Abstract
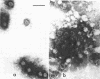
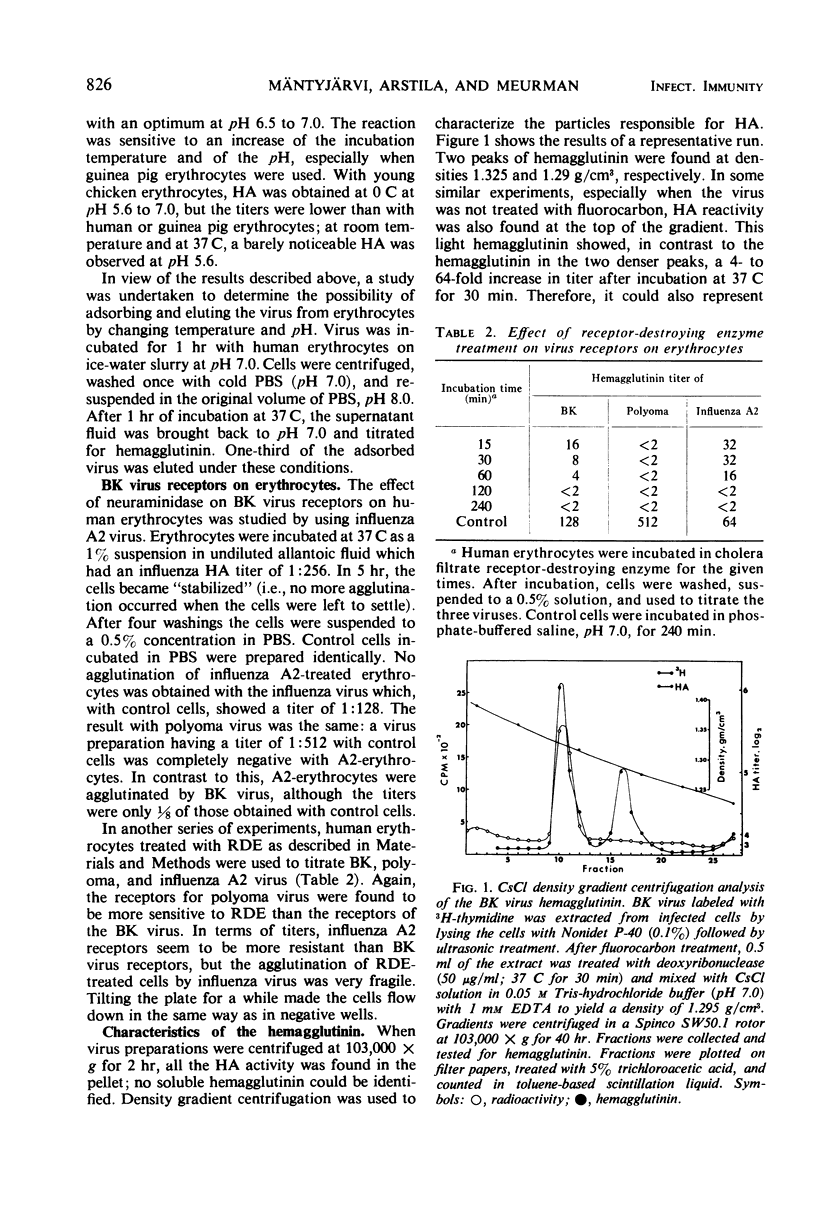
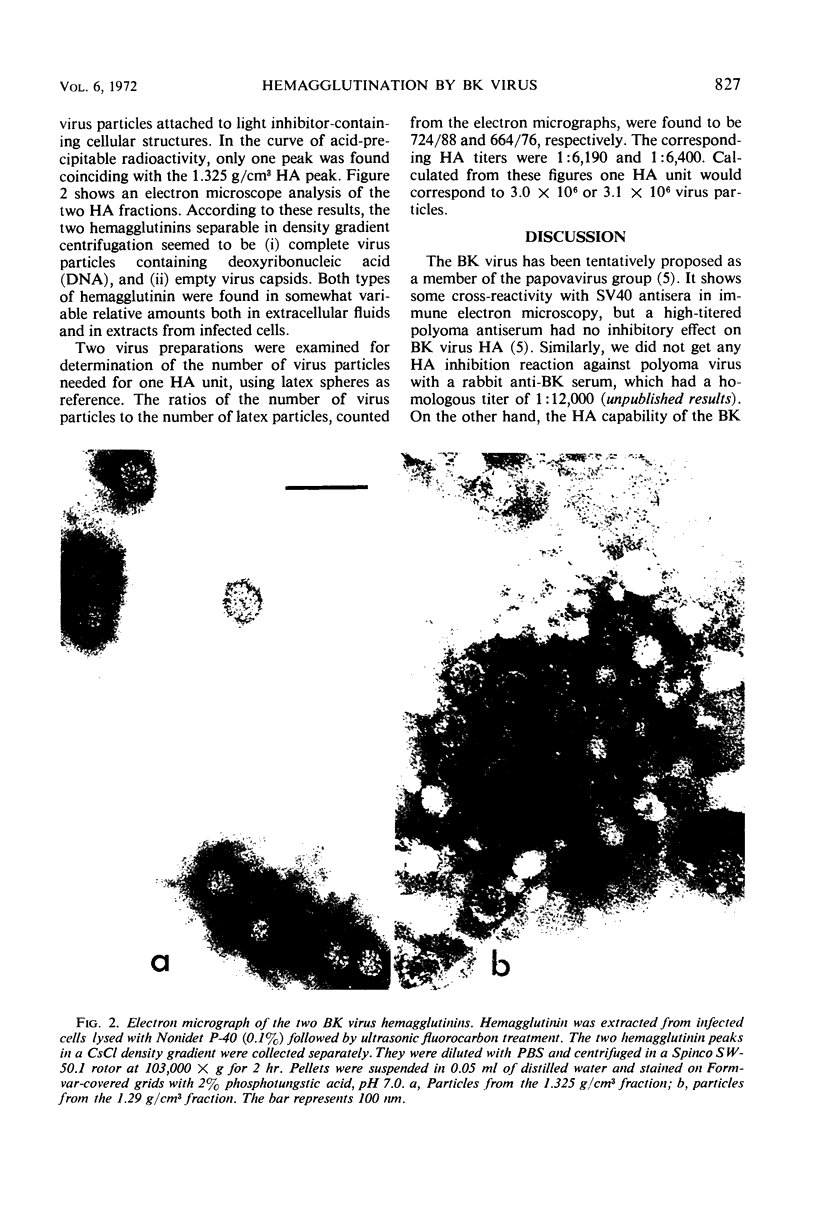
Some characteristics of hemagglutination (HA) by the BK virus, a new candidate for the papovavirus group, have been studied. Hemagglutinin prepared from cell cultures was found to be partially masked by inhibitors which could be dissociated from the virus by incubation at 37 C or by fluorocarbon extraction. Optimal conditions for HA are outlined. In routine tests, 0.5% human erythrocytes were used. The reaction was carried out at pH 7.0 on ice-water slurry. BK hemagglutinin receptors on human erythrocytes were found to be more resistant to neuraminidase than polyoma receptors. By gradient centrifugation analysis, two types of particles were found to be responsible for HA: (i) full, deoxyribonucleic acid-containing particles with a density of 1.325 g/cm3 and (ii) empty capsids with a density 1.29 g/cm3. Based on particle counting, one HA unit was calculated to correspond to 3 × 106 virus particles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL P., CRAWFORD L. V. Physical characteristics of polyoma virus. III. Correlation with biological activities. Virology. 1963 Apr;19:470–474. doi: 10.1016/0042-6822(63)90040-0. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M., WATSONDH The physical characteristics of polyoma virus. I. Two types of particle. Virology. 1962 Oct;18:170–176. doi: 10.1016/0042-6822(62)90002-8. [DOI] [PubMed] [Google Scholar]
- EDDY B. E., ROWE W. P., HARTLEY J. W., STEWART S. E., HUEBNER R. J. Hemagglutination with the SE polyoma virus. Virology. 1958 Aug;6(1):290–291. doi: 10.1016/0042-6822(58)90078-3. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J. The use of fluorocarbon for "unmasking" polyoma virus hemagglutinin. Virology. 1959 Nov;9:488–489. doi: 10.1016/0042-6822(59)90141-2. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- HARTLEY J. W., ROWE W. P., CHANOCK R. M., ANDREWS B. E. Studies of mouse polyoma virus infection. IV. Evidence for mucoprotein erythrocyte receptors in polyoma virus hemagglutination. J Exp Med. 1959 Jul 1;110(1):81–91. doi: 10.1084/jem.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY J. W., ROWE W. P. Unmasking of mouse polyoma virus hemagglutinin by heat and RDE. Virology. 1959 Feb;7(2):249–250. doi: 10.1016/0042-6822(59)90194-1. [DOI] [PubMed] [Google Scholar]
- HIRST G. K. Receptor destruction by viruses of the mumps-NDV-influenza group. J Exp Med. 1950 Feb;91(2):161–175. doi: 10.1084/jem.91.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]