Abstract
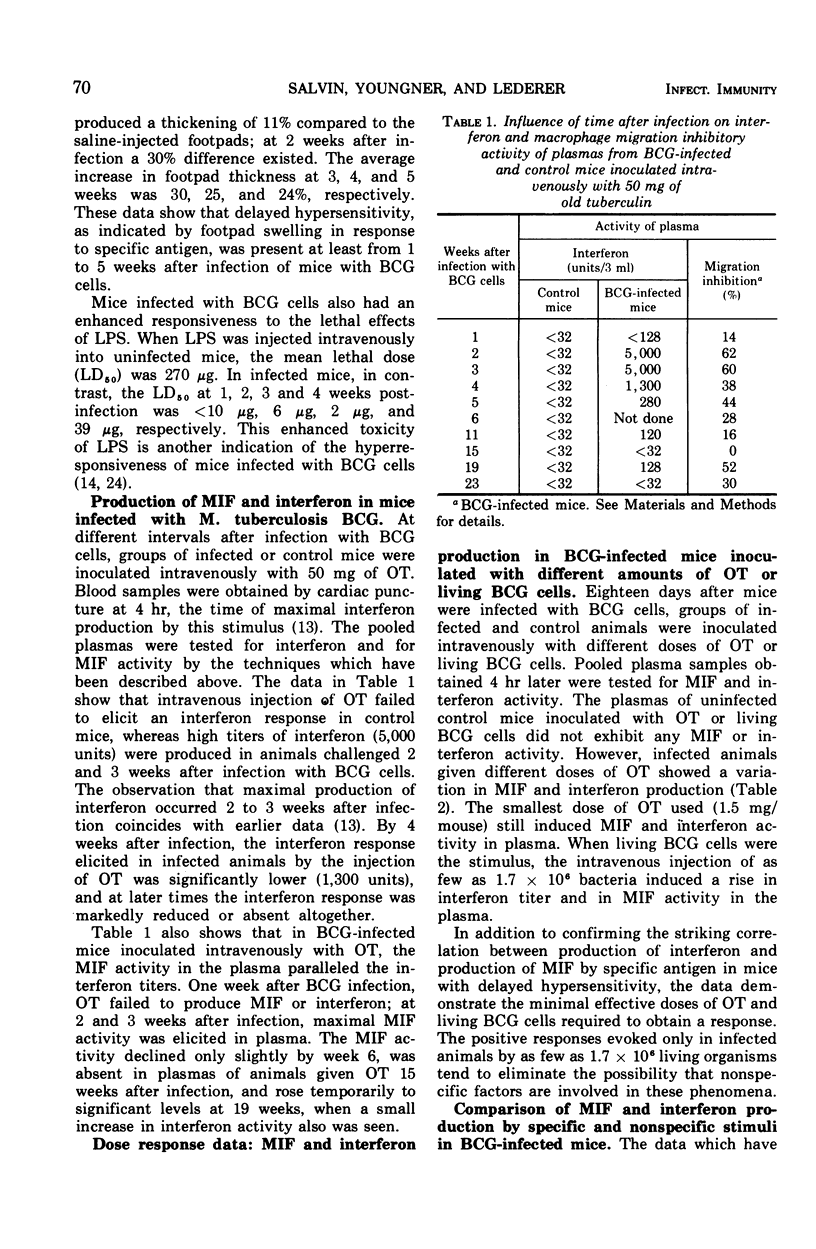
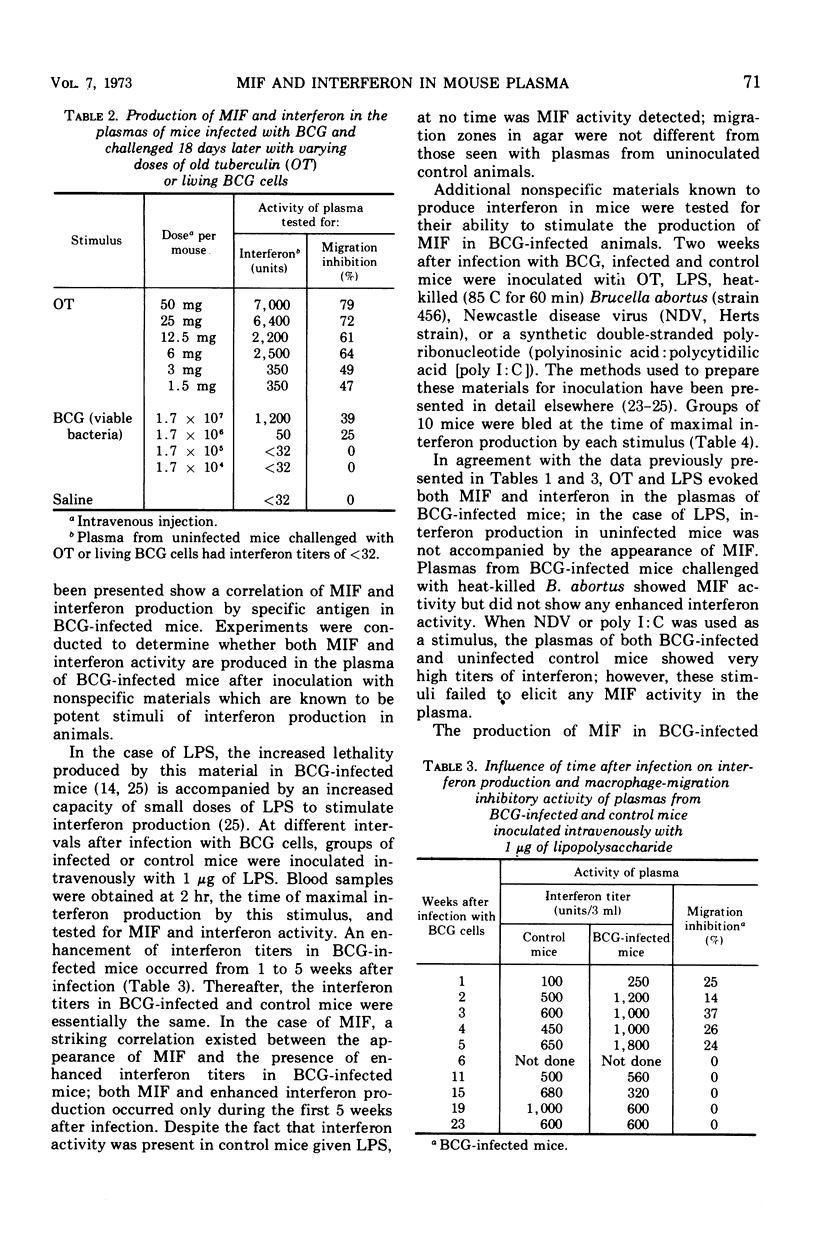
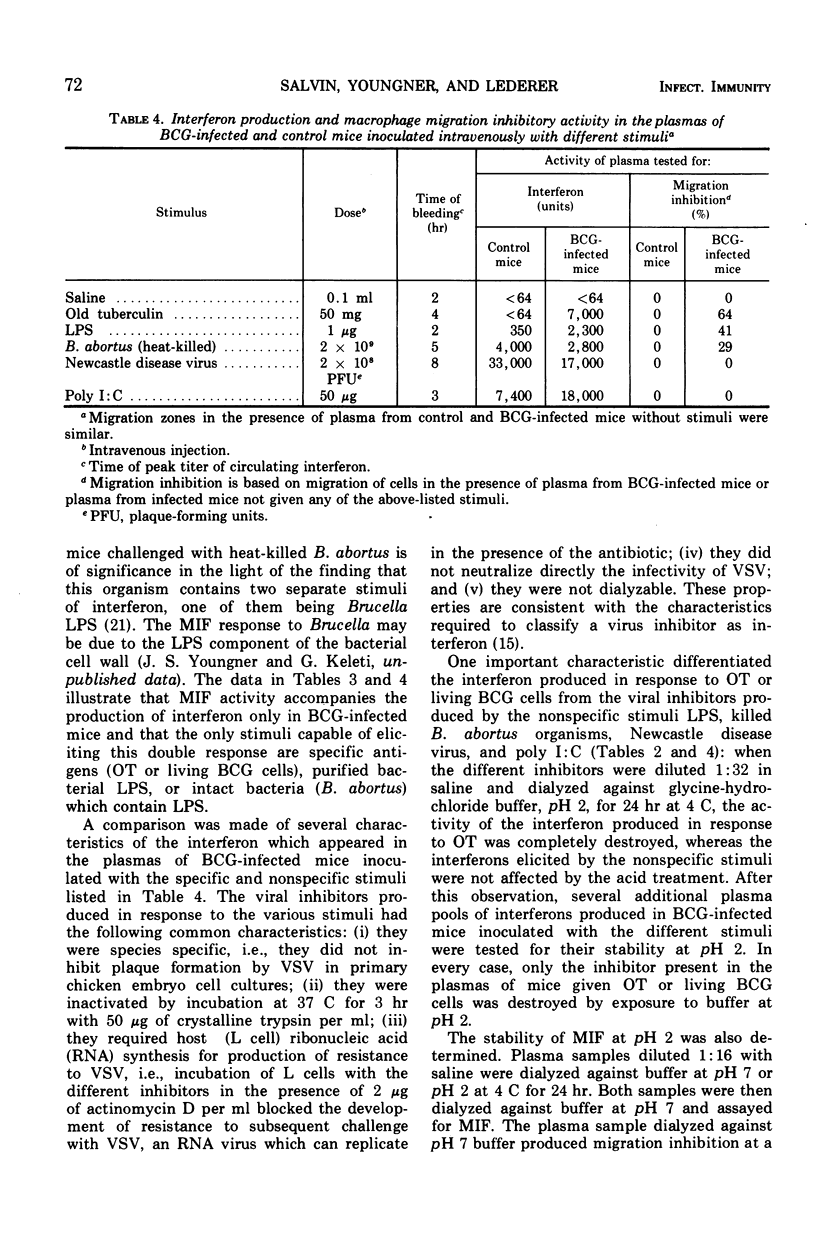
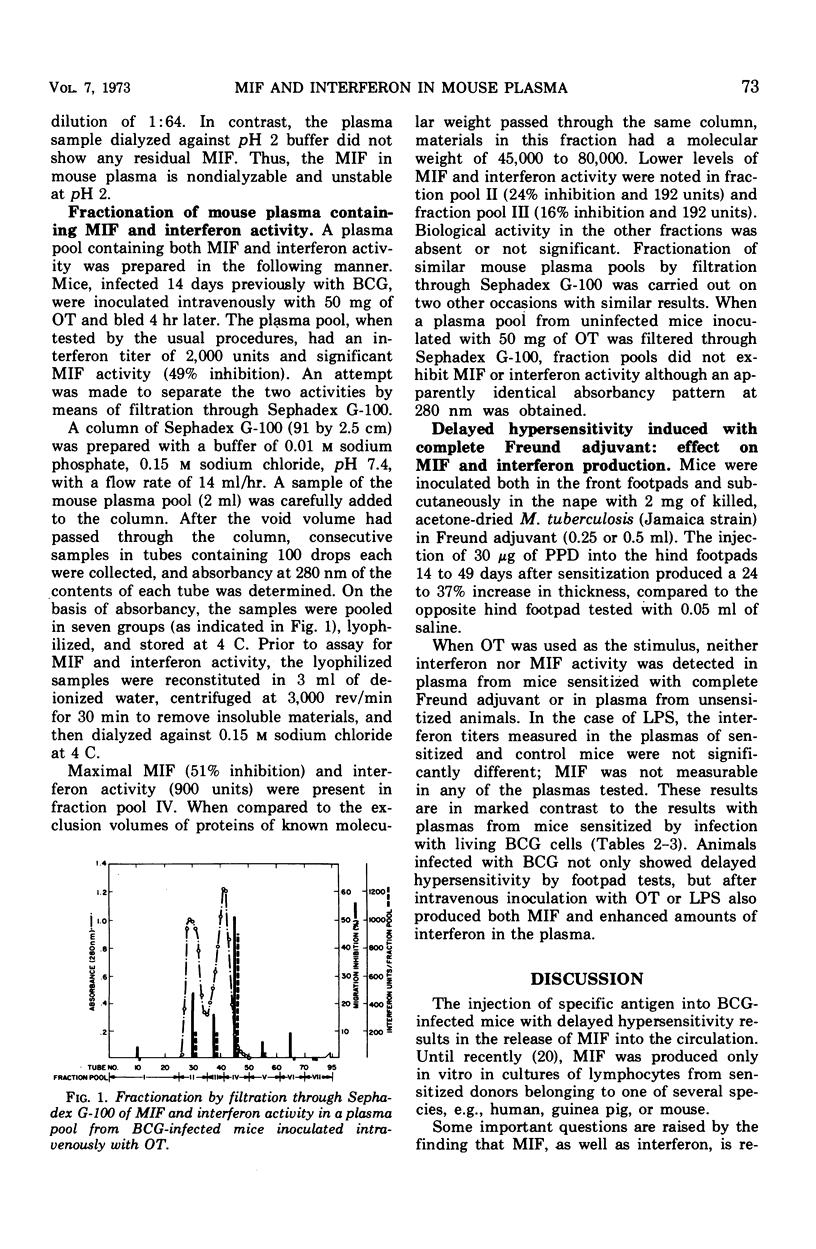
When mice infected with Mycobacterium tuberculosis strain BCG were inoculated intravenously with old tuberculin (OT) or living BCG cells, both migration inhibitory factor (MIF) and interferon appeared in the circulation within a few hours. In such animals, which showed delayed hypersensitivity by footpad tests, as little as 1.5 mg of OT or as few as 1.7 × 106 bacteria per mouse were capable of eliciting circulating MIF and interferon. Uninfected animals inoculated with large doses of OT or living BCG cells did not produce MIF or interferon. When nonspecific stimuli such as bacterial lipopolysaccharide (LPS; from Salmonella typhimurium strain LT-2), heat-killed Brucella abortus, Newcastle disease virus (NDV), and polyinosinic acid:polycytidilic acid (poly I:C) were inoculated intravenously into BCG-infected mice, MIF was produced in the circulation of animals challenged with LPS or Brucella but not in those challenged with NDV or poly I:C, although all the stimuli were capable of eliciting an interferon response. The interferon elicited in BCG-infected mice by specific antigen differed in at least one