Significance
Microalgae accumulate valuable compounds under conditions adverse to growth. For example, nutrient starvation causes accumulation of triacylglycerols but also induces cellular quiescence, characterized by the reversible cessation of growth. Among other factors, this inverse relationship between biomass productivity and triacylglycerol accumulation has long hampered efforts toward the efficient generation of biofuel feedstocks from microalgae. The discovery of a mutant and corresponding protein of Chlamydomonas reinhardtii affecting the orderly transition of algal cells from quiescence to normal growth provides mechanistic insights to address this problem. Quiescent cells also are found in plants and animals. Thus, understanding how Chlamydomonas CHT7 affects the exit out of quiescence promises to provide important insights into the regulation of cellular behavior in multicellular organisms as well.
Keywords: algae, lipid metabolism, nutrient stress, cellular quiescence, transcriptome
Abstract
Microalgae are prolific photosynthetic organisms that have the potential to sustainably produce high-value chemical feedstocks. However, an industry based on microalgal biomass still is faced with challenges. For example, microalgae tend to accumulate valuable compounds, such as triacylglycerols, only under stress conditions that limit growth. To investigate the fundamental mechanisms at the base of this conundrum—the inverse relationship between biomass production and storage compound accumulation—we applied a combination of cell biological and genetic approaches. Conceptually, nutrient deprivation, which commonly is used to induce the accumulation of triacylglycerol in microalgae, leads to a state of cellular quiescence defined by a halt of cell divisions that is reversible upon nutrient resupply. To identify factors that govern cellular quiescence, we screened for mutants of the model alga Chlamydomonas reinhardtii that, in contrast to wild-type cells placed under conditions of nitrogen deprivation, were unable to degrade triacylglycerols following nitrogen resupply. One of the mutants described here in detail, compromised hydrolysis of triacylglycerols 7 (cht7), was severely impaired in regrowth following removal of different conditions inducing cellular quiescence. The mutant carries a deletion affecting four genes, only one of which rescued the quiescence phenotype when reintroduced. It encodes a protein with similarity to mammalian and plant DNA binding proteins. Comparison of transcriptomes indicated a partial derepression of quiescence-related transcriptional programs in the mutant under conditions favorable to growth. Thus, CHT7 likely is a repressor of cellular quiescence and provides a possible target for the engineering of high-biomass/high-triacylglycerol microalgae.
Nutrient deprivation of microalgal cultures provides a facile experimental tool to induce and study triacylglycerol (TAG) accumulation in lipid droplets and is used in biotechnological settings for the production of high-value oils (1, 2). In particular, responses to the withdrawal of nitrogen (N) have been studied widely in the model unicellular green alga Chlamydomonas reinhardtii, and a comprehensive picture of N-sparing mechanisms during N deprivation is emerging through integrated global analysis of transcripts, proteins, and metabolites (3–5). Mechanisms of lipid droplet formation following N deprivation and proteins associated with lipid droplets are being explored (6–8), and mutants have become available that provide mechanistic insights in vivo into specific aspects of the lipid biosynthetic machinery of C. reinhardtii required for TAG accumulation (9–11).
From a cell biological viewpoint, N deprivation induces cellular quiescence, a reversible state of the cell cycle during which cell divisions temporarily cease and cells are reprogrammed to adjust metabolism for survival of the adverse condition (12). In C. reinhardtii, metabolic changes during N deprivation-induced quiescence include the partial degradation and reorganization of the photosynthetic apparatus and the protein biosynthetic machinery, induction of lipase and autophagy genes, and accumulation of carbon storage compounds (3–5). N deprivation also induces gametogenesis (13), allowing cells of opposite mating types to fuse and form thick-walled zygospores that can survive temporary harsh conditions. The goal of this study was to gain mechanistic insights into the regulation of N deprivation-induced quiescence in C. reinhardtii and to answer the question of how changes in the metabolic status of the algal cell induced by N deprivation affect progression through the cell cycle or the entry and exit into and out of quiescence. Toward this end, we searched for mutants of C. reinhardtii unable to rapidly exit quiescence, readjust their metabolism, and resume growth following N resupply after a period of N deprivation. As proxy for the metabolic status of the cell, we focused on TAG degradation following N resupply and identified a mutant and corresponding protein that met the criteria for a repressor of quiescence in C. reinhardtii.
Results
Isolation of compromised hydrolysis of triacylglycerols Mutants.
