Significance
Contamination of water and foods with arsenic (As) poses a threat to millions of people worldwide. Because the rice grain is the major source of As intake, reducing the transfer of As from soil to the grain is a pressing public health issue. We found that a member of the Oryza sativa C-type ATP-binding cassette transporter (OsABCC) family, OsABCC1, detoxifies As and reduces the amount of As in the rice grain. OsABCC1 in the upper nodes of rice plants restricts the distribution of As to the grain by sequestering it in the vacuoles of the phloem companion cells of diffuse vascular bundles directly connected to the grain. Our work suggests a strategy for limiting As accumulation in rice grains and thereby reducing human As exposure.
Keywords: vacuolar sequestration, ABC transporter, arsenic, rice, node
Abstract
Arsenic (As) is a chronic poison that causes severe skin lesions and cancer. Rice (Oryza sativa L.) is a major dietary source of As; therefore, reducing As accumulation in the rice grain and thereby diminishing the amount of As that enters the food chain is of critical importance. Here, we report that a member of the Oryza sativa C-type ATP-binding cassette (ABC) transporter (OsABCC) family, OsABCC1, is involved in the detoxification and reduction of As in rice grains. We found that OsABCC1 was expressed in many organs, including the roots, leaves, nodes, peduncle, and rachis. Expression was not affected when plants were exposed to low levels of As but was up-regulated in response to high levels of As. In both the basal nodes and upper nodes, which are connected to the panicle, OsABCC1 was localized to the phloem region of vascular bundles. Furthermore, OsABCC1 was localized to the tonoplast and conferred phytochelatin-dependent As resistance in yeast. Knockout of OsABCC1 in rice resulted in decreased tolerance to As, but did not affect cadmium toxicity. At the reproductive growth stage, the As content was higher in the nodes and in other tissues of wild-type rice than in those of OsABCC1 knockout mutants, but was significantly lower in the grain. Taken together, our results indicate that OsABCC1 limits As transport to the grains by sequestering As in the vacuoles of the phloem companion cells of the nodes in rice.
Arsenic (As) is a highly toxic metalloid that is classified as a nonthreshold class-1 carcinogen (1, 2). Long-term exposure to As in humans causes a number of diseases, including hyperpigmentation, keratosis, and skin and internal cancers (3). Due to As contamination of drinking water and soil from both anthropogenic and geogenic sources, millions of people worldwide suffer from As toxicity. This problem is particularly serious in countries in South and Southeast Asia, such as India and Bangladesh, where groundwater, which is used both as a drinking water supply and for irrigating rice, contains high concentrations of As (4). Therefore, reducing the As concentration in drinking water and foods is a critical goal for promoting human health.
Rice (Oryza sativa L.), a staple food of half of the world’s human population, is a major dietary source of As (5, 6). A recent cohort study in West Bengal, India showed that high concentrations of As in rice are associated with elevated genotoxic effects in humans (7). Rice accumulates As in the shoots and grains more efficiently than do other cereal crops such as wheat (Triticum aestivum) and barley (Hordeum vulgare) (8, 9). This higher efficiency has been attributed to the increased bioavailability of As under flooded conditions (such as those found in paddy fields) and the efficient As uptake system in rice (10–12). In the anaerobic paddy field, As is mainly present in the form of arsenite, which is taken up by two silicon (Si) transporters—namely, Lsi1 (low silicon 1), a Si influx transporter, and Lsi2 (low silicon 2), a Si efflux transporter (11). These transporters take up both arsenite and silicic acid, which have similar chemical properties. Although other transporters are also able to transport arsenite (11, 13, 14), Lsi1 and Lsi2 are the major transporters of arsenite in rice due to their high expression in the roots (11). Furthermore, the distinct cellular localization of Lsi1 and Lsi2 enables rice to take up Si and As more efficiently than other cereal crops (11, 15). Lsi1 and Lsi2 are localized to both the exodermis and endodermis of rice roots (16, 17). Whereas Lsi1 is localized to the distal side of these two cell layers, Lsi2 is localized to the proximal side, and the accumulation of high levels of Si (and most likely As as well) in rice depends on the cooperation of these two transporters (18).
After arsenite is taken up by the root cells, some of it is immediately released into the rhizosphere, a process that is partially mediated by Lsi1, a bidirectional channel (19). However, the remaining As is sequestered into the root vacuoles or translocated to the shoots and delivered to various organs (20). Phloem cells in the nodes contain high levels of As and appear to play important roles in delivering As to the grains (21), suggesting that this tissue might sequester As and thereby reduce delivery of As to the grain. Sequestration of As in phloem cells requires the presence of a vacuolar As transporter in this tissue. However, transporters involved in the vacuolar sequestration of As have hitherto not been identified in rice. Recently, two different types of vacuolar As transporters were discovered in the As hyperaccumulator fern (Pteris vittata) and Arabidopsis thaliana. PvACR3 from P. vittata compartmentalizes As into vacuoles, and knockdown of this gene results in As hypersensitivity (22). Thus, PvACR3 has been proposed to function as a transporter that is essential for As tolerance in the P. vittata gametophyte. Orthologs of PvACR3 have not been identified in angiosperms. However, two transporters (AtABCC1 and AtABCC2) belonging to the ATP-binding cassette (ABC) family were found to sequester As into the vacuoles in Arabidopsis (23). Both AtABCC1 and AtABCC2 transport phytochelatin (PC)–As complexes and atabcc1 atabcc2 double knockout plants exhibited As hypersensitivity (23). These findings indicate that AtABCC1 and AtABCC2 play a major role in As detoxification. Recently, vacuoles isolated from barley were shown to have a pattern of PC2–As transport similar to that of Arabidopsis vacuoles (24), suggesting that similar ABC transporters are involved in vacuolar sequestration in monocotyledonous plants such as barley and rice.
