresponsiveness to mildly painful stimuli, measured by a tail flick test administered postnatally (Rogers et al. 2004). Thus, there is evidence that prenatal or neonatal alcohol exposure alters sensory responsivity to tactile, auditory, visual and olfactory stimuli in rodent studies.
Summary
Sensory processing problems have been shown in both standardized parent ratings and laboratory tests of auditory and visual processing in children exposed to alcohol during pregnancy compared with non-exposed children. Exposed children showed reduced attention and orienting as well as reduced habituation to repeated sensory stimuli and demonstrated exaggerated sensory responses to non-noxious stimuli. Recall that fetal alcohol-exposed human neonates showed reduced habituation to auditory and visual stimuli in comparison to non-exposed neonates (Streissguth et al. 1983) and showed reduced adaptability during the NBAS compared with non-exposed infants (Coles et al. 1985). Primate studies used a laboratory assessment adapted from the human test and reported exaggerated aversive responses to non-noxious tactile stimuli in monkeys exposed to alcohol throughout pregnancy compared with non-exposed monkeys. Rodents developmentally exposed to alcohol not only showed reduced habituation to repeated sensory stimuli and hyper-responsivity to mildly painful stimuli but also alterations in somatosensory cortex activity and reduced cellular density in the somatosensory cortex, compared with non-exposed rats. The overall picture of reduced habituation to repeated stimulation as well as hyper-responsivity suggests that regulatory brain mechanisms may be impaired. Whether this goes beyond the findings of altered somatosensory cortex documented in rodents will be addressed in a later section.
Learning Task Performance
Effects on learning tasks have been well documented in human, primate, and rodent models of prenatal and/or neonatal alcohol exposure (Clarren et al. 1992; Coles et al. 1991; Mattson et al. 2010; Schneider et al. 2001b).
Human Studies
One of the primary effects of prenatal alcohol exposure in humans is poor school performance (Howell et al. 2006). In the search for the core deficits responsible, researchers have come to emphasize failure of “executive control” (Coles et al. 1997; Green et al. 2009; Kodituwakku et al. 1995; Mattson et al. 1999, 2010; Rasmussen and Bisanz 2009; Vaurio et al. 2008). Executive function refers to voluntary planned behavior that requires the representation of the goal in working memory as well as the inhibition of task-inappropriate responses. Measures of executive function and spatial processing are particularly sensitive to prenatal alcohol exposure (Mattson et al. 2010). Deficits in an aspect of executive function termed cognitive flexibility, the ability to attend to multiple criteria at the same time and to shift attention during a task, has been shown in prenatal alcohol exposed children (Coles et al. 1997; Kodituwakku et al. 1995; Jacobson 1998; Mattson et al. 1999; Schonfeld et al. 2001). The brain areas thought to be involved in executive function tasks are primarily the basal ganglia and prefrontal cortex, part of the frontal-striatal loop (Casey et al. 1997).
Primate Studies
As mentioned earlier, learning task performance was measured in Clarren et al. (1992) study of 3- 6- and 24-week prenatal alcohol exposed (1.8 g/kg/ week) pig-tailed macaques. The 24-week alcohol-exposed monkeys took two to three times more trials to criterion than controls on learning and memory tests in the Wisconsin General Test Apparatus. The 24-week exposure group searched twice as many boxes to locate the food reward on the Hamilton Search Task as controls (Clarren et al. 1992).
Learning and memory in moderate dose alcohol-exposed monkeys at adolescence (32- to 34-month-old) was assessed using the nonmatch-to-sample task (NMS) in the Wisconsin General Test Apparatus (Harlow 1959; Schneider et al. 2001b). The NMS task involves both working memory and executive control. It requires the monkey to learn the rule that the nonmatching or novel object conceals the reward, shift attention from the previously seen object to the novel one, and remember whether an object was seen on the immediately preceding trial (working memory). Animals were also tested with 30-, 60-, and 120-s delays. Testers blind to conditions also rated five behavior categories after each session: irritability, activity, stereotypies, inhibition, and impulsivity. Monkeys exposed to alcohol throughout pregnancy took significantly more trials to reach the learning criterion in the NMS task compared with non- exposed monkeys. However, their performance was not affected by the time delays after initial learning. The alcohol-exposed monkeys were also rated as more irritable during testing. The monkeys exposed to both prenatal alcohol exposure and prenatal stress were rated as higher on stereotypies and activity level. Further, higher scores on irritability, activity, stereotypies and impulsivity were correlated with worse performance (more trials to criterion) during task acquisition (Schneider et al. 2001b). Thus, prenatal alcohol exposure, even at moderate levels, induced acquisition deficits on the NMS task and increased behavioral disturbances. Moreover, prenatal stress in combination with prenatal alcohol exposure induced more severe acquisition deficits and higher rates of behavioral stereotypy. These findings not only underscore the importance of evaluating prenatal alcohol exposure in the context of other risk factors in developmental outcomes but also show effects analogous to the executive control difficulties in children with FAS and FASD.
Rodent Studies
Rodent learning tasks that measure some aspects of executive function or inhibitory control have been used to assess the cognitive effects of alcohol exposure. For example, most rodent tests of executive function focus on inhibitory control, including reversal learning and passive avoidance. Animals are trained to produce a response, after which they are tested on either their ability to inhibit the learned response in the presence of a negative stimulus (passive avoidance) or to learn the opposite response (reversal). Prenatally alcohol-exposed rodents have been found to be deficient in reversal learning in some studies (Lee and Rabe 1999; Mihalick et al. 2001). Timing of alcohol exposure with respect to brain development may be one key factor. For example, Thomas and colleagues (1997) confirmed that exposure on postnatal day 6 resulted in reversal deficits in juvenile rats. O’Leary-Moore and colleagues (2006) found that prenatal exposure failed to impair reversal learning but confirmed that postnatal alcohol treatment (days 4–9) did.
