Abstract
The purpose of these experiments was to study the early response of mouse foot pads to Mycobacterium leprae. To accomplish this, mice were inoculated in both foot pads with large and small numbers of organisms. The animals were sacrificed at intervals from 2 hr to 27 days after inoculation. The microscopical results, which utilized normal BALB/c and thymectomized-irradiated B6C3F1 mice, showed that the tissue responded first with an influx of polymorphonuclear cells and later lymphocytes and monocytes. The latter formed a diffuse infiltrate in the tissues. Under conditions where growth normally occurred, the mononuclear cell infiltrate did not persist. The organisms were found within phagocytic cells and the interstitial space. They were always contained within a phagosome and often fused with lysosomes. Most of the organisms appeared to be degenerating at all of the times studied. No organisms were observed in striated muscle fibers of tissues studied.
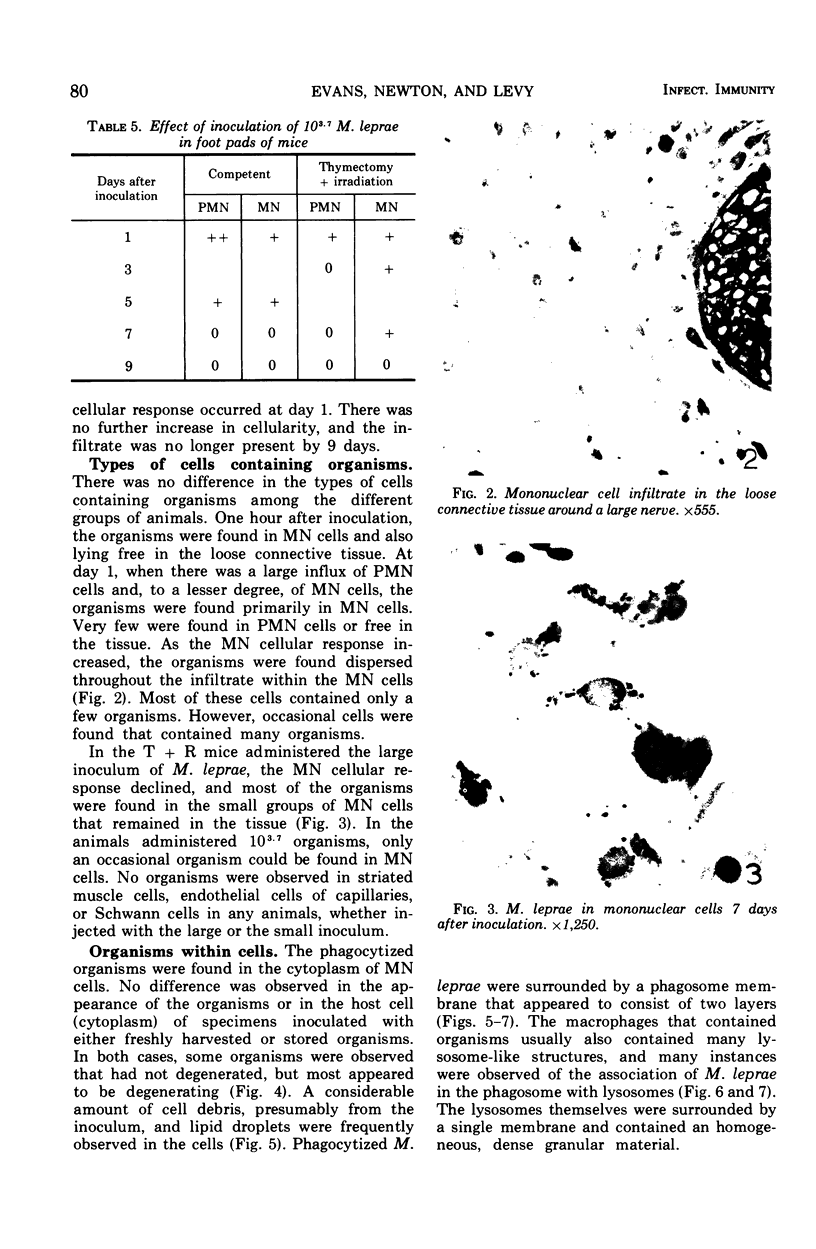
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri M. M., Weddell A. G., Rees R. J. Infection of murine striated muscle with Mycobacterium leprae: a study by light and electron microscopy. J Pathol. 1972 Feb;106(2):73–80. doi: 10.1002/path.1711060203. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Levy L. Ultrastructural changes in cells of the mouse footpad infected with Mycobacterium leprae. Infect Immun. 1972 Feb;5(2):238–247. doi: 10.1128/iai.5.2.238-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- Levy L. Death of Mycobacterium leprae in mice, and the additional effect of dapsone administration. Proc Soc Exp Biol Med. 1970 Dec;135(3):745–749. doi: 10.3181/00379727-135-35134. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. Cellular immunity. Ann Inst Pasteur (Paris) 1971 Mar;120(3):428–437. [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- McRae D. H., Shepard C. C. Relationship Between the Staining Quality of Mycobacterium leprae and Infectivity for Mice. Infect Immun. 1971 Jan;3(1):116–120. doi: 10.1128/iai.3.1.116-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E., Rees R. J., Weddell A. G. Site of multiplication of human leprosy bacilli inoculated into the foot-pads of mice. Nature. 1965 May 1;206(983):521–522. doi: 10.1038/206521a0. [DOI] [PubMed] [Google Scholar]
- Pearson J. M., Rees R. J., Weddell A. G. Mycobacterium leprae in the striated muscle of patients with leprosy. Lepr Rev. 1970 Jul;41(3):155–166. doi: 10.5935/0305-7518.19700023. [DOI] [PubMed] [Google Scholar]
- Rees R. J. Enhanced susceptibility of thymectomized and irradiated mice to infection with Mycobacterium leprae. Nature. 1966 Aug 6;211(5049):657–658. doi: 10.1038/211657a0. [DOI] [PubMed] [Google Scholar]
- Rees R. J., Weddell A. G. Biology of the mycobacterioses. Experimental models for studying leprosy. Ann N Y Acad Sci. 1968 Sep 5;154(1):214–236. doi: 10.1111/j.1749-6632.1968.tb16711.x. [DOI] [PubMed] [Google Scholar]
- SHEPARD C. C., MCRAE D. H. MYCOBACTERIUM LEPRAE IN MICE: MINIMAL INFECTIOUS DOSE, RELATIONSHIP BETWEEN STAINING QUALITY AND INFECTIVITY, AND EFFECT OF CORTISONE. J Bacteriol. 1965 Feb;89:365–372. doi: 10.1128/jb.89.2.365-372.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Congdon C. C. Increased growth of Mycobacterium leprae in thymectomized-irradiated mice after foot pad inoculation. Int J Lepr Other Mycobact Dis. 1968 Apr-Jun;36(2):224–227. [PubMed] [Google Scholar]
- Spector W. G. The granulomatous inflammatory exudate. Int Rev Exp Pathol. 1969;8:1–55. [PubMed] [Google Scholar]
- Weddell A. G., Palmer E., Rees R. J. The fate of Mycobacterium leprae in CBA mice. J Pathol. 1971 Jun;104(2):77–92. doi: 10.1002/path.1711040202. [DOI] [PubMed] [Google Scholar]