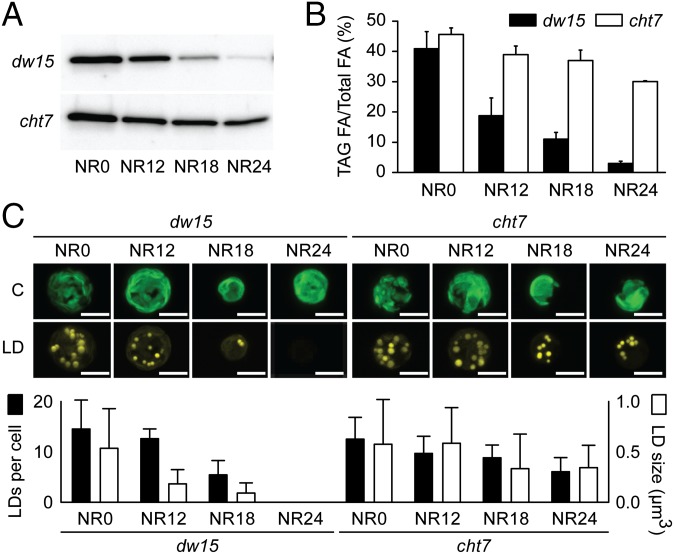
The amount of TAG in C. reinhardtii coincides with the abundance of the major lipid droplet-associated protein (MLDP) (7) (Fig. S1A). This correlation was used to identify mutants of C. reinhardtii with altered TAG degradation after the resupply of N to N-deprived cells. An immuno-dot blot-based assay for MLDP allowed us to screen indirectly for TAG abundance in a mutant population generated by random insertion of a selectable marker (Fig. S1B). Insertion lines were cultured in N-replete medium for 48 h, followed by 48 h of N deprivation, and then resupplied with N. Typically, 24 h after N resupply, most of the TAG accumulated during N deprivation was hydrolyzed and MLDP was degraded in the parental line (PL), dw15 (Fig. 1A). Putative mutants with a persistent MLDP immunosignal after 24 h N resupply were designated compromised hydrolysis of triacylglycerols (cht). Among the initial 1,760 insertion lines, eight putative cht mutants were identified, with cht7 showing the greatest delay in MLDP degradation (Fig. 1A). Importantly, TAG content also decreased with a severe delay in response to N resupply in cht7 (Fig. 1B). Lipid droplets observed following Nile red staining of cells of the PL started to decrease in size after 12 h of N resupply, and by 24 h, these cells were virtually devoid of lipid droplets (Fig. 1C). In contrast, cht7 cells retained lipid droplets even after 24 h.
Fig. 1.
Phenotypes of cht7. (A) Immunoblot of MLDP in the PL (dw15) and cht7 mutant following N resupply (NR) at times indicated (hours). (B) TAG degradation in dw15 and cht7 following N resupply. (C) Confocal microscopy images (Top) and lipid droplet quantification (Bottom) of Nile red-stained dw15 and cht7 cells following N resupply. Chlorophyll fluorescence (C) and Nile red fluorescence of lipid droplets (LD); scale bar, 5 µm. Lipid droplets of 5–20 cells, depending on the sample, were counted and their area was quantified with imaging software and their volume calculated. No lipid droplets were observed in dw15 cells at NR24. SD is indicated.
CHT7 Encodes a CXC Domain DNA Binding Protein Present in the Nucleus.
The cht7 mutant was crossed to a line of the opposite mating type. Abundance of TAG in meiotic progeny following N resupply cosegregated with the antibiotic marker, suggesting that a single nuclear mutation was responsible for the lipid phenotype (Fig. S2A). DNA/DNA hybridization blots confirmed the presence of a single insertion (Fig. S2B). With SiteFinding-PCR (14), the flanking sequences on both ends of the inserted hygromycin B marker were mapped and a 18,087-bp deletion affecting four predicted genes was discovered (Fig. 2A). Complementation of the defect was accomplished following transformation with a genomic fragment containing gene 1 bracketed by 1,000 bp on either side (Fig. S2 C and D). Expression of this gene in the complemented lines was confirmed by the presence of the CHT7 protein (Fig. S3B). Therefore, loss of gene 1 (Cre11.g481800, C. reinhardtii genome v5.3.1; CHT7) is the cause for the delayed TAG degradation following N resupply in cht7.
Fig. 2.
CHT7 is a CXC domain-containing protein present in the nucleus. (A) Structure of the cht7 genomic locus with gene 1 corresponding to CHT7. Black boxes, exons; white boxes, untranslated regions; arrow points, 3′-ends of ORFs. (B) Different CXC-domain proteins from different species: At, Arabidopsis thaliana; Ce, Caenorhabditis elegans; Cr, C. reinhardtii; Dm, Drosophila melanogaster; Gm, Glycine max; Hs, Homo sapiens. Black boxes, CXC domains. (C) Nuclear localization of CHT7-GFP by confocal microscopy in N-replete (+N) or N-deprived (−N) cells. The PL (dw15) and a transgenic line expressing EGFP-tagged CHT7 under the regulation of the endogenous CHT7 promoter in the cht7 mutant background (CHT7-GFP:cht7) are shown. The nuclear CHT7-GFP signal is marked by white arrows. The nuclei were stained with Hoechst 33342 (marked by yellow arrows). (D) Enrichment of CHT7 in nuclear extracts shown by immunoblot. Five micrograms of protein was loaded into each well. Markers: chloroplast (CP), OE33; nucleus (N), histone 3 (H3). CBB, Coomassie Brilliant Blue; WCL, whole-cell lysate.
The predicted CHT7 protein contains two cysteine-rich motifs comprising CXC domains (Pfam 03638) (15), initially defined in human tesmin (16) and Arabidopsis TSO1 (17) (Fig. 2B). TSO1 and other CXC proteins, e.g., soybean CPP1 (18), human LIN54 (19), and Caenorhabditis elegans Lin54 (20), have been shown to bind zinc (21) and specific DNA sequences through their CXC domains. To determine whether CHT7 is located in the nucleus, we added a C-terminal green fluorescent protein (GFP) to CHT7, thereby increasing its size by 30 kDa, and introduced it into the cht7 background (Fig. S3B). Phenotypes of cht7 were rescued in CHT7-GFP:cht7 transgenic lines (Fig. S3C), indicating that CHT7-GFP is functional and present in its correct location. The nucleus was visualized using the DNA-binding dye Hoechst 33342 (Fig. 2C, yellow arrows; Fig. S3A). Aside from strong chlorophyll fluorescence delineating the chloroplast, in N-replete CHT7-GFP:cht7 transgenic lines we observed only GFP-specific signals associated with the nucleus (Fig. 2C, white arrows). After 48 h of N deprivation, the cells were lysed when exposed to the Hoechst dye. Therefore, we examined the location of CHT7 in N-deprived cells without nuclear staining. GFP signals still were observed in the nucleus (Fig. 2C). Because of the ambiguities related to chlorophyll fluorescence, we used cell fractionation in combination with markers and confirmed the nuclear location of CHT7 and its absence from chloroplasts (Fig. 2D).