For passive avoidance, prenatal exposure to alcohol resulted in deficits measured on postnatal day 18 or day 23 in females (Abel 1982; Barron and Riley 1990; Driscoll et al. 1982; Riley et al. 1979) as well as on postnatal day 50 (Abel 1982; Riley et al. 1979). However, passive avoidance deficits did not to persist into adulthood (Abel 1982). Again, the developmental timing of exposure appears to be important. Exposure during the entire gestational period or late gestation (gestation days 14–20) induced inhibition deficits while exposure on gestation days 7–13 did not (Driscoll et al. 1982).
Summary
Children with FAS and FASD show clear deficits in executive function and inhibitory control that are paralleled in both rodent and primate studies. Prenatal alcohol-exposed primates required more trials to reach the learning criterion on non-match-to-sample (executive function). Depending on the developmental timing, developmentally exposed rodents showed reduced response inhibition and impaired reversal learning.
Dopaminergic Underpinnings of Executive Function Effects of Prenatal Alcohol Exposure
Human Studies
A decade of neuroscience research has explored the neurological underpinnings of prenatal alcohol effects, including poor response inhibition, executive function, attention, hyperactivity, and compromised sensory processing. Human studies in fetal alcohol exposed children compared with controls using MRI and Diffusion Tensor Imaging (DTI) have found deficits in overall brain size, abnormalities of grey and white matter particularly in the frontal lobes, abnormal development of the cerebellum, volume deficits in the basal ganglia, and increased cortical thickness (Astley et al. 2009; Chen et al. 2003; Mattson et al. 1996, 1999, Riley and McGee 2005; Sowell et al. 1996, 2001; 2008).
Frontal-striatal circuitry, which is dependent on dopamine (DA) function and involved in inhibitory control and executive function in humans, has been shown to be disrupted by fetal alcohol exposure (Crocker et al. 2011; Kodituwakku et al. 1995; Mattson et al. 1999; Sari et al. 2010). Several lines of evidence suggest that hypofunction of the DA system may be related to the attention deficits and hyperactivity problems reported in children with FASD. For example, a reduction in the DA metabolite homovanillic acid in cerebrospinal fluid has been found in children with ADHD (Shaywitz et al. 1977). Moreover, the caudate nucleus, one of the major DA output areas, is disproportionately smaller in both ADHD and FAS children (Castellanos et al. 1994; Mattson et al. 1994). DA system regulation and alterations in behavioral function produced by DA dysregulation could contribute to the high prevalence of mental health problems in individuals with FASD (Nestler et al. 2002; Streissguth et al. 1996).
Primate Studies
Experimental animal studies show that prenatal alcohol exposure can cause permanent changes in brain DA function, which, in turn, could underlie behavioral deficits. Clarren’s group, working with pig-tailed macaques, found dose-dependent prenatal alcohol effects on striatal dopamine (DA) neurochemistry (Astley et al. 1995). There was a negative correlation between offspring striatal DA (measured by tritiated spiroperiodol) and maternal blood alcohol concentration in pig-tailed macaques exposed to high dose alcohol (Clarren et al. 1990). Also, groups receiving 2.5 g/kg/week dose and above also showed a 12% deficit in Purkinje cell numbers in the cerebellum (after sacrifice) but did not show discernable facial dysmorphology, indicating that neuronal damage can occur without the full FAS diagnosis (Bonthius et al. 1996). Alcohol-treated pig-tailed macaques showed more axons in the corpus callosum than controls, suggesting that the pruning of callosal axons that normally occurs in developing macaques was partially blocked by prenatal alcohol exposure (Miller et al. 1999b). Thus, the work from Clarren’s group suggests that cognitive deficits could be induced by DA dysfunction in the striatum or by overabundance of axons and dendrites, presumably from interference with substances that regulate typical primate neuronal growth (Miller et al. 1999b).
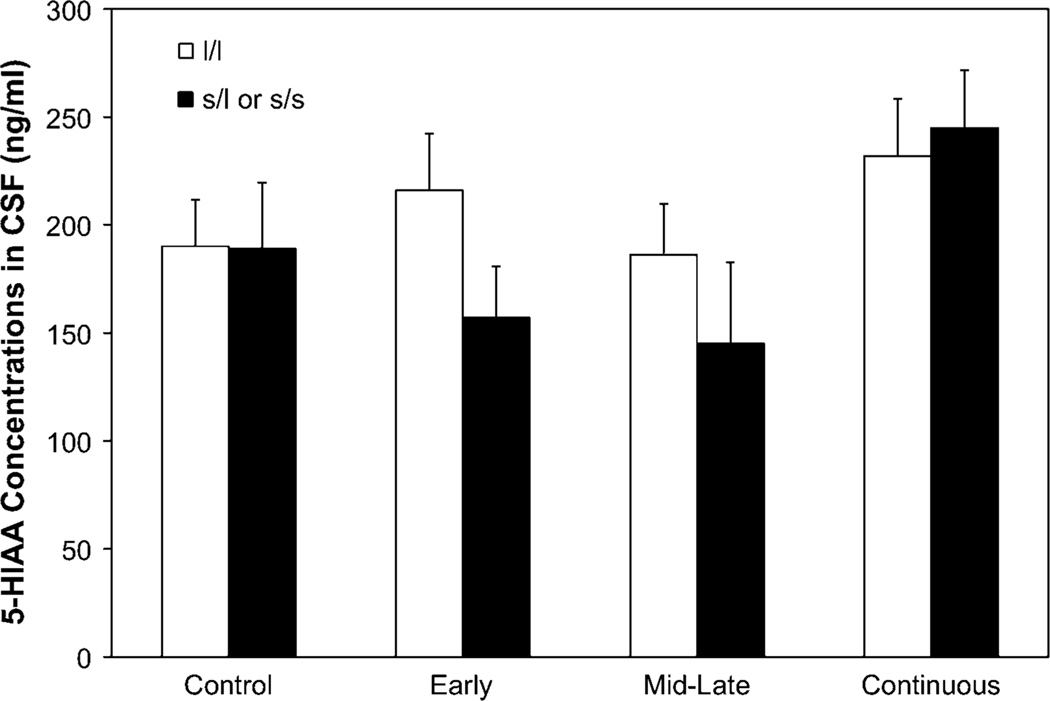
Schneider and colleagues (2011) examined dopamine and serotonin function in rhesus macaques exposed to moderate level alcohol during pregnancy compared with non-exposed monkeys. Serotonin and dopamine metabolites were measured in cerebrospinal fluid (CSF) during social separations from cage-mates for 3 days when they were 30 months of age. Monkeys exposed to alcohol during early or mid-to-late gestation and who carried the short variant of the serotonin transporter allele showed lower concentrations of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in CSF both at baseline and after separation from cage mates. A similar trend was found at baseline (pre-separation) for the DA metabolite, homovanillic acid (HVA) (Schneider et al. 2011) (see Fig. 4). Although these are in vivo measures, a drawback is that the measurement of CSF metabolites reflects global CNS changes not linked to specific brain areas.