In the present study, we report that an ABC transporter, OsABCC1, is important for the vacuolar sequestration of As and therefore for reducing As accumulation in rice grains. OsABCC1, which is the only member of the ABC transporter family in the rice genome to exhibit a high degree of similarity to AtABCC1 and AtABCC2, forms a distinct cluster from other members in this family (Fig. S1) (23). Our detailed functional analysis revealed that OsABCC1 is involved in As detoxification and, more importantly, in reducing As levels in the rice grain by sequestering it in the node cell vacuoles.
Results
Expression Patterns of OsABCC1.
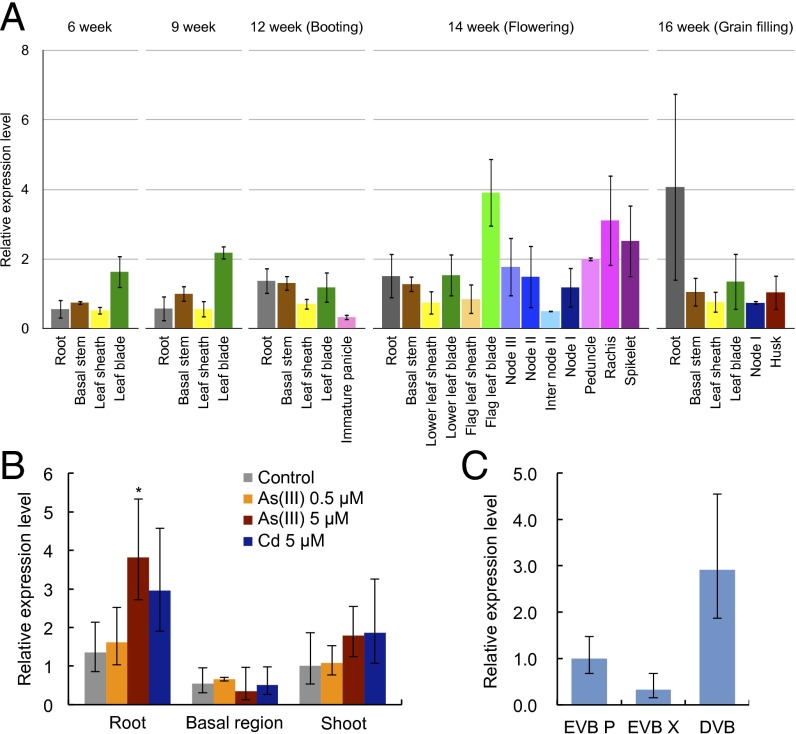
OsABCC1 shared 70% and 72% amino acid sequence identity, respectively, with AtABCC1 and AtABCC2 (Fig. S1 A and B). The expression pattern of OsABCC1 was investigated in different organs throughout the growth period of rice cultivated in paddy fields. OsABCC1 was expressed in all organs; at the vegetative growth stage, it was expressed in the roots, basal nodes, and leaves (Fig. 1A), and at the reproductive growth stage, in the roots, nodes, leaves, peduncles, rachis, and spikelets (Fig. 1A).
Fig. 1.
Expression pattern of OsABCC1. (A) Relative expression of OsABCC1 in various organs at different growth stages. Rice was grown in a paddy field, and various tissues were sampled at different growth stages. (B) Effect of As on OsABCC1 expression. Rice seedlings (17-d-old) were exposed to a solution containing 0, 0.5, or 5 µM arsenite or 5 µM Cd for 24 h. Significant differences from WT at *P < 0.05 by Tukey’s test. (C) Expression of OsABCC1 in different vascular tissues of node I separated by LMD at the milky stage. The expression level was determined by quantitative real-time RT-PCR. Expression relative to node I at the flowering stage (A), to the control roots (B), and to EVB P (C) is shown. HistoneH3 and Actin were used as internal standards. Data are means ± SD of three biological replicates. DVB, diffuse vascular bundle; EVB P, enlarged vascular bundle phloem; EVB X, enlarged vascular bundle xylem.
Whereas OsABCC1 expression in the roots was not affected by low levels of As exposure (0.5 µM), it was slightly up-regulated by 5 µM As (Fig. 1B). By contrast, in the basal node and shoot, the expression of OsABCC1 was unaffected by both low and high As concentrations (Fig. 1B). However, publicly available microarray data in different rice cultivars showed that OsABCC1 expression was consistently up-regulated by As in roots as well as in other organs (Fig. S2). This difference may be attributed to the high concentration of As (1 mg⋅L−1) used in the microarray analysis. To test this possibility, we evaluated OsABCC1 expression in rice plants exposed to high concentrations of As (50 µM). The expression in shoot and root was indeed up-regulated after 6 and 24 h of exposure (Fig. S3). However, OsABCC1 expression was not significantly affected by exposure to both low and high levels of cadmium (Cd) in any organ (Fig. 1B and Fig. S3).