Absence of CHT7 Affects the Exit from Quiescence but Not Cell Viability.
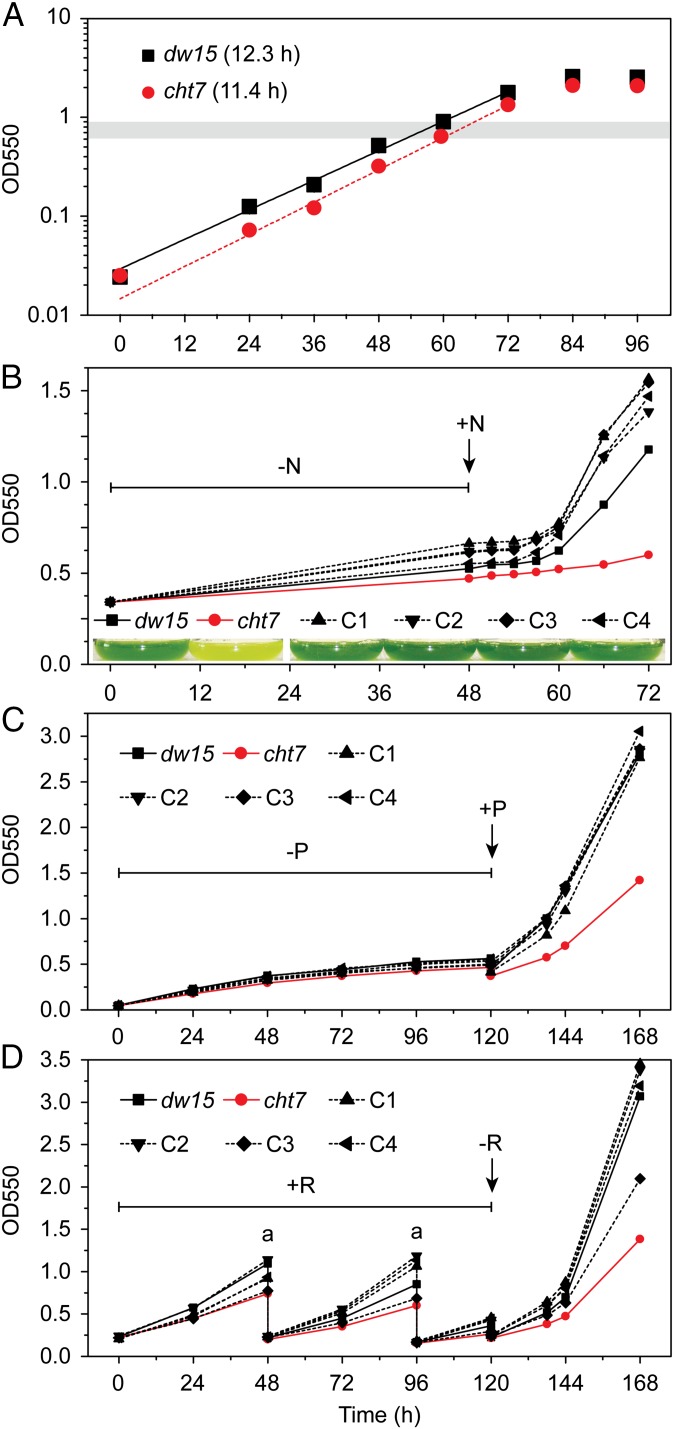
Growth of the cht7 mutant was normal in N-replete medium under standard conditions (Fig. 3A). However, when cht7 cells in liquid cultures had been deprived of N, which then was resupplied, growth was severely delayed (Fig. 3B). This delay in growth and the delay in TAG degradation following N resupply (Fig. 1B) suggested that the cht7 mutant struggles to reverse quiescence because of its inability to correctly perceive the N status of cells during resupply of N, because cht7 cells simply lose viability following N deprivation, or because of a general defect in the regulation of quiescence. To rule out the possibility of a specific N-sensing defect in cht7, we tested induction of quiescence by phosphate deprivation (Fig. 3C and Fig. S4A). The cht7 mutant also showed delayed regrowth when phosphate was resupplied to a deprived culture, suggesting a more general defect than N sensing. In yeast and many other organisms, the nutrient status of the cell to a large extent is integrated with progression through the cell cycle by the TOR (target of rapamycin) signaling pathway (22). Typically, rapamycin treatment induces quiescence, and we tested the effect of its removal in cht7. A saturating concentration of rapamycin (1 μM) as established for C. reinhardtii (23) doubled the time needed for division of the PL (23.9 h) and slightly more so for cht7 (30.1 h) (Fig. S4B). A striking difference was seen when rapamycin was removed: the cht7 mutant was much slower to regrow, similar to the effect of nutrient resupply (Fig. 3D and Fig. S4C). Thus, the defect in cht7 affects the ability of cells to exit quiescence, regardless of how it is induced.
Fig. 3.