Fig. 4.
Effect of prenatal alcohol exposure and genotype on cerebrospinal fluid concentrations of 5-HIAA averaged across time (baseline and post-separation). White bars represent monkeys homozygous for the long rh5-HTTLPR allele. Black bars represent monkeys carrying the short (s/l or s/s) allele. From “Moderate prenatal alcohol exposure and serotonin genotype interact to alter CNS serotonin function in rhesus monkey offspring,” M. L. Schneider, C. F. Moore, C. S. Barr, J. A. Larson, and G. W. Kraemer, in press, Alcoholism: Clinical and Experimental Research, 35, p. 5. Copyright 2011. Reprinted with permission
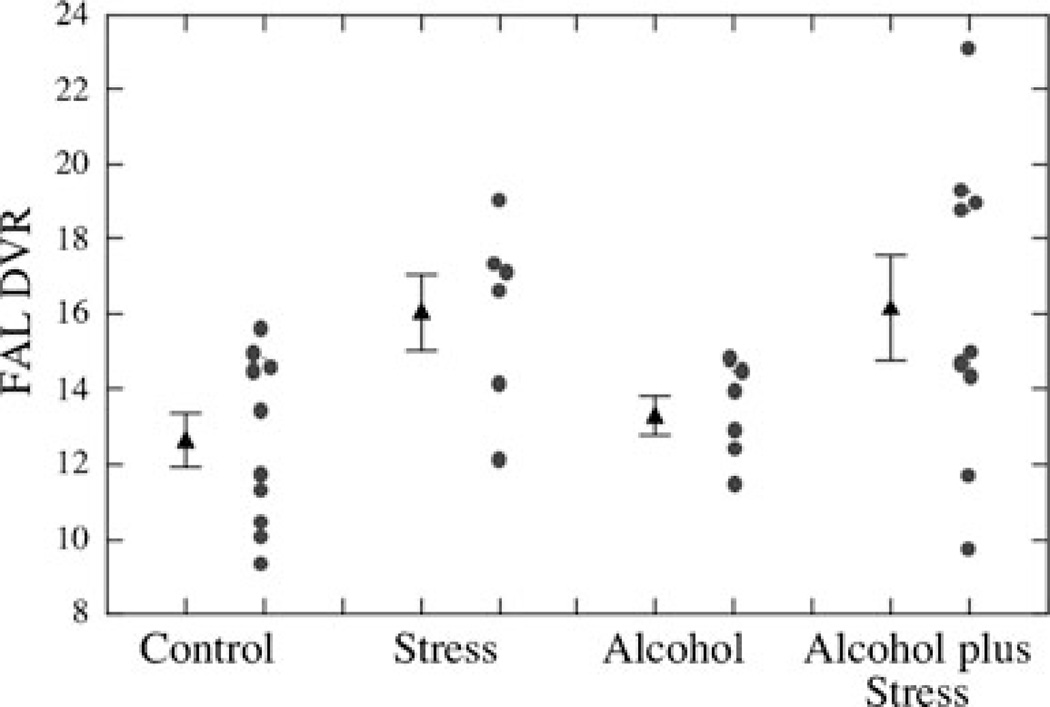
Using the same animals, DA system function was examined during adulthood with high-resolution positron emission tomography (PET) targeting dopaminergic receptor availability in the striatum (Roberts et al. 2004). Monkeys exposed to alcohol in combination with prenatal stress showed increased striatal D2R availability compared with non-exposed monkeys (Roberts et al. 2004) (see Fig. 5). This finding agrees with the findings for the DA metabolite, HVA. DA receptors upregulate when there is less DA in the synapse (Donnan et al. 1991). Moreover, increased striatal D2R availability was positively associated with trials to reach criterion on the non-match-to-sample cognitive task and negatively associated with tester’s rating of behavioral inhibition during task performance (Roberts et al. 2004). Thus, these data provide evidence for a link between reduced inhibitory control and upregulated striatal DA receptor availability induced by prenatal alcohol exposure. These findings are consistent with those of Clarren and Bowden (1982) showing that DA receptor availability measured by tritiated spiroperiodol after sacrifice was significantly increased in the putamen in prenatal alcohol-exposed pig-tailed macaques compared with controls.
Fig. 5.
Comparisons of dopaminergic parameters measured with PET in control versus alcohol plus stress groups. Group means and S.E.M. are shown. DVR for FAL binding on postsynaptic D2 receptors is shown (p=0.007). From “Prenatal stress, moderate fetal alcohol, and dopamine system function in rhesus monkeys,” A. D. Roberts, C. F. Moore, O. T. DeJesus, T. E. Barnhart, J. A. Larson, J. Mukherjee, et al., 2004, Neurotoxicology and Teratology, 26, p. 173. Copyright 2004. Reprinted with permission
Rodent Studies
Both prenatal and neonatal alcohol exposure have been shown to disrupt the DA system, including a reduction in spontaneous activity in DA neurons, enhanced response to DA agonists and decreased DA metabolite homovanillic acid (HVA) in DA neurons (Choong and Shen 2004; Druse et al. 1990; Zhou et al. 2003). Rodent studies provide some evidence that prenatal alcohol-induced DA system deficits might underlie some of the behavioral problems associated with FASD. For example, hyperactivity during early development can be produced by chemical depletion of midbrain DA neurons in neonatal rats (Erinoff et al. 1979; Shaywitz et al. 1976) or by administering alcohol prenatally (Bond 1984; Shaywitz et al. 1979). Of course, a variety of other neurological effects from developmental alcohol exposure have been documented in rodents, including adverse effects on the hippocampus, cerebellum, somatosensory cortex, and olfactory bulb (Barnes and Walker 1981; Bonthius et al. 1992; Gil-Mohapel et al. 2010; Hamre and West 1993; Maier and West 2001; Miller and Potempa 1990; Pierce et al. 1999; Uban et al. 2010). However, the DA system has been emphasized here because of its importance in executive and inhibitory control, areas that FASD children show marked deficits.