The tissue specificity of OsABCC1 expression in highly developed vascular bundles of the uppermost node I at the flowering stage was investigated using laser microdissection (LMD). Higher expression of OsABCC1 was found in the diffuse vascular bundles (DVBs) than in the enlarged vascular bundles (EVBs) (Fig. 1C). In the EVBs, expression of OsABCC1 was higher in the phloem region (EVB P) than in the xylem region (EVB X) (Fig. 1C). The expression patterns of two other genes in the same samples, OsLsi6 in EVB X and OsHMA2 in EVB P and DVB (Fig. S4), matched the results from the previous reports (25, 26) and indicated the purity of the tissues sampled.
Cell Specificity of OsABCC1 Expression.
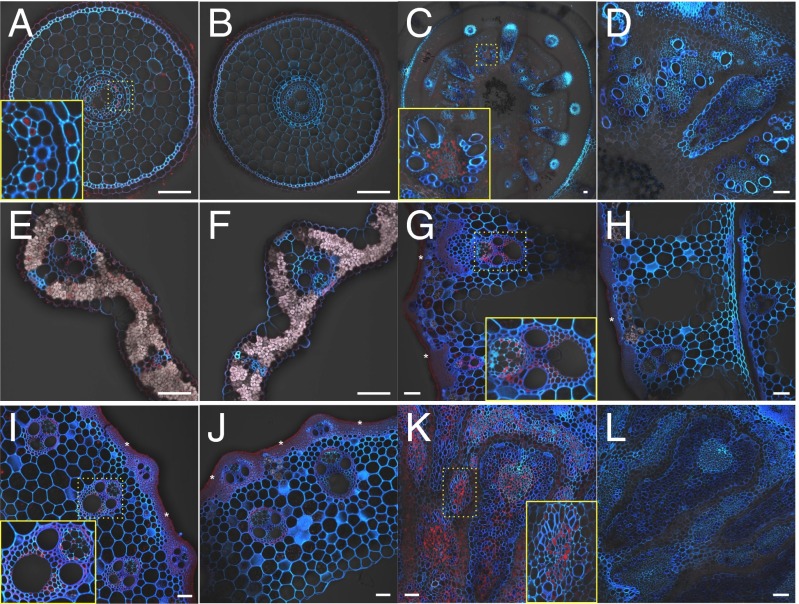
To investigate the cell specificity of OsABCC1 expression, we generated transgenic lines carrying GUS under the control of the OsABCC1 promoter. Immunostaining with a GUS antibody showed that the GUS signal was present in the exodermis and phloem region of the roots, basal nodes, and leaf blades at the vegetative growth stage (Fig. 2 A, C, and E). At the reproductive stage, the signal was also observed in vascular tissues mainly in the phloem region of leaf sheaths, internodes, and node I (Fig. 2 G, I, and K). No signal was observed in any tissues of wild type (Fig. 2 B, D, F, H, J, and L), demonstrating the specificity of the antibody.
Fig. 2.
Cell specificity of OsABCC1 expression. Immunostaining with a GUS antibody was performed in transgenic rice carrying the OsABCC1 promoter fused with GUS (A, C, E, G, I, and K) or WT as negative control (B, D, F, H, J, and L) in different organs at the vegetative (A–F) and reproductive growth (G–L) stages. Red color indicates the GUS antibody-specific signal. Blue color indicates cell wall autofluorescence. Asterisks indicate crosstalk of background autofluorescence from a particular cell wall. (A and B) Root. (C and D) Basal node. (E and F) Leaf blade. (G and H) Leaf sheath. (I and J) Internode. (K and L) Node I. Insets in A, C, G, I, and K represent regions magnified in yellow dotted boxes, respectively. (Scale bar, 50 µm.)
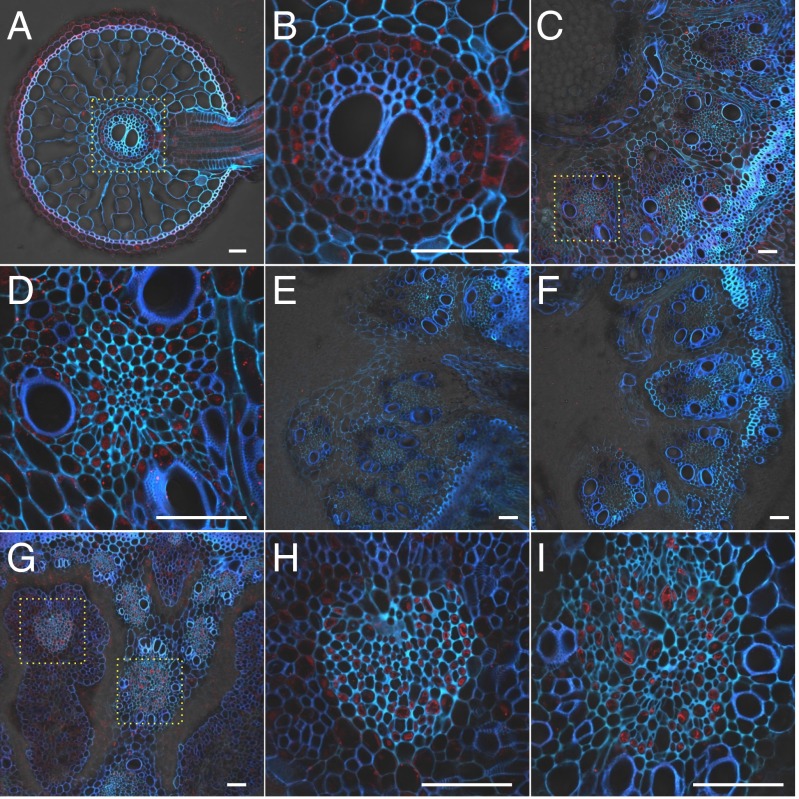
The cell specificity of OsABCC1 localization to the roots, basal node, and node I was further investigated by immunostaining with an OsABCC1 antibody. Signal was observed in the exodermis and pericycle of roots (Fig. 3 A and B), and in the phloem companion cells of both the EVBs and DVBs at the basal node and node I (Fig. 3 C, D, and G–I). The signal was stronger in the DVB than in the EVB. No signal was observed in the OsABCC1 knockout mutants (Fig. 3 E and F), indicating the specificity of this antibody. OsABCC1 expression in phloem cells of the node, which is important for delivering minerals to the grain, suggests that OsABCC1 might regulate As accumulation in the grain.