Growth of cht7 affected by different treatments. (A) Growth of cht7 and PL (dw15) on N-replete medium (OD at 550 nm). Doubling times are given in parentheses. The gray bar indicates the OD at which N-replete samples were taken for all experiments in this study. Averages of three biological replicates are given, with SDs for all points being smaller than 3%. (B) Growth of dw15, cht7, and four independent complementation lines (C1–4) during N deprivation (−N) followed by N resupply (+N) measured at the times indicted. (Inset) Cultures at 24 h following N resupply. (C) Growth during phosphate deprivation (−P) followed by P resupply (+P). (D) Growth in the medium with 1 μM rapamycin (+R) followed by the removal of rapamycin (−R). Cultures were diluted twice (a) with medium containing 1 μM rapamycin to avoid reaching stationary phase.
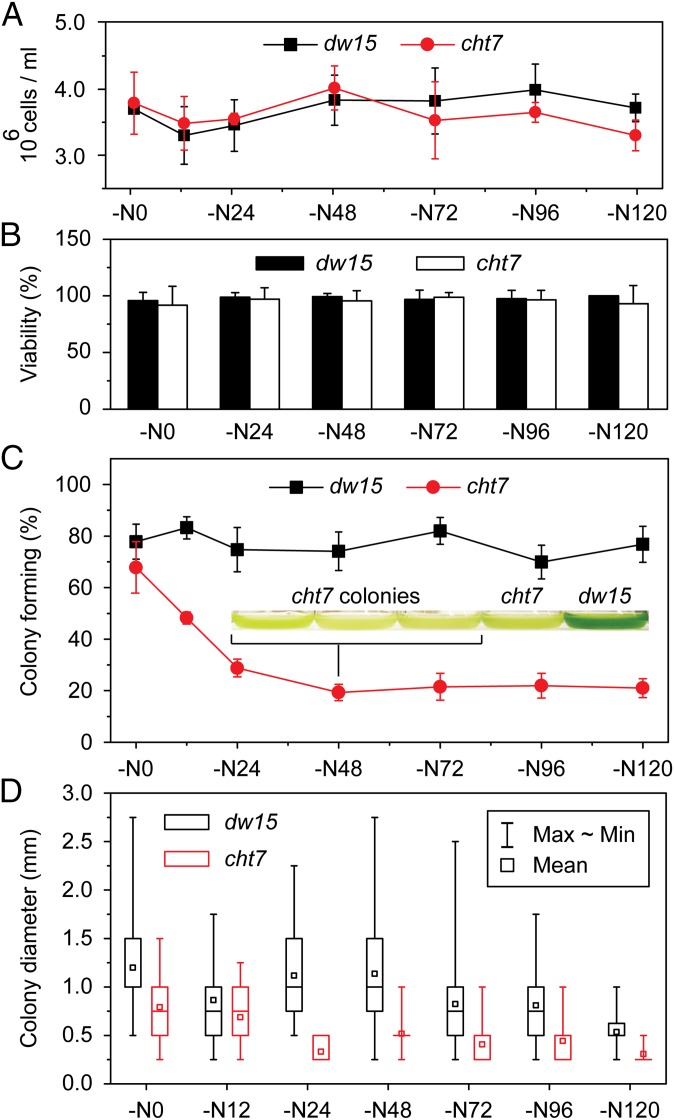
One trivial explanation for the growth phenotype is that most cht7 cells cannot survive N deprivation. The number and integrity of cells were assessed by using a hemocytometer and SYTOX Green, which does not penetrate living cells but stains the nuclear DNA of nonviable or partially lysed cells (24). Parallel cultures of PL and cht7 had a similar number of intact cells (Fig. 4A), and cells were similarly viable (Fig. 4B) during the 5 d following N deprivation. Moreover, following N deprivation, cht7 cells changed their metabolism to accumulate TAG (Fig. 1B, NR0) and they decreased RNA and protein synthesis (Fig. S4 D and E). In addition, cht7 cells increased RNA and protein synthesis following N resupply in parallel to the PL (Fig. S4 D and E). It should also be noted that cht7 cells were capable of normal mating following N deprivation. Therefore, the mutant had no defect in gametogenesis. Thus, we concluded that cht7 cells had not lysed, were viable, and were metabolically active and mating competent following N deprivation. However, when plated on agar-solidified N-replete medium at different times of N deprivation, the efficiency of cht7 colony formation (observed 11 d after plating and again after an additional 14 d with no further increase in numbers) decreased during the first 24 h following N deprivation to ∼20%, compared with 80% for the PL (Fig. 4C), and remained there. Presumably, during the first 24 h as cht7 cells become increasingly N-deprived, they enter quiescence, after which only 20% of the cells pass an apparent threshold or checkpoint, allowing for colony formation. These cht7 colonies had a decreased diameter (Fig. 4D) consistent with a delay in resumption of cell division. When the cht7 colonies were transferred to N-replete liquid medium, they grew at a rate similar to that of PL but again showed a delay in regrowth after N deprivation followed by resupply, recapitulating the original cht7 phenotype (Fig. 4C, Inset). Together, these observations are consistent with a regulatory defect in cht7 preventing most cht7 cells from orderly progression out of quiescence to resume normal growth, even though they are viable and metabolically active during N deprivation.
Fig. 4.