Summary
Rodent and primate experimental studies provide evidence that prenatal alcohol exposure can produce permanent changes in brain DA function. Lower DA metabolism and upregulation of receptors may underlie some of the behavioral effects, including attention deficits and hyperactivity, sensory and motor deficits and compromised executive function shown in human, primate, and rodent studies. Primate studies using high resolution PET have shown increased striatal D2 receptor availability in monkeys exposed to the combination of alcohol and stress during pregnancy compared with non-exposed controls, which are likely to underlie behavioral effects. These rodent and primate findings are translatable to human findings of reductions of basal ganglia volume in people with FAS and suggest that reduced DA function may underlie frontal-striatal circuitry deficits in people with FASD.
Conclusion
Both human correlational and animal experimental research show altered function beginning in the neonatal period. Later in life deficits in attention and hyperactivity, sensory processing and habituation, executive function, and inhibitory control were shown as a result of prenatal alcohol exposure. Experimental data from rodent and primate studies, together with correlational data from humans, suggest that altered DA function may underlie some of the behavioral deficits observed in people with FASD. Moreover, primate studies show that the behavioral and dopamine effects can result from even moderate dose prenatal alcohol exposure, equivalent to approximately two drinks per day for an average size woman. In primates, the most severe effects of fetal loss and behavioral impairments were found under conditions either of high dose prenatal alcohol (greater than approximately 200 mg/dL) or when the developing fetus was exposed to both prenatal alcohol and prenatal stress. Both animal and human research suggests that some individuals may be more susceptible to the adverse effects of prenatal alcohol exposure due to their heritable predispositions. Finally, the primate moderate dose behavioral findings fill a translational gap between human correlation results and rodent research. In sum, this overview of behavioral outcomes of prenatal alcohol exposure in animal models and the relation of those phenomena to findings in humans shows clearly that attributing causality to prenatal alcohol exposure for at least some of the human phenomena is warranted.
Acknowledgement
This study was supported by AA10079 and AA12277 from the National Institute of Alcoholism and Alcohol Abuse to M. L. Schneider.
Contributor Information
Mary L. Schneider, Email: schneider@education.wisc.edu, Harlow Center for Biological Psychology, University of Wisconsin-Madison, 22 North Charter Street, Madison, WI 53715, USA; Department of Kinesiology, University of Wisconsin-Madison, Madison, WI, USA; Department of Psychology, University of Wisconsin-Madison, Madison, WI, USA.
Colleen F. Moore, Department of Psychology, University of Wisconsin-Madison, Madison, WI, USA
Miriam M. Adkins, Department of Kinesiology, University of Wisconsin-Madison, Madison, WI, USA
References
- Abel EL. In utero alcohol exposure and developmental delay of response inhibition. Alcoholism, Clinical and Experimental Research. 1982;6(3):369–376. doi: 10.1111/j.1530-0277.1982.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Berman RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicology and Teratology. 1994;16(5):467–470. doi: 10.1016/0892-0362(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicology and Teratology. 1995;17(4):445–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Als H. A synactive model of neonatal behavioral organization. Physical & Occupational Therapy in Pediatrics. 1986;6(3):3–53. [Google Scholar]
- Altshuler HL, Shippenberg TS. A subhuman primate model for fetal alcohol syndrome research. Neurobehavioral Toxicology and Teratology. 1981;3(2):121–126. [PubMed] [Google Scholar]
- Astley SJ, Weinberger E, Shaw DW, Richards TL, Clarren SK. Magnetic resonance imaging and spectroscopy in fetal ethanol exposed Macaca nemestrina. Neurotoxicology and Teratology. 1995;17(5):523–530. doi: 10.1016/0892-0362(95)00012-g. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres AJ. Sensory integration and learning disorders. Los Angeles: Western Psychological Services; 1972. [Google Scholar]
- Ayres AJ. Sensory integration and praxis tests manual. Los Angeles: Western Psychology Services; 1989. [Google Scholar]
- Baranek GT, Berkson G. Tactile defensiveness in children with developmental disabilities: Responsiveness and habituation. Journal of Autism and Developmental Disorders. 1994;24(4):457–471. doi: 10.1007/BF02172128. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Walker DW. Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Brain Research. 1981;227(3):333–340. doi: 10.1016/0165-3806(81)90071-7. [DOI] [PubMed] [Google Scholar]
- Barron S, Riley EP. Passive avoidance performance following neonatal alcohol exposure. Neurotoxicology and Teratology. 1990;12(2):135–138. doi: 10.1016/0892-0362(90)90125-v. [DOI] [PubMed] [Google Scholar]
- Barron S, Riley EP. The effects of prenatal alcohol exposure on behavioral and neuroanatomical components of olfaction. Neurotoxicology and Teratology. 1992;14(4):291–297. doi: 10.1016/0892-0362(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Barron S, Kelly SJ, Riley EP. Neonatal alcohol exposure alters suckling behavior in neonatal rat pups. Pharmacology, Biochemistry and Behavior. 1991;39(2):423–427. doi: 10.1016/0091-3057(91)90202-d. [DOI] [PubMed] [Google Scholar]
- Barron S, Segar TM, Yahr JS, Baseheart BJ, Willford JA. The effect of neonatal ethanol and/or cocaine exposure on isolation-induced ultrasonic vocalizations. Pharmacology, Biochemistry and Behavior. 2000;67(1):1–9. doi: 10.1016/s0091-3057(00)00304-x. [DOI] [PubMed] [Google Scholar]
- Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2):401–409. doi: 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Lundahl KR, Caldwell J, Gilliam DM. Ethanol teratogenesis in the C57BL/6J, DBA/2J. and A/J inbred mouse strains. Alcohol. 1997;14(4):389–395. doi: 10.1016/s0741-8329(97)87950-5. [DOI] [PubMed] [Google Scholar]
- Bond NW. Prenatal alcohol exposure in rodents: A review of its effects on offspring activity and learning ability. Australian Journal of Psychology. 1981;33(3):331–344. [Google Scholar]