Fig. 3.
Cellular localization of OsABCC1. Immunostaining with an antibody against OsABCC1 was performed in different organs of rice at the vegetative (A–F) and reproductive growth (G–I) stage. (A and B) WT root. (C and D) WT basal node. (E and F) abcc1-1 and abcc1-2 mutant basal node, respectively. (G–I) WT node I. Red color indicates the OsABCC1 antibody-specific signal. Blue color indicates cell wall autofluorescence. Yellow dotted boxes in A, C, and G represent regions magnified in B (root stele), D, H (phloem of EVB), and I (DVB), respectively. (Scale bar, 50 µm.)
Tonoplast Localization of OsABCC1.
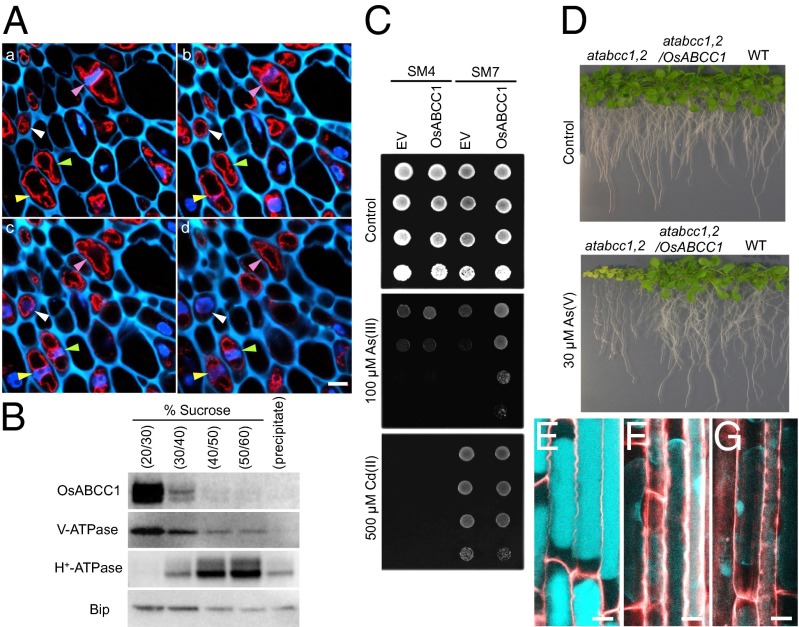
The subcellular localization of OsABCC1 was investigated by both immunohistological and Western blot analysis using an antibody against OsABCC1. The antibody cross-reacted with a major band corresponding to the molecular size of OsABCC1 in the microsomal fraction of wild-type rice but not of the mutant rice (Fig. S5). Although the antibody detected another band in both the wild type and the mutant, it was minor compared with the major band. This result indicated the high specificity of the antibody to OsABCC1. Immunohistochemical observation using double staining with DAPI for nuclei and OsABCC1 antibody revealed that endogenous OsABCC1 protein was localized to a ring-like structure in phloem companion cells in four consecutive optical sections with 2.5-µm intervals in node I (Fig. 4A). The ring-like signals were separately localized from the nucleus, and their size was larger than that of the nucleus. This localization is different from that of cytosol and other cytosolic small organelles, which are localized at the peripheral region of the cells, especially around the nucleus. This result shows that the OsABCC1 protein was localized to the tonoplast rather than to other organelle membranes. This subcellular localization was further confirmed by sucrose density gradient fractionation of microsomal proteins and immunoblot analysis with the same antibody. OsABCC1 showed the same fractionation pattern with V-type ATPase, a marker protein of tonoplast membrane (Fig. 4B), but different from the pattern of H+-ATPase or binding immunoglobulin protein (BiP), which represents the marker protein for the plasma membrane and ER, respectively.
Fig. 4.