Viability and colony formation of cht7 during N deprivation. (A) Cell concentration of PL dw15 and cht7 during N deprivation (−N) at times (h) indicated, determined by using a hemocytometer. The averages of three to six measurements and SDs are indicated. (B) SYTOX Green viability staining of dw15 and cht7 cells shown in A at times indicated. Approximately 10–20 images showing 5–10 cells each were examined and averaged per time point. SD between images is indicated. (C) Colony formation of N-deprived (−N) dw15 and cht7 plated on N-replete solid medium at times indicated and incubated for 11 d. The average percentage of plated cells forming a colony on three plates is given. SD is indicated. (Inset) Three liquid cultures derived from cht7 colonies from this experiment (−N48), the original cht7 mutant, and dw15 PL first grown in N-replete medium then N deprived, followed by N resupply and growth for 24 h, recapitulating the original phenotype. (D) Size of the colonies formed by the N-deprived cells after plating on N-replete medium. All colonies on one plate were analyzed, with n ranging from 6 to 70 (usually fewer for cht7). Mean, maximum, minimum, and quartile values are indicated. Data points in all panels (A–D) were obtained in the same experiment.
Absence of CHT7 Partially Derepresses Quiescence-Associated Transcriptional Programs.
Because CHT7 resembles known DNA binding proteins, we asked whether a change in global transcriptional profiles during or even before entering quiescence might explain the observed phenotypes of cht7. Global transcript profiles of cht7 and the PL were compared by RNA sequencing (RNA-Seq) during midlog phase of an N-replete culture and after 48 h of N deprivation [Fig. S5A; for complete datasets and enriched Gene Ontology (GO) categories, refer to Datasets S1 and S2]. To confirm the findings obtained by RNA-Seq and to test for effects specific to the loss of CHT7, the expression of selected genes was tested by quantitative PCR (qPCR) in the PL, cht7, and multiple complementation lines. The expression of selected genes observed by RNA-Seq (three independent biological repeats) was comparable to that measured by qPCR (correlation coefficient R2 = 0.8065; Fig. S5B).
In line with previously reported transcriptional changes for C. reinhardtii (3–5) following N deprivation, 2,647 genes were up-regulated and 3,346 down-regulated in PL (dw15) N-deprived cells compared with PL N-replete cells (Fig. 5A, blue circles; twofold cutoff, P < 0.05). Comparing cht7 N-replete with PL N-replete cells, 1,477 genes were found up-regulated and 1,491 down-regulated in cht7 (Fig. 5A, yellow circles). Most strikingly, there was a substantial overlap in genes up-regulated (573) and down-regulated (894) between the two comparisons (Fig. 5A, intersecting blue and yellow circles; Dataset S3). In other words, a subset of genes, i.e., 49% of all genes that were misregulated in cht7 during N-replete conditions, were expressed as if the cells had already entered quiescence. Examples are genes involved in photosynthesis, such as PSBS1, MCA1, NAB1, and LHCBM4, and genes related to flagellum assembly, such as FA1, FA2, FAP139, and IFT46 (Fig. 5B; Fig. S5 C–E for qPCR confirmation). Autophagy is a hallmark of quiescence, and autophagy markers APG8 (Cre16.g689650) (25, 26) and APG3 (Cre02.g102350) were constitutively expressed in cht7, although at lower levels than in the PL following N deprivation. To further explore this pattern of transcriptional alterations in N-replete cht7, we asked whether these misregulated genes represent meaningful biological functions. In the PL N-deprived versus PL N-replete comparison (Fig. 5A, blue circles), differentially expressed genes were enriched in 68 GO categories, with 18 GO categories associated with flagellum assembly and 21 with photosynthesis (Dataset S2). Differentially expressed genes in the cht7 N-replete versus PL N-replete comparison (Fig. 5A, yellow circles) fell into six enriched GO categories, one of which was associated with flagellum assembly and two with photosynthesis. Indeed, virtually every gene involved in photosynthesis and 171 of 320 genes associated with flagellum assembly tended to be differentially regulated in the same manner in both comparisons (Fig. S5 A, F, and G), but not necessarily to the same extent. In most cases, differential gene expression was less pronounced in the cht7 N-replete versus PL N-replete comparison than in the PL N-deprived versus PL N-replete comparison (Fig. 5B; Fig. S5 A, F, and G; and Dataset S3).
Fig. 5.
Global gene expression comparison of N-replete and -deprived cells of PL dw15 and cht7. (A) Large circles: total number of genes changed in expression in a comparison of dw15 N deprived for 48 h and dw15 N replete (never deprived of N). Small circles: total number of genes changed in expression in a comparison of the cht7 mutant N replete and dw15 N replete. Circle size is proportional to the number of genes. (B) RNA-Seq log2 (fold change) of representative genes encoding the indicated proteins related to photosynthesis (PS), flagellum (FL), or autophagy (AP).
CHT7 Levels Remain Constant in Response to the N Supply, and CHT7 Is in a Large Complex.
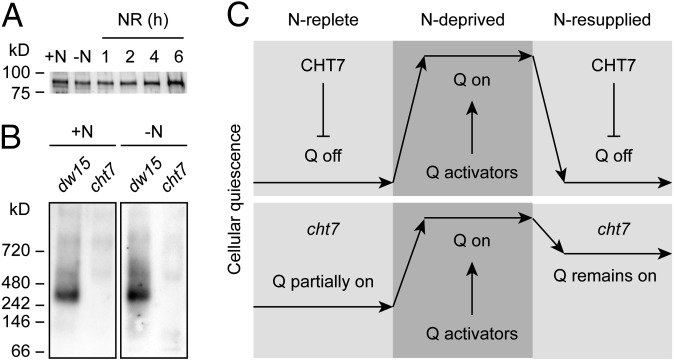
One may hypothesize that to exert its effects on gene expression related to quiescence, CHT7 abundance changes in response to the N supply. However, immunoblotting indicated that CHT7 protein abundance (see Fig. S6A for purity of CHT7 antiserum) was relatively constant during the conditions tested, which included N deprivation and several time points following resupply of N (Fig. 6A). Using blue native (BN) gel electrophoresis, we also determined that CHT7 is part of a larger protein complex that does not change in apparent size or abundance following N deprivation (Fig. 6B; see Fig. S6B for 2D electrophoresis).