- Bond NW. Behavioural teratology: Fetal alcohol exposure and hyperactivity. In: Bond NW, editor. Animal models in psychopathology. Sydney: Academic; 1984. [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, West JR. Early postnatal alcohol exposure acutely and permanently reduces the number of granule cells and mitral cells in the rat olfactory bulb: A stereological study. The Journal of Comparative Neurology. 1992;324(4):557–566. doi: 10.1002/cne.903240408. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, Astley SJ, Clarren SK, West JR. Purkinje cell deficits in nonhuman primates following weekly exposure to ethanol during gestation. Teratology. 1996;53(4):230–236. doi: 10.1002/(SICI)1096-9926(199604)53:4<230::AID-TERA5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. The Journal of Neuroscience. 1993;13(7):2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT. A biology of misfortune: Stress reactivity, social context, and the ontogeny of psychopathology in early life. In: Masten A, editor. Multilevel dynamics in developmental psychopathology: Pathways to the future. 34th ed. Minneapolis: University of Minnesota; 2007. pp. 45–82. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Developmental Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brazelton TB. Assessment of the infant at risk. Clinical Obstetrics and Gynecology. 1973;16(1):361–375. doi: 10.1097/00003081-197303000-00020. [DOI] [PubMed] [Google Scholar]
- Brickson M, Bachevalier J. Visual recognition in infant rhesus monkeys: Evidence for a primitive memory process. Society for Neuroscience Abstracts. 1984;10:137. [Google Scholar]
- Brien JF, Loomis CW, Tranmer J, McGrath M. Disposition of ethanol in human maternal venous blood and amniotic fluid. Journal of American Obstetrics and Gynecology. 1983;146(2):181–186. doi: 10.1016/0002-9378(83)91050-5. [DOI] [PubMed] [Google Scholar]
- Brookes K, Mill J, Guindalini C, Curran S, Xu X, Knight J, et al. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Archives of General Psychiatry. 2006;63:74–81. doi: 10.1001/archpsyc.63.1.74. [DOI] [PubMed] [Google Scholar]
- Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, et al. Effects of prenatal alcohol exposure at school age. II. Attention and behavior. Neurotoxicology and Teratology. 1991;13(4):369–376. doi: 10.1016/0892-0362(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behavior Genetics. 2005;35(1):85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcoholism, Clinical and Experimental Research. 2005;29(3):443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sudgen K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Eckburg P, Marsh WL, Waituzis AC, Kaysen D, et al. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. The American Journal of Psychiatry. 1994;151(12):1791–1796. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- Chen JS, Driscoll CD, Riley EP. Ontogeny of suckling behavior in rats prenatally exposed to alcohol. Teratology. 1982;26(2):145–153. doi: 10.1002/tera.1420260206. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Maier SE, Parnell SE, West JR. Alcohol and the developing brain: Neuroanatomical studies. Alcohol Research & Health. 2003;27(2):174–180. [PMC free article] [PubMed] [Google Scholar]
- Chernoff GF. The fetal alcohol syndrome in mice: Maternal variables. Teratology. 1980;22(1):71–75. doi: 10.1002/tera.1420220110. [DOI] [PubMed] [Google Scholar]
- Choong KC, Shen RY. Methylphenidate restores ventral tegmental area dopamine neuron activity in prenatal ethanol-exposed rats by augmenting dopamine neurotransmission. The Journal of Pharmacology and Experimental Therapeutics. 2004;309(2):444–451. doi: 10.1124/jpet.103.060657. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Developmental Psychopathology. 1996;8:597–600. [Google Scholar]
- Clarren SK, Astley SJ. Pregnancy outcomes after weekly oral administration of ethanol during gestation in the pig-tailed macaque: Comparing early gestational exposure to full gestational exposure. Teratology. 1992;45(1):1–9. doi: 10.1002/tera.1420450102. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Bowden DM. Fetal alcohol syndrome: A new primate model for binge drinking and its relevance to human ethanol teratogenesis. The Journal of Pediatrics. 1982;101(5):819–824. doi: 10.1016/s0022-3476(82)80340-5. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. The New England Journal of Medicine. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Bowden DM, Astley SJ. Pregnancy outcomes after weekly oral administration of ethanol during gestation in the pig-tailed macaque (Macaca nemestrina) Teratology. 1987;35(3):345–354. doi: 10.1002/tera.1420350309. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ, Bowden DM, Lai H, Milam AH, Rudeen PK, et al. Neuroanatomical and neurochemical abnormalities in nonhuman primate infants exposed to weekly doses of ethanol during gestation. Alcoholism, Clinical and Experimental Research. 1990;14(5):674–683. doi: 10.1111/j.1530-0277.1990.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ, Gunderson VM, Spellman D. Cognitive and behavioral deficits in nonhuman primates associated with very early embryonic binge exposures to ethanol. The Journal of Pediatrics. 1992;121(5):789–796. doi: 10.1016/s0022-3476(05)81917-1. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Crispen HR, Shirtcliff EA, Schneider ML. Challenges to maternal wellbeing during pregnancy impact temperament, attention, ad neuromotor responses in the infant rhesus monkey. Developmental Psycho-biology. 2010;52(7):625–637. doi: 10.1002/dev.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Smith I, Fernhoff PM, Falek A. Neonatal neurobehavioral characteristic as correlates of maternal alcohol use during gestation. Alcoholism, Clinical and Experimental Research. 1985;9(5):454–460. doi: 10.1111/j.1530-0277.1985.tb05582.x. [DOI] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effect of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicology and Teratology. 1991;13(4):357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism, Clinical and Experimental Research. 1997;21(1):150–161. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcoholism, Clinical and Experimental Research. 2002;26(2):263–271. [PubMed] [Google Scholar]
- Crocker N, Nguyen TT, Mattson SM. Review of neuropsychological and behavioral effects of heavy prenatal alcohol exposure. Neuropsychology Review. 2011;21(2) doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Chang WP, Gavin WJ. Maturation of sensory gating performance in children with and without sensory processing disorders. International Journal of Psychophysiology. 2009;72(2):187–197. doi: 10.1016/j.ijpsycho.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Chou SP, Pickering RP. Subgroup variation in US drinking patterns: Result of the 1992 national longitudinal alcohol epidemiological study. Journal of Substance Abuse. 1995;7(3):331–344. doi: 10.1016/0899-3289(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Development. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Woodhouse DG, Kaczmarczyk SJ, Holder JE, Paxinos G, Chilco PJ, et al. Evidence for plasticity of the dopaminergic system in parkinsonism. Molecular Neurobiology. 1991;5:421–433. doi: 10.1007/BF02935563. [DOI] [PubMed] [Google Scholar]