Subcellular localization of OsABCC1 and As resistance mediated by OsABCC1. (A) Immunostaining analysis of subcellular localization of OsABCC1. Double staining with OsABCC1 antibody and DAPI was performed in node I of rice at the flowering stage. OsABCC1 antibody (red) and DAPI (nucleus; blue) fluorescence and cell wall autofluorescence (cyan) at four consecutive optical sections of 2.5-µm intervals (a–d) of the same sample are shown. Different colored arrowheads indicate the position of nuclei in each cell. (Scale bar, 5 µm.) (B) Immunoblot analysis of OsABCC1 localization. Microsomes extracted from rice roots were fractionated by sucrose density gradient. Polyclonal antibodies of anti-OsABCC1, anti–V-ATPase (tonoplast marker), anti–H+-ATPase (plasma membrane marker), and BiP (ER marker) were used. (C) Phytochelatin-dependent As resistance mediated by OsABCC1. Yeast strains SM4 and SM7 transformed with empty vector (EV) or OsABCC1 were cultured in half-strength SD agar plates with or without As(III) or Cd(II) at 30 °C for 3 d. (D) Recovery of As resistance in the Arabidopsis atabcc1 atabcc2 double mutant (atabcc1,2) by the introduction of OsABCC1. Wild-type (WT), atabcc1,2, and atabcc1,2 Arabidopsis lines transformed with OsABCC1 were grown on half-strength MS agar media supplemented with or without 30 µM As (V) for 3 wk. (E–G) Subcellular localization of thiol compounds. Seedlings (3-d-old) of WT rice (E), the osabcc1-1 mutant (F), and the osabcc1-2 mutant (G) were exposed to 0.5 µM As(III) for 3 h and then the roots were stained with monobromobimane for thiol compounds (cyan) and propidium iodide for the cell wall (red). Plasmolysis was induced by placing roots in 8% mannitol. Merged images are shown. (Scale bar, 10 µm.)
OsABCC1 Enhances As Resistance in Yeast and Arabidopsis.
The effect of OsABCC1 on As resistance was assayed in two yeast strains—that is, SM4 and SM7. SM4 is a budding yeast mutant strain lacking YCF1 [the yeast vacuolar As(GS)3 transporter] and three other vacuolar ABCC-type ABC transporters, whereas SM7 is a transgenic strain carrying the wheat PC synthase gene TaPCS1 in the SM4 background (23). In the absence of As, yeast growth was similar in the empty vector and OsABCC1-expressing lines in both the SM4 and SM7 backgrounds (Fig. 4C). Expression of OsABCC1 in SM4 had little effect on tolerance to As (Fig. 4C). However, expression of OsABCC1 in SM7 significantly enhanced tolerance to As (Fig. 4C). These results indicate that OsABCC1 specifically enhances As resistance through the PC-dependent pathway. OsABCC1 expression did not appear to affect Cd tolerance, although TaPCS1 expression greatly enhanced Cd tolerance in SM7 (Fig. 4C).
To examine whether OsABCC1 promotes As resistance in plants, similar to AtABCC1 and AtABCC2 in Arabidopsis, OsABCC1 was introduced into the Arabidopsis atabcc1atabcc2 double mutant under the control of the 35S promoter. In the absence of As, growth was similar in the wild type, atabcc1atabcc2, and atabcc1atabcc2 transformed with OsABCC1 (Fig. 4D). By contrast, in the presence of As, growth of the double mutant was inhibited, whereas expression of OsABCC1 rescued the As-induced growth inhibition of the double mutant (Fig. 4D). These results indicate that OsABCC1, like AtABCC1 and AtABCC2 in Arabidopsis, functions in As detoxification.
Subcellular Localization of Thiol Compounds.
Because As forms a complex with thiol compounds (e.g., PC), we investigated the subcellular localization of thiol compounds in the roots of rice plants using monobromobimane, a fluorescence dye for thiol compounds. After exposure to As, thiol compounds were localized to the vacuoles, which occupy most of the cell volume in the roots of wild-type rice (Fig. 4E). By contrast, the thiol compounds in the roots of the osabcc1-1 and osabcc1-2 mutants were detected in the thin layer of cytosol and nucleus (Fig. 4 F and G).
Knockout of OsABCC1 Resulted in Increased As Sensitivity.
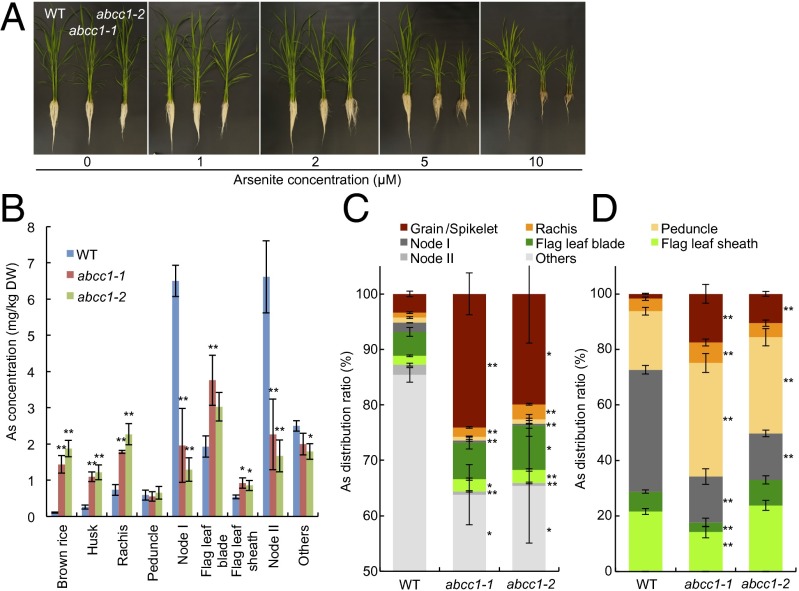
To test whether OsABCC1 is involved in As resistance in rice, we obtained two independent T-DNA insertion lines (Fig. S6 A and B). RT-PCR analysis showed that no OsABCC1 transcript was present in either of the lines, indicating that these are true knockout lines (Fig. S6C). In the absence of As, the growth of the two knockout lines was similar to that of wild-type rice (cv. Dongjin). However, in the presence of As (up to 10 µM), the growth of both the roots and shoots of the knockout lines was inhibited to a greater extent than was that of the wild-type rice (Fig. 5A and Fig. S7). In an independent experiment, we found that the shoot growth of the two knockout lines was completely inhibited in the presence of 50 µM As (Fig. S8A). These results indicate that OsABCC1 is involved in As tolerance in rice.