Fig. 6.
Abundance of CHT7 and hypothesis for CHT7 function. (A) Anti-CHT7 immunoblot showing CHT7 protein levels in the presence (+N) and absence of N (−N) or after N resupply (NR) at times (hours) indicated. Equal amounts of protein were loaded. (B) Immunodetection of a high molecular weight CHT7 complex by BN-PAGE. Whole-cell lysates were loaded at equal protein, and cht7 was used as a negative control to discriminate against nonspecific signals. dw15, PL of cht7; +N, N replete; –N, N deprived. (C) We hypothesize that CHT7 acts as a repressor in safeguarding against the premature activation of global transcriptional changes associated with quiescence (Q) during N-replete growth and also to fully revert quiescence after N resupply. Activators of quiescence are postulated to fully turn on quiescence following N deprivation, but their inactivation after N resupply is insufficient in the absence of CHT7 to restore growth.
Discussion
Regulatory proteins that participate in the integration of the metabolic status of the cell with cell cycle activity are of fundamental biological importance and also are potential targets to maximize algal biomass and its TAG content by engineering. The unicellular alga C. reinhardtii provides an excellent genetic model for a photosynthetic eukaryotic cell, in which nutrient status may be manipulated readily to induce and reverse cellular quiescence. Hence, we isolated a mutant, cht7, affected in the reversal of N deprivation-induced quiescence, i.e., the degradation of lipid droplet protein MLDP and TAG, and the regrowth of algal mutant cultures when N is resupplied. We have ruled out several trivial explanations for this phenotype, including loss of viability of the mutant during N deprivation or a specific deficiency in an N-signaling pathway. The delay in regrowth in response to phosphate refeeding and after removal of rapamycin-induced quiescence of cht7 (Fig. 3 C and D) points to a more general defect in cht7 in the integration of the nutrient status of the cell with the cell cycle.
CHT7 likely is localized in the nucleus and contains a putative CXC DNA binding motif (Fig. 2) consistent with its possible role as a regulator of gene expression. Thus, we hypothesized and subsequently showed that CHT7 affects transcriptional programs associated with N deprivation-induced quiescence. A subset of genes normally up- or down-regulated is misregulated in cht7 under N-replete conditions, consistent with a partial derepression of transcriptional programs characteristic for quiescence. The extent of these changes in the expression of individual genes, as well as the number of genes affected, is smaller than observed following full induction of quiescence in response to N deprivation in the PL, which may explain the apparent lack of a growth phenotype of cht7 under the N-replete conditions tested (Fig. 3A). Thus, although quiescence programs are fully off under N-replete conditions in the PL, they are partially on in cht7, as summarized in the model in Fig. 6C. Based on this observation, CHT7 appears to act as a repressor of a subfraction of the transcriptional program associated with quiescence. During N deprivation, activators likely come into play to establish full quiescence to the same extent in cht7 and the PL, as they behave similarly: for example, accumulating TAG and ceasing to divide without loss of viability. However, the delayed growth of cht7 following N resupply suggests that CHT7 is needed to turn off quiescence-associated programs to reestablish growth rapidly (Fig. 6C). One may postulate that any number of repressors and activators of quiescence have to be balanced out during quiescence exit and that the absence of CHT7 in the mutant shifts this balance. Assuming the involvement of multiple inputs and regulatory components besides CHT7 to govern quiescence, the apparent threshold phenomenon documented in the ability of ∼20% of N-deprived cht7 cells to “escape deep quiescence” (Fig. 4C) seems plausible.
The delay in regrowth of the cht7 mutant when resupplied with N following deprivation is similar to the phenotype of the mat3 mutant (27). MAT3 is a C. reinhardtii ortholog of the mammalian retinoblastoma tumor suppressor protein (Rb) (28). Both CHT7 and Rb/MAT3 are present in the C. reinhardtii nucleus throughout the cell cycle (29). However, the absence of Rb/MAT3 leads to drastically reduced cell size, an essential cue in C. reinhardtii for decisions made during cell cycle progression, whereas cht7 cell size is normal (Fig. 2C and Fig. S3A). In general, Rb interacts directly with DNA-binding proteins, such as members of the E2F and DP protein families, to repress genes required for cell cycle progression during quiescence (28, 29). CXC domain proteins in animals have been found in large multiprotein complexes involved in transcriptional regulation that also contain Rb (28). Thus, the fact that CHT7 is a CXC domain protein present in a large complex (Fig. 6B) leads to intriguing questions regarding the precise regulatory function of CHT7, such as whether CHT7 and Rb/MAT3 might cooperate in the regulation of quiescence.