- Downing C, Balderrama-Durbin C, Hayes J, Johnson TE, Gilliam D. No effect of prenatal alcohol exposure on activity in three inbred strains of mice. Alcohol and Alcoholism. 2009;44(1):25–33. doi: 10.1093/alcalc/agn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CD, Chen JS, Riley EP. Passive avoidance performance in rats prenatally exposed to alcohol during various periods of gestation. Neurobehavioral Toxicology and Teratology. 1982;4(1):99–103. [PubMed] [Google Scholar]
- Druse ML, Tajuddin N, Kuo A, Connerty M. Effects of in utero ethanol exposure on the developing dopaminergic system in rats. Journal of Neuroscience Research. 1990;27(2):233–240. doi: 10.1002/jnr.490270214. [DOI] [PubMed] [Google Scholar]
- Dunn W. The sensory profile. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Dursun I, Jakubowska-Doğru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult wistar rats. Pharmacology, Biochemistry and Behavior. 2006;85(2):345–355. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Elton RH, Wilson ME. Changes in ethanol consumption by pregnant pigtailed macaques. Journal of Studies on Alcohol. 1977;38(11):2181–2183. doi: 10.15288/jsa.1977.38.2181. [DOI] [PubMed] [Google Scholar]
- Erinoff L, MacPhail RC, Heller A, Seiden LS. Age-dependent effects of the 6-hydroxydopamine on locomotor activity in the rat. Brain Research. 1979;164:195–205. doi: 10.1016/0006-8993(79)90015-5. [DOI] [PubMed] [Google Scholar]
- Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. In: Lipsitt LP, editor. Advances in infancy research. Vol. 2. New York: Ablex; 1983. [Google Scholar]
- Fifer WP, Fingers ST, Youngman M, Gomez-Gribben E, Myers MM. Effects of alcohol and smoking during pregnancy on infant autonomic control. Developmental Psychobiology. 2009;51(3):234–242. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Porges SW. The relationship between neonatal heart period patterns and developmental outcome. Child Development. 1985;56(1):28–37. [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marijuana, cigarettes, and alcohol. Neurotoxicology and Teratology. 1992;14(5):299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Giknis ML, Damjanov J. The teratogenic and embryonic effects of alcohol in four mouse strains. Teratology. 1980;21:7. [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Research Reviews. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, et al. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB) Journal of Child Psychology and Psychiatry. 2009;50(6):688–697. doi: 10.1111/j.1469-7610.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Gunderson VM, Grant-Webster KS, Sackett GP. Deficits in visual recognition in low birth weight infant pigtailed monkeys (Macaca nemestrina) Child Development. 1989;60(1):119–127. [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcoholism, Clinical and Experimental Research. 1993;17(3):610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Harlow HF. The development of learning in the rhesus monkey. American Scientist. 1959;47:459–479. [Google Scholar]
- Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism - basic research and clinical implications. Journal of Neural Transmission. 1997;104(10):1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: A longitudinal follow-up. Journal of Pediatric Psychology. 2006;31(1):116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Phillips JS. Postnatal binge ethanol exposure affects habituation of the cardiac orienting response to an olfactory stimulus in preweanling rats. Alcoholism, Clinical and Experimental Research. 2004;28(1):123–130. doi: 10.1097/01.ALC.0000108650.02216.1A. [DOI] [PubMed] [Google Scholar]
- Jacobson SW. Specificity of neurobehavioral outcomes associated with prenatal alcohol exposure. Alcoholism, Clinical and Experimental Research. 1998;22(2):313–320. doi: 10.1111/j.1530-0277.1998.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SP, Sehgal P, Bronson R, Door B, Burnap J. Comparisons between an oral and intravenous method to demonstrate the in utero effects of ethanol in the monkey. Neurobehavioral Teratology and Toxicology. 1980;2:253–258. [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. Neonatal correlates of prenatal exposure to smoking, caffeine, and alcohol. Infant Behavior & Development. 1984;7:253–265. [Google Scholar]
- Jirikowic T, Olson HC, Kartin D. Sensory processing, school performance, and adaptive behavior of young school-age children with fetal alcohol spectrum disorders. Physical & Occupational Therapy in Pediatrics. 2008;28(2):117–136. doi: 10.1080/01942630802031800. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith MJ. Recognition of fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcoholism, Clinical and Experimental Research. 2004;28(3):489–496. doi: 10.1097/01.alc.0000117837.66107.64. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcoholism, Clinical and Experimental Research. 1995;19(6):1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kooistra L, Crawford S, Gibbard B, Ramage B, Kaplan BJ. Differentiating attention deficits in children with fetal alcohol spectrum disorder or attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2010;52(2):205–211. doi: 10.1111/j.1469-8749.2009.03352.x. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Moore CF, Newman TK, Barr CS, Schneider ML. Moderate level fetal alcohol exposure and serotonin transporter gene promoter polymorphism affect neonatal temperament and limbic-hypothalamic-pituitary-adrenal axis regulation in monkeys. Biological Psychiatry. 2008;63(3):317–324. doi: 10.1016/j.biopsych.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesman-Dwyer S, Keller LS, Streissguth AP. Naturalistic observations of newborns: effects of maternal alcohol intake. Alcoholism, Clinical and Experimental Research. 1978;2(2):171–177. doi: 10.1111/j.1530-0277.1978.tb04718.x. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Bonner HC, Newsom RJ, Kelly SJ. Effects of alcohol exposure during development on play behavior and c-Fos expression in response to play behavior. Behavior and Brain Research. 2008;188(1):209–218. doi: 10.1016/j.bbr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Rabe A. Infantile handling eliminates reversal learning deficit in rats prenatally exposed to alcohol. Alcohol. 1999;18(1):49–53. doi: 10.1016/s0741-8329(98)00067-6. [DOI] [PubMed] [Google Scholar]