Fig. 5.
Phenotypic analysis of osabcc1 mutants. (A) The As tolerance test. Seventeen-day-old seedlings of wild-type (WT) rice and two OsABCC1 knockout lines (abcc1-1 and abcc1-2) were exposed to a nutrient solution containing different As concentrations. After 2 wk, the plants were photographed. (B and C) The As concentration (B) and distribution (C) in different organs at the ripening stage. Both wild-type rice and two knockout mutants were grown in soil containing 1.3 mg As per 1 kg soil. Different organs were sampled and subjected to As determination. The distribution of As in organs above node I was calculated; specifically, the amount of As accumulated in each organ versus the total As accumulated in the aboveground parts was determined. Data are means ± SD of three biological replicates. (D) Short-term As distribution in different organs. The plants were cut at the second upper internode below node II and then fed with 10 µM As(III) solution at the cut end. After 24 h, each organ was separately harvested for As determination. Data are means ± SD of four to five biological replicates. Significant differences from WT at *P < 0.05 and **P < 0.01 by Tukey’s test.
Cd tolerance was also compared between wild-type rice and the two knockout lines. However, there was no difference in the growth between these lines, regardless of whether the plants were subjected to low or high Cd concentrations (Fig. S8).
Analysis of As accumulation showed that the two mutants accumulated less As in the shoots compared with the wild-type rice at all As concentrations tested (Fig. S9A). However, in the roots, the mutants showed different patterns of accumulation that depended on the external As concentrations. At low As concentrations (<2 µM), the mutants accumulated more As than did wild-type rice; however, similar and lower levels were found at 5 µM and 10 µM As, respectively (Fig. S9B).
Knockout of OsABCC1 Resulted in Increased As Allocation to the Grain.
When grown to maturity in soil supplied with 1.3 mg As per 1 kg soil, the mutants accumulated much less As in nodes I and II, but more As in the flag leaf, rachis, husk, and brown rice compared with the wild-type rice (Fig. 5B). Notably, in the brown rice, the mutants’ As concentrations were 13- to 18-fold higher than those of wild-type rice. An analysis of the As distribution in the organs of the shoot showed that more As was delivered to the grain in the two mutants than in the wild type (20–24% vs. 3.4%) (Fig. 5C).
Because OsABCC1 is also expressed in other organs, such as roots, to exclude the possibility of an OsABCC1 effect in the roots on As distribution, we investigated the role of OsABCC1 in As distribution to the grains using a short-term (1-d) feeding experiment with arsenite and rubidium (Rb) (as a control) from the second upper internode at the milky stage. Only 1.7% of total As was allocated to the grain in wild-type rice (Fig. 5D). By contrast, 10.6–17.5% of As was allocated to the grain in the mutants. An opposite trend was found in the distribution of As to node I: More As accumulated in node I of wild-type rice than of the two mutants (Fig. 5D). There was only little difference in the distribution of Rb between wild-type rice and the two mutants (Fig. S10). These results are consistent with our experiment described above (Fig. 5 B and C), indicating that OsABCC1 plays an important role in limiting As transport to the rice grain.
Discussion
As in the form of arsenite inhibits plant growth by binding to vicinal sulfhydryl groups of proteins, thereby causing structural changes or loss of catalytic functions (27). Detoxification of As in plants has been proposed to occur via the formation of complexes with thiol compounds and the subsequent sequestration of these complexes into the vacuoles (20). Our results indicate that OsABCC1 is involved in As detoxification by sequestering As–PC into the vacuoles. OsABCC1 is localized to the tonoplast of rice cells (Fig. 4 A and B) and enhances As resistance in yeast expressing PC synthase (Fig. 4C). Knockout of OsABCC1 resulted in significantly decreased As tolerance (Fig. 5A and Figs. S7 and S8). The introduction of OsABCC1 into the Arabidopsis atabcc1atabcc2 double mutant complemented As-mediated root growth inhibition (Fig. 4D), indicating that OsABCC1 can rescue the function of AtABCC1 and AtABCC2, which sequester As into Arabidopsis vacuoles. Furthermore, thiol compounds were detected in the vacuoles of wild-type roots, but in the cytosol of osabcc1 mutant roots (Fig. 4 E–G). Therefore, the function of OsABCC1 in rice is similar to that of AtABCC1 and AtABCC2 in Arabidopsis (23). However, in contrast to AtABCC1 and AtABCC2, which are also involved in Cd detoxification (28), OsABCC1 did not confer Cd tolerance in rice (Fig. S8). This difference may be attributed to the presence of OsHMA3 in rice. OsHMA3, a member of the P-type ATPase family, is a tonoplast-localized Cd2+ transporter (29) and is responsible for the sequestration of Cd into the vacuoles of rice roots. Although a homolog of OsHMA3 exists in Arabidopsis (AtHMA3), its function has been lost due to mutation in the Columbia ecotype (30). Another possible explanation for this difference involves the different stabilities of As– and Cd–thiol complexes in the cells. Cd–PC complexes are stable in neutral and alkaline conditions, as found in the cytosol, but not in acidic environments (31). By contrast, As–PC complexes are stable under the acidic conditions found in root vacuoles (32). Therefore, the formation of Cd–thiol complexes in the cytosol results in strong Cd detoxification (24), whereas As–PC complexes must be sequestered into vacuoles for the complete detoxification of As.