The persistence of CHT7 before, during, and after quiescence (Fig. 6A) suggests that fluctuation of its abundance likely is not part of the regulatory mechanism determining entry and exit into and out of quiescence. Changes in abundance or size of the CHT7 complex during quiescence also were not observed (Fig. 6B). Movement of CHT7 in and out of the nucleus also seems an unlikely mechanism to modulate CHT7, as we observed the protein in the nucleus before and during N deprivation (Fig. 2C). However, we currently cannot rule out posttranslational modifications of CHT7 depending on the nutritional status of the cell that may modulate its possible DNA binding preferences.
As summarized in Fig. 6C, all current data point toward a role of CHT7 as a repressor of a subset of transcriptional programs associated with nutrient deprivation-induced quiescence. Its activity likely is balanced by other regulatory factors, such as activators of quiescence. Its loss causes partial derepression of quiescence-associated transcriptional programs during N-replete conditions; CHT7 apparently is not needed for full establishment of quiescence during N deprivation, but its absence prevents the orderly and rapid exit from quiescence following N refeeding. As such, CHT7 is a candidate regulatory factor involved in the integration of the metabolic status of the cell and cell division, and its discovery provides a unique entry point for a more in-depth study of the regulation of cellular quiescence and the discovery of additional factors involved.
Methods
Strains, Genetic Analysis, and Growth Conditions.
C. reinhardtii cell wall-less strain dw15 (cw15, nit1, mt+) is referred to as the wild type (with regard to CHT7) PL throughout. Transgenic complemented lines of cht7 carrying a genomic fragment containing the intact CHT7 gene with 1-kb flanking sequences excised from the BAC 21K10 were generated. Cells were grown in Tris–acetate–phosphate (TAP) medium (30) under continuous light (70–80 μmol⋅m−2⋅s−1) at 22 °C or ambient room temperature (∼22 °C) for solid media (SI Methods).
Generation of MLDP and CHT7 Antibodies and Protein Analysis.
MLDP and CHT7 antibodies were raised against recombinant proteins in rabbits by Cocalico Biologicals, Inc. Antibody purification and quality control, as well as immunoblot analysis and protein gel electrophoresis, were done using standard procedures (SI Methods).
Mutant Screen.
Insertional mutagenesis was done as described previously (9), with modifications using a shorter fragment. The mutant screen was performed using an MLDP immunoblot procedure (Fig. S1 and SI Methods).
Confocal Microscopy.
Nile red staining was used to visualize lipid droplets and Hoechst 33342 to detect DNA in nuclei. To observe subcellular localization of CHT7, pMN24-CHT7-GFP was constructed to express the translational fusion under the control of the endogenous promoter and terminator. Oligonucleotide primers used are listed in Table S1. Confocal images were collected (SI Methods).
Phenotyping Assays.
Growth under different N and P regimes and following rapamycin treatment, plating, and viability assays, as well as lipid assays, were performed using established methods (SI Methods).
Standard DNA and RNA Procedures.
C. reinhardtii genomic DNA was isolated and DNA hybridizations were performed using standard procedures. To identify the locus disrupted by insertional mutagenesis, SiteFinding-PCR (14) was used with minor modifications. Templates for qPCR were prepared and qPCR was conducted using standard protocols. Oligonucleotide primers used in this study are listed in Table S1 and qPCR primers in Table S2 (SI Methods).
Illumina RNA Sequencing and Bioinformatics.
Three biologically independent sets of samples were prepared for each treatment at different times and submitted to the Michigan State University Research Technologies Service Facility (rtsf.natsci.msu.edu/) for single-end sequencing on an Illumina Genome Analyzer II (Illumina). The filtered sequence data were deposited at the National Center for Biotechnology Information Sequence Read Archive (www.ncbi.nlm.nih.gov/Traces/sra/) with the BioProject ID PRJNA241455 for the Illumina dataset. RNA abundance in the samples was computed using the CLC Genomics Workbench (www.clcbio.com/corporate/about-clc-bio/), version 5.5.1. Genome sequence and annotations were downloaded from the Joint Genome Institute (JGI; www.phytozome.net/chlamy.php). C. reinhardtii version 5.3.1 was used. Differential expression was determined by using the numbers of mapped reads overlapping with annotated C. reinhardtii genes as inputs to DESeq, version 1.10.1 (31). GO analysis of RNA-Seq data was performed with Goseq, version 1.10.1 (32) (SI Methods).
Supplementary Material
Acknowledgments
We thank Matt D. Larson from Michigan State University (MSU) Research Technology Support Facility and Nick A. Thrower from MSU Great Lakes Bioenergy Research Center for bioinformatics support. This work was supported by the US Air Force Office of Scientific Research [Grant FA9550-11-1-0264 (to C.B.)], by a Strategic Partnership grant from the MSU Foundation (to C.B. and B.B.S.), and by MSU AgBioResearch (C.B.). J.W. was supported by a Royal Thai Government Scholarship (Ministry of Science and Technology) and T.T. was supported in part by the Plant Biotechnology for Health and Sustainability Training Program at MSU (NIH T32 GM110523).
Footnotes
Conflict of interest statement: E.R.M. currently is an employee of Synthetic Genomics, Inc.
This article is a PNAS Direct Submission.
Data deposition: The filtered sequence data reported in this paper were deposited at the National Center for Biotechnology Information Sequence Read Archive, www.ncbi.nlm.nih.gov/Traces/sra/ (with BioProject ID PRJNA241455 for the Illumina dataset).
See Commentary on page 15610.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414567111/-/DCSupplemental.