important property from the viral inhibitor produced by the nonspecific stimuli. The interferon which appeared after injection of OT or living BCG cells was destroyed by treatment at pH 2 for 24 hr at 4C, whereas the interferons produced after injection of the nonspecific stimuli were stable under the same conditions. The MIF activity in plasma from sensitized mice inoculated with specific antigen was also destroyed by treatment at pH 2. When mouse plasma containing both MIF and interferon activity was filtered through Sephadex G-100, both mediators were excluded in the same peak fractions. Sensitization of mice with complete Freund adjuvant instead of infection with BCG cells produces a different pattern of response. Although hypersensitive to specific antigen by footpad swelling tests, mice sensitized with complete Freund adjuvant failed to produce MIF or interferon when they were inoculated intravenously with OT or living BCG cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBOS R. J., PIERCE C. H., SCHAEFER W. B. Antituberculous immunity induced in mice by vaccination with living cultures of attenuated tubercle bacilli. J Exp Med. 1953 Feb 1;97(2):207–220. doi: 10.1084/jem.97.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. PPD-stimulated interferon: in vitro macrophage-lymphocyte interaction in the production of a mediator of cellular immunity. Cell Immunol. 1971 Dec;2(6):602–613. doi: 10.1016/0008-8749(71)90008-6. [DOI] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Leukocytes and interferon in the host response to viral infections. II. Enhanced interferon response of leukocytes from immune animals. J Bacteriol. 1966 Jun;91(6):2185–2191. doi: 10.1128/jb.91.6.2185-2191.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. A., Cooperband S. R., Kibrick S. Immune specific induction of interferon production in cultures of human blood lymphocytes. Science. 1969 Jun 20;164(3886):1415–1417. doi: 10.1126/science.164.3886.1415. [DOI] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A. 1967 Aug;58(2):782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C. Induction of circulating interferon by synthetic anionic polymers of known composition. Nature. 1967 Apr 22;214(5086):416–417. doi: 10.1038/214416a0. [DOI] [PubMed] [Google Scholar]
- Pick E., Brostoff J., Krejci J., Turk J. L. Interaction between "sensitized lymphocytes" and antigen in vitro. II. Mitogen-induced release of skin reactive and macrophage migration inhibitory factors. Cell Immunol. 1970 May;1(1):92–109. doi: 10.1016/0008-8749(70)90063-8. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The susceptibility of mice to bacterial endotoxins. J Exp Med. 1961 Mar 1;113:559–570. doi: 10.1084/jem.113.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvin S. B., Nishio J. Communications. "In vitro" cell reactions in delayed hypersensitivity. J Immunol. 1969 Jul;103(1):138–141. [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. The production by antigen-stimulated lymphocytes of a leukotactic factor distinct from migration inhibitory factor. Cell Immunol. 1970 Jul;1(2):162–174. doi: 10.1016/0008-8749(70)90003-1. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-Like Virus-Inhibitor Induced in Human Leukocytes by Phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. doi: 10.1126/science.149.3681.310. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Takahashi Y. Macrophage migration inhibition by serum from desensitized animals previously sensitized with tubercle bacilli. Nat New Biol. 1971 Oct 27;233(43):261–263. doi: 10.1038/newbio233261a0. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Hallum J. V. Interferon production in mice by double-stranded synthetic polynucleotides: induction or release? Virology. 1968 May;35(1):177–179. doi: 10.1016/0042-6822(68)90320-6. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]