Because rice grains are a major source of As intake (5), reducing the As concentration in the grain is an important objective of efforts to reduce As poisoning. A physiological study showed that transport of arsenite to the grain occurs mainly via phloem (33). However, the underlying molecular mechanism for this process was hitherto unknown. In this study, we found that OsABCC1 plays an important role in preventing As transport to the grain. Knockout of OsABCC1 resulted in a greater increase in As concentration in the brown rice (Fig. 5 B and C). Allocation of mineral elements, including As, to the grains is mediated by transporters in the nodes of rice (34). There are two major vascular bundles in the node—namely, the EVBs and DVBs. At the uppermost node (node I), EVBs are connected to the lower node and the flag leaf, whereas the DVB initiates at the node and is connected to the panicle (34). Efficient delivery of minerals to the grains requires the intervascular transfer of mineral elements from EVBs to DVBs. OsABCC1 is mainly localized to the tonoplast of phloem companion cells (Figs. 2, 3, and 4 A and B). Moreover, As was present at high concentrations in the nodes of wild-type rice (Fig. 5 B–D). These results indicate that As was sequestered into the phloem cells before translocation to the grain. In addition to its expression in the phloem region of upper nodes, OsABCC1 is also expressed in the phloem region of other organs, including basal nodes, leaf sheaths, and peduncles (Figs. 2 and 3). Localization of OsABCC1 to these organs also likely reduces As movement up to the grain by sequestering As into the vacuoles of the phloem companion cells. This notion is supported by a previous report describing As accumulation in the phloem region of these organs (21).
Knockout of OsABCC1 did not decrease As accumulation in the roots, but rather reduced As accumulation in the shoots compared with wild-type rice at relatively low As concentrations (Fig. S9). This is different from findings for OsHMA3, a tonoplast-localized Cd transporter (29). Loss of function of OsHMA3 resulted in decreased Cd accumulation in the roots, but increased Cd accumulation in the shoots. This difference may be attributed to the existence of different forms of and transporters for As and Cd. In contrast to Cd, which is likely sequestered by OsHMA3 into the vacuoles in the ionic form and loaded into the xylem in the same form by other transporter(s) (26, 29), As is sequestered by OsABCC1 into the root vacuoles most likely in the form of As–PC complexes (Fig. 4C), but loaded into the xylem in the ionic form (i.e., not bound to thiols) by Lsi2, a silicic acid and arsenite efflux transporter (11). One possibility is that knockout of OsABCC1 results in increased toxicity, which induces the biosynthesis of thiol compounds, such as glutathione, which bind to As. Because thiol–As complexes are not translocated to the shoots by Lsi2, As might accumulate in the roots of osabcc1 mutants (Fig. S9). At higher As concentrations (>5 µM), because the roots were severely damaged in the mutants (Fig. 5A and Fig. S7), the As concentration change may be indirectly caused by increased toxicity.
In conclusion, OsABCC1 is not only involved in As detoxification but, more importantly, is also involved in reducing the allocation of As to the rice grain. Particularly during the reproductive growth stage, the localization of OsABCC1 to the tonoplast of the phloem companion cells of vascular bundles at the upper nodes effectively reduces the translocation of As to the grains, thereby protecting the next generation of plants (which will grow out of the grains) from accumulating high levels of As. Overexpression of OsABCC1 may be used as a strategy to breed As-tolerant and low As-accumulating rice cultivars in the future.
Materials and Methods
Plant Materials, Growth Conditions, and Phenotypic Analysis.
Wild-type rice (cv. Dongjin) and two independent OsABCC1 T-DNA insertion lines were grown hydroponically or in a pot filled with 3.5 kg of soil containing 1.3 mg As⋅kg−1 in a greenhouse at 25–30 °C under natural light. At different growth stages, various organs were separately harvested and subjected to As determination by inductively coupled plasma mass spectrometry. The As tolerance was evaluated by exposing the plants to different As concentrations. The root length was measured before treatment and again after 48 h of treatment. The growth was also compared between wild-type rice and two independent OsABCC1 T-DNA insertion lines after 2 wk of exposure to different As concentrations. The distribution of As was investigated by feeding As to the cut end of the second upper internode of rice for 1 d at the milky growth stage.
Functional Characterization of OsABCC1.