- Lee KT, Mattson SN, Riley EP. Classifying children with heavy prenatal alcohol exposure using measures of attention. Journal of the International Neuropsychological Society. 2004;10(2):271–277. doi: 10.1017/S1355617704102142. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, et al. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: Alternative biallelic variation in rhesus monkeys. Journal of Neural Transmission. 1997;104(11–12):1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23(1):49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Martin DC, Martin JC, Streissguth AP. Sucking frequency and amplitude in newborns as a function of maternal drinking and smoking. Currents in Alcoholism. 1979;5:359–366. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism, Clinical and Experimental Research. 1998;22(2):279–297. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcoholism, Clinical and Experimental Research. 2002;26(6):875–882. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, et al. A decrease in the size of the basal ganglia following prenatal alcohol exposure: A preliminary report. Neurotoxicology and Teratology. 1994;16(3):283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism, Clinical and Experimental Research. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism, Clinical and Experimental Research. 1999;23(11):1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, et al. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2010;34(9):1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, Hagerman R. Sensory modulation disruption, electrodermal responses, and functional behaviors. Developmental Medicine and Child Neurology. 1999;41:608–615. doi: 10.1017/s0012162299001267. [DOI] [PubMed] [Google Scholar]
- Melcer T, Gonzalez D, Somes D, Riley EP. Neonatal alcohol exposure and early development of motor skills in alcohol preferring and nonpreferring rats. Neurotoxicology and Teratology. 1995;17(2):103–110. doi: 10.1016/0892-0362(94)00058-l. [DOI] [PubMed] [Google Scholar]
- Meyer LS, Riley EP. Behavioral teratology of alcohol. In: Vorhees CV, editor. Handbook of behavioral teratology. New York: Plenum; 1986. [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicology and Teratology. 2001;23(5):453–462. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Miller LJ. Miller Assessment for Preschoolers (MAP) San Antonio: Psychological Corporation; 1988. [Google Scholar]
- Miller MW. Effects of prenatal exposure to ethanol on callosal projection neurons in rat somatosensory cortex. Brain Research. 1997;766(1–2):121–128. doi: 10.1016/s0006-8993(97)00533-7. [DOI] [PubMed] [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. The Journal of Comparative Neurology. 1990;293(1):92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, et al. Electrodermal responses to sensory stimuli in individuals with Fragile X syndrome: A preliminary report. American Journal of Medical Genetics. 1999a;83(4):268–279. [PubMed] [Google Scholar]
- Miller MW, Astley SJ, Clarren SK. Number of axons in the corpus callosum of the mature Macaca nemestrina: Increases caused by prenatal exposure to ethanol. The Journal of Comparative Neurology. 1999b;412(1):123–131. [PubMed] [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcoholism, Clinical and Experimental Research. 1990;14(5):656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, Hannigan JH. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38(2):99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- O’Malley KD, Nanson JL. Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Canadian Journal of Psychiatry. 2002;47(4):349–354. doi: 10.1177/070674370204700405. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Jacobson SW, Weinberg J, Grunau RE, Molteno CD, Jacobson JL. Prenatal alcohol exposure alters biobehavioral reactivity to pain in newborns. Alcoholism, Clinical and Experimental Research. 2010;34(4):681–692. doi: 10.1111/j.1530-0277.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parush S, Sohmer H, Steinberg A, Kaitz M. Somatosensory function in boys with ADHD and tactile defensiveness. Physiology & Behavior. 2007;90:553–558. doi: 10.1016/j.physbeh.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Williams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcoholism, Clinical and Experimental Research. 1999;23(10):1650–1659. [PubMed] [Google Scholar]
- Popova EN. Structure of the sensorimotor area of the cerebral cortex in the offspring of alcoholized rats. Neuroscience and Behavioral Physiology. 2004;34(7):663–669. doi: 10.1023/b:neab.0000036004.15139.36. [DOI] [PubMed] [Google Scholar]
- Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Progress in Brain Research. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Development of cerebral cortex in human and nonhuman primates. In: Lewis M, editor. Child and adolescent Psychiatry. Baltimore: Williams and Wilkins; 1995. pp. 9–29. [Google Scholar]
- Rasmussen C, Bisanz J. Executive functioning in children with Fetal Alcohol Spectrum Disorders: Profiles and age-related differences. Child Neuropsychology. 2009;15(3):201–215. doi: 10.1080/09297040802385400. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Experimental Biology and Medicine. 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Riley EP, Rockwood GA. Alterations in suckling behavior in preweanling rats exposed to alcohol prenatally. Nutrition and Behavior. 1984;1:289–299. [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR. Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology. 1979;62(1):47–52. doi: 10.1007/BF00426034. [DOI] [PubMed] [Google Scholar]
- Riley EP, Barron S, Melcer T, Gonzalez D. Alterations in activity following alcohol administration during the third trimester equivalent in P and NP rats. Alcoholism, Clinical and Experimental Research. 1993;17(6):1240–1246. doi: 10.1111/j.1530-0277.1993.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Moore CF, DeJesus OT, Barnhart TE, Larson LA, Mukherjee J, et al. Prenatal stress, moderate fetal alcohol, and dopamine system function in rhesus monkeys. Neurotoxicology and Teratology. 2004;26(2):169–178. doi: 10.1016/j.ntt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rogers DT, Barron S, Littleton JM. Neonatal ethanol produces a hyperalgesia that extends into adolescence, and is associated with increased analgesic and rewarding properties of nicotine in rats. Psychopharmacology. 2004;171(2):204–211. doi: 10.1007/s00213-003-1574-z. [DOI] [PubMed] [Google Scholar]
- Rutter M, Sroufe LA. Developmental psychopathology: concepts and challenges. Development and Psychopathology. 2000;12(3):265–296. doi: 10.1017/s0954579400003023. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Developmental systems and psychopathology. Development and Psychopathology. 2000;12(3):297–312. doi: 10.1017/s0954579400003035. [DOI] [PubMed] [Google Scholar]