References
- 1.Hu Q, et al. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008;54(4):621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- 2.Liu B, Benning C. Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotechnol. 2013;24(2):300–309. doi: 10.1016/j.copbio.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Blaby IK, et al. Systems-level analysis of nitrogen starvation-induced modifications of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. Plant Cell. 2013;25(11):4305–4323. doi: 10.1105/tpc.113.117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller R, et al. Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol. 2010;154(4):1737–1752. doi: 10.1104/pp.110.165159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmollinger S, et al. Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell. 2014;26(4):1410–1435. doi: 10.1105/tpc.113.122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodson C, Roth R, Wang ZT, Goodenough U. Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryot Cell. 2011;10(12):1592–1606. doi: 10.1128/EC.05242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moellering ER, Benning C. RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot Cell. 2010;9(1):97–106. doi: 10.1128/EC.00203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen HM, et al. Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: With focus on proteins involved in lipid metabolism. Proteomics. 2011;11(21):4266–4273. doi: 10.1002/pmic.201100114. [DOI] [PubMed] [Google Scholar]
- 9.Li X, et al. A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell. 2012;24(11):4670–4686. doi: 10.1105/tpc.112.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen HM, et al. The green microalga Chlamydomonas reinhardtii has a single ω-3 fatty acid desaturase that localizes to the chloroplast and impacts both plastidic and extraplastidic membrane lipids. Plant Physiol. 2013;163(2):914–928. doi: 10.1104/pp.113.223941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle NR, et al. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J Biol Chem. 2012;287(19):15811–15825. doi: 10.1074/jbc.M111.334052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valcourt JR, et al. Staying alive: Metabolic adaptations to quiescence. Cell Cycle. 2012;11(9):1680–1696. doi: 10.4161/cc.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck CF, Acker A. Gametic differentiation of Chlamydomonas reinhardtii: Control by nitrogen and light. Plant Physiol. 1992;98(3):822–826. doi: 10.1104/pp.98.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan G, et al. SiteFinding-PCR: A simple and efficient PCR method for chromosome walking. Nucleic Acids Res. 2005;33(13):e122. doi: 10.1093/nar/gni124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Punta M, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40(Database issue):D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugihara T, Wadhwa R, Kaul SC, Mitsui Y. A novel testis-specific metallothionein-like protein, tesmin, is an early marker of male germ cell differentiation. Genomics. 1999;57(1):130–136. doi: 10.1006/geno.1999.5756. [DOI] [PubMed] [Google Scholar]
- 17.Hauser BA, He JQ, Park SO, Gasser CS. TSO1 is a novel protein that modulates cytokinesis and cell expansion in Arabidopsis. Development. 2000;127(10):2219–2226. doi: 10.1242/dev.127.10.2219. [DOI] [PubMed] [Google Scholar]
- 18.Cvitanich C, et al. CPP1, a DNA-binding protein involved in the expression of a soybean leghemoglobin c3 gene. Proc Natl Acad Sci USA. 2000;97(14):8163–8168. doi: 10.1073/pnas.090468497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmit F, Cremer S, Gaubatz S. LIN54 is an essential core subunit of the DREAM/LINC complex that binds to the cdc2 promoter in a sequence-specific manner. FEBS J. 2009;276(19):5703–5716. doi: 10.1111/j.1742-4658.2009.07261.x. [DOI] [PubMed] [Google Scholar]
- 20.Tabuchi TM, et al. Chromosome-biased binding and gene regulation by the Caenorhabditis elegans DRM complex. PLoS Genet. 2011;7(5):e1002074. doi: 10.1371/journal.pgen.1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen SU, et al. The conserved cysteine-rich domain of a tesmin/TSO1-like protein binds zinc in vitro and TSO1 is required for both male and female fertility in Arabidopsis thaliana. J Exp Bot. 2007;58(13):3657–3670. doi: 10.1093/jxb/erm215. [DOI] [PubMed] [Google Scholar]
- 22.Gray JV, et al. “Sleeping beauty”: Quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2004;68(2):187–206. doi: 10.1128/MMBR.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crespo JL, Díaz-Troya S, Florencio FJ. Inhibition of target of rapamycin signaling by rapamycin in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol. 2005;139(4):1736–1749. doi: 10.1104/pp.105.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M, Murata Y, Mizusawa M, Iwahashi H, Oka S-I. A simple and rapid dual fluorescence viability assay for microalgae. Microbiol Cult Collect. 2004;20(2):53–59. [Google Scholar]
- 25.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Pérez ME, Florencio FJ, Crespo JL. Inhibition of target of rapamycin signaling and stress activate autophagy in Chlamydomonas reinhardtii. Plant Physiol. 2010;152(4):1874–1888. doi: 10.1104/pp.109.152520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armbrust EV, Ibrahim A, Goodenough UW. A mating type-linked mutation that disrupts the uniparental inheritance of chloroplast DNA also disrupts cell-size control in Chlamydomonas. Mol Biol Cell. 1995;6(12):1807–1818. doi: 10.1091/mbc.6.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadasivam S, DeCaprio JA. The DREAM complex: Master coordinator of cell cycle-dependent gene expression. Nat Rev Cancer. 2013;13(8):585–595. doi: 10.1038/nrc3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson BJ, et al. Regulation of the Chlamydomonas cell cycle by a stable, chromatin-associated retinoblastoma tumor suppressor complex. Plant Cell. 2010;22(10):3331–3347. doi: 10.1105/tpc.110.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris EH. Chlamydomonas Sourcebook. Academic Press; New York: 1989. [Google Scholar]
- 31.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010;11(2):R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.