OsABCC1 expression was investigated in different organs at different growth stages by real-time RT-PCR. Different tissues of node I (i.e., the node directly beneath the panicle) were collected by LMD and subjected to expression analysis. The cell specificity of OsABCC1 expression was examined by immunostaining with an antibody against GUS in transgenic lines carrying the OsABCC1 promoter–GUS fusion. The cellular and subcellular localization of OsABCC1 was further investigated by immunostaining and immunoblot analysis using an OsABCC1 antibody. The As resistance test was performed in two yeast mutant strains, SM4 and SM7, and in the Arabidopsis abcc1 abcc2 double knockout mutant.
For other methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sanae Rikiishi, Akemi Morita, Tomoko Haruna, and Kaoru Ishii for their technical assistance. This research was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan [22119002 and 24248014 (to J.F.M.)], by the Global Research Laboratory Program of Korea (to E.M. and Y.L.), and by the Next-Generation Bio-Green 21 Program of the Republic of Korea Rural Development Administration [Plant Molecular Breeding Center, Grant PJ008128 (to G.A.)].
Footnotes
Conflict of interest statement: OsABCC1 is included in the PCT (Patent Cooperation Treaty)/KR11/05691 entitled “composition for phytochelatin transport.”
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414968111/-/DCSupplemental.
References
- 1.Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Public health. Arsenic epidemiology and drinking water standards. Science. 2002;296(5576):2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- 2.Abernathy CO, et al. Arsenic: Health effects, mechanisms of actions, and research issues. Environ Health Perspect. 1999;107(7):593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argos M, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. Lancet. 2010;376(9737):252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brammer H, Ravenscroft P. Arsenic in groundwater: A threat to sustainable agriculture in South and South-east Asia. Environ Int. 2009;35(3):647–654. doi: 10.1016/j.envint.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Meharg AA, et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol. 2009;43(5):1612–1617. doi: 10.1021/es802612a. [DOI] [PubMed] [Google Scholar]
- 6.Zhu YG, et al. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ Sci Technol. 2008;42(13):5008–5013. doi: 10.1021/es8001103. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee M, et al. High arsenic in rice is associated with elevated genotoxic effects in humans. Sci Rep. 2013;3:2195. doi: 10.1038/srep02195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams PN, et al. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol. 2007;41(19):6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- 9.Su YH, McGrath SP, Zhao FJ. Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil. 2010;328(1-2):27–34. [Google Scholar]
- 10.Xu XY, McGrath SP, Meharg AA, Zhao FJ. Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol. 2008;42(15):5574–5579. doi: 10.1021/es800324u. [DOI] [PubMed] [Google Scholar]
- 11.Ma JF, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA. 2008;105(29):9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytol. 2009;181(4):777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- 13.Mitani-Ueno N, Yamaji N, Zhao FJ, Ma JF. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J Exp Bot. 2011;62(12):4391–4398. doi: 10.1093/jxb/err158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bienert GP, et al. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008;6:26. doi: 10.1186/1741-7007-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitani N, Chiba Y, Yamaji N, Ma JF. Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell. 2009;21(7):2133–2142. doi: 10.1105/tpc.109.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma JF, et al. A silicon transporter in rice. Nature. 2006;440(7084):688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 17.Ma JF, et al. An efflux transporter of silicon in rice. Nature. 2007;448(7150):209–212. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 18.Ma JF, Yamaji N, Mitani-Ueno N. Transport of silicon from roots to panicles in plants. Proc Jpn Acad, Ser B, Phys Biol Sci. 2011;87(7):377–385. doi: 10.2183/pjab.87.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao FJ, et al. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol. 2010;186(2):392–399. doi: 10.1111/j.1469-8137.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- 21.Moore KL, et al. Combined NanoSIMS and synchrotron X-ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol. 2014;201(1):104–115. doi: 10.1111/nph.12497. [DOI] [PubMed] [Google Scholar]
- 22.Indriolo E, Na G, Ellis D, Salt DE, Banks JA. A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell. 2010;22(6):2045–2057. doi: 10.1105/tpc.109.069773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song WY, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA. 2010;107(49):21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song WY, et al. Phytochelatin-metal(loid) transport into vacuoles shows different substrate preferences in barley and Arabidopsis. Plant Cell Environ. 2014;37(5):1192–1201. doi: 10.1111/pce.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaji N, Ma JF. A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell. 2009;21(9):2878–2883. doi: 10.1105/tpc.109.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaji N, Xia J, Mitani-Ueno N, Yokosho K, Feng Ma J. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013;162(2):927–939. doi: 10.1104/pp.113.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133(1):1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 28.Park J, et al. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012;69(2):278–288. doi: 10.1111/j.1365-313X.2011.04789.x. [DOI] [PubMed] [Google Scholar]
- 29.Ueno D, et al. Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA. 2010;107(38):16500–16505. doi: 10.1073/pnas.1005396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel M, et al. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009;149(2):894–904. doi: 10.1104/pp.108.130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johanning J, Strasdeit H. A coordination-chemical basis for the biological function of the phytochelatins. Angew Chem Int Ed. 1998;37(18):2464–2466. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2464::AID-ANIE2464>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Schmöger MEV, Oven M, Grill E. Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 2000;122(3):793–801. doi: 10.1104/pp.122.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carey A-M, et al. Grain unloading of arsenic species in rice. Plant Physiol. 2010;152(1):309–319. doi: 10.1104/pp.109.146126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaji N, Ma JF. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014;19(9):556–563. doi: 10.1016/j.tplants.2014.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.