- Sari Y, Hammad LA, Saleh MM, Rebec GV, Mechref Y. Alteration of selective neurotransmitters in fetal brains of prenatally alcohol-treated C57BL/6 mice: Quantitative analysis using liquid chromatography/tandem mass spectrometry. International Journal of Developmental Neuroscience. 2010;28(3):263–269. doi: 10.1016/j.ijdevneu.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): Developmental changes, behavioral stability, and early experience. Infant Behavior & Development. 1992;15(2):155–177. [Google Scholar]
- Schneider ML, Moore CF, Suomi SJ, Champoux M. Laboratory assessment of temperament and environmental enrichment in rhesus monkey infants (Macaca mulatta) American Journal of Primatology. 1991;25:137–155. doi: 10.1002/ajp.1350250302. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induces attention and neuromotor impairments in primate infants. Child Development. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Becker EF. Timing of moderate alcohol exposure during pregnancy and neonatal outcome in rhesus monkeys (Macaca mulatta) Alcoholism, Clinical and Experimental Research. 2001;25(8):1238–1245. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: Learning and behavior in adolescent rhesus monkeys. Alcoholism, Clinical and Experimental Research. 2001;25(9):1383–1392. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: Perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary-adrenocortical axis response to stress is rhesus monkeys. Child Development. 2004;75(1):96–109. doi: 10.1111/j.1467-8624.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Champoux M, Moore CF. Neuro-behavioral assessment of nonhuman primate neonates. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. New York: Springer; 2006. pp. 215–247. [Google Scholar]
- Schneider ML, Moore CF, Gajewski LL, Larson JA, Roberts AD, Converse AK, et al. Sensory processing disorder in a primate model: evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child Development. 2008;79(1):100–113. doi: 10.1111/j.1467-8624.2007.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Larson JA, Barr CS, DeJesus OT, Roberts AD. Timing of moderate level prenatal alcohol exposure influences gene expression of sensory processing behavior in rhesus monkeys. Frontiers in Integrative Neuroscience. 2009;3:30. doi: 10.3389/neuro.07.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barr CS, Larson JA, Kraemer GW. Moderate prenatal alcohol exposure and serotonin genotype interact to alter CNS serotonin function in rhesus monkey offspring. Alcoholism, Clinical and Experimental Research. 2011;35(5):1–9. doi: 10.1111/j.1530-0277.2010.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. Journal of Studies on Alcohol. 2001;62(2):239246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Scott WJ, Jr, Fradkin R. The effects of prenatal ethanol in cynomolgus monkeys. Teratology. 1984;29(1):49–56. doi: 10.1002/tera.1420290107. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Yager RD, Klopper JH. Selective brain dopamine depletion in developing rats: An experimental model of minimal brain dysfunction. Science. 1976;191(4224):305–308. doi: 10.1126/science.942800. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Cohen DJ, Bowers MB. CSF monoamine metabolites in children with minimal brain dysfunction: evidence for alteration of brain dopamine. A preliminary report. The Journal of Pediatrics. 1977;90(1):67–71. doi: 10.1016/s0022-3476(77)80766-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Gordon JW, Klopper JH, Zelterman DA, Irvine J. Ontogenesis of spontaneous activity and habituation of activity in the rat pup. Developmental Psychobi-ology. 1979;12(4):359–367. doi: 10.1002/dev.420120410. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thomas JD, Riley ED, Ehlers CL. Neurophysiological consequences of neonatal ethanol exposure in the rat. Alcohol. 2004;34(2–3):187–196. doi: 10.1016/j.alcohol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reductions in lobules I-V. Alcoholism, Clinical and Experimental Research. 1996;20(1):31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: Effects of heavy prenatal alcohol exposure. Neurology. 2001;57(2):235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cerebral Cortex. 2008;18(1):136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton scale. Child Development. 1983;54(4):1109–1118. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Parrish-Johnson JC, Kirchner GL, Martin DC. Attention, distraction, and reaction time at age 7 years and prenatal alcohol exposure. Neurobehavioral Toxicology and Teratology. 1986;8(6):717–725. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein F. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE). Final report to the Centers for Disease Control and Prevention. August 1996. (Tech. Rep. No. 96–06) Seattle, WA: University of Washington Press; 1996. [Google Scholar]
- Suomi SJ, Higley JD. Rationale and methodologies for developing nonhuman primate models of prenatal drug exposure. NIDA Research Monograph. 1991;114:291–302. [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Developmental Psychobiology. 1996;29(5):433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Weinert SP, Sharif S, Riley EP. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcoholism, Clinical and Experimental Research. 1997;21(7):1218–1225. [PubMed] [Google Scholar]
- Uban KA, Silwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, et al. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Hormones and Behavior. 2010;58(5):835–843. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulug S, Riley EP. The effect of methylphenidate on overactivity in rats prenatally exposed to alcohol. Neurobehavioral Toxicology and Teratology. 1983;5(1):35–39. [PubMed] [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development. 2004;75(4):1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2008;14(1):119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30(8):724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Waltman R, Iniquez ES. Placental transfer of ethanol and its elimination at term. Obstetrics and Gynecology. 1972;40(2):180–185. [PubMed] [Google Scholar]
- West JR. Use of a pup in a cup model to study brain development. The Journal of Nutrition. 1993;123(2 suppl.):382–385. doi: 10.1093/jn/123.suppl_2.382. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Synaptogenesis in monkey somatosensory cortex. Cerebral Cortex. 1991;1(6):510–523. doi: 10.1093/cercor/1.6.510. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li TK. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Developmental Brain Research. 2003;144(1):43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]