Abstract
In vertebrates, a variety of cell types generate a primary cilium. Cilia are implicated in determination and differentiation of a wide variety of organs and during embryonic development. However, there is little information on the presence or function of primary cilia in the mammalian testis. Therefore, the objective of this study was to characterize expression of primary cilia in the developing pig testis. Testicular tissue from pigs at 2 to 10 weeks of age was analyzed for primary cilia by immunocytochemistry. Expression of primary cilia was also analyzed in testicular tissue formed de novo from a single cell suspension ectopically grafted into a mouse host. Functionality of primary cilia was monitored based on cilia elongation after exposure to lithium. Analysis showed that the primary cilium is present in testis cords as well as in the interstitium of the developing pig testis. Germ cells did not express primary cilia. However, we identified Sertoli cells as one of the somatic cell types that produce a primary cilium within the developing testis. Primary cilium expression was reduced from the second to the third week of pig testis development in situ and during de novo morphogenesis of testis tissue from a single cell suspension after xenotransplantation. In vitro, primary cilia were elongated in response to lithium treatment. These results indicate that primary cilia on Sertoli cells may function during testicular development. De novo morphogenesis of testis tissue from single cell suspensions may provide an accessible platform to study and manipulate expression and function of primary cilia.
Keywords: Mammalian testis, cilium, Sertoli cells, morphogenesis
INTRODUCTION
Testis development can be divided into three stages: foetal, infantile/juvenile, and pubertal. During the foetal and pubertal stages, testis development comprises a series of highly regulated molecular and cellular processes and drastic morphological changes. For instance, testis cords are formed at the foetal stage, which involves localization and differentiation of foetal Sertoli cells, formation of clusters by Sertoli cells and primordial germ cells, and encapsulation of the clusters by peritubular myoid cells [for reviews see (McLaren 2000; Chen et al. 2009)]. As the cords are forming, foetal Leydig cells start to differentiate and localize to the interstitial spaces between the sex cords. The differentiated Sertoli cells and Leydig cells, together with other cell types, hormones, such as Mullerian inhibitory substance (MIS), testosterone and insulin-like 3 protein, promote masculinisation of the foetal testis and establish the essential testis structure (Hughes et al. 1999).
The infantile/juvenile stage occurs between the foetal stage and the pubertal stage. This is a stage of relative quiescence: no major morphological changes occur in Sertoli cells and germ cells. Nevertheless, in this stage, both Sertoli cells and germ cells proliferate many fold. As well, at this stage, pituitary gonadotrophins (in particular follicle-stimulating hormone) participate in the regulation of the cell proliferation process (Griswold et al. 1977; Orth 1984). Ultimately, it is the number of Sertoli cells produced at this stage that determines the magnitude of production of mature spermatozoa and also the size of the testis in adulthood [for review see (Petersen and Soder 2006)].
We previously established a xenotransplantation model that recapitulates testicular morphogenesis de novo from isolated testis cells ectopically grafted into a mouse host, mimicking all three stage of testis development mentioned above (Honaramooz et al. 2007). In this model, a single cell suspension is isolated from neonatal pig testes, and transplanted ectopically into a site underneath the back skin of immune-deficient mice. With time, these cells form testis cords, and subsequently support germ cell differentiation into spermatozoa. This model provides a versatile tool in deciphering testis development and spermatogenesis by allowing manipulation of cell types integral to testis formation using molecular, cellular, genetic, and/or biochemical approaches.
Two different types of cilia have been described in mammalian cells: motile and non motile cilia. Their basic structure is similar and both have important roles during development, tissue morphogenesis and to sustain homeostasis. The motile cilium is present in specialized cell types where its main function is to promote movement of fluid resulting in flow and clearance. In the testis, the sperm flagellum is a motile cilium. Different from motile cilia, primary cilia lack the pair of central microtubules and dynein arms. The primary cilium is an antenna-like structure that protrudes from the cell surface into the extracellular environment. Many signalling proteins, such as platelet-derived growth factor receptor alpha, polycystins and Patched, are concentrated at the ciliary membrane (Brailov et al. 2000; Handel et al. 1999; Pazour et al. 2002; Rohatgi et al. 2007; Schneider et al. 2005; Yoder et al. 2002). In addition, some extracellular matrix receptors are also localized to the membrane of primary cilia (McGlashan et al. 2006; McGlashan et al. 2008). During embryonic development, this sensory organelle was found to be required for survival and patterning of the mouse embryo (Nonaka et al. 1998; Huangfu and Anderson 2005). More recently, this organelle was also implicated in determination, differentiation and maturation of a wide variety of organs within the body, including the central nervous system, skeleton, heart and blood vessels, kidney, and liver (Tasouri and Tucker 2011; Willaredt et al. 2013). During formation of these organs, primary cilia function as sensory organelles, playing a pivotal role in integrating signals derived from a number of signalling transduction pathways, such as hedgehog, wingless, fibroblast growth factor, platelet-derived growth factor, and planar cell polarity (Goetz and Anderson 2010). At present, however, there is no clear indication whether or not primary cilia are expressed and function during testis development. Spermatogenesis is a complex process that involves interaction of many different cell types in which proliferation and differentiation must be coordinated and regulated. We hypothesized that the primary cilium, a sensory organelle expressed in a large number of vertebrate cells, is expressed in testis and plays an important role in testis organogenesis.
To address this hypothesis, we investigated primary cilia expression during the infantile/juvenile stage of testis development using the pig as a model. We identified that testicular somatic cells express primary cilia. We used the xenotransplantation animal model to explore the relationship between primary cilia and testis development. We found a reduction in primary cilium number on Sertoli cells after the second week of testis development both in situ and in de novo formed tissue. These findings demonstrate that Sertoli cells express primary cilia in a dynamic, temporal pattern, during testis development.
MATERIALS AND METHODS
Preparation and cultivation of testis cells
Testis cells were obtained from testes of 6-day-old piglets using a two step enzymatic digestion as described (Honaramooz et al. 2002). First, seminiferous tubules were isolated by digestion of pig testes with collagenase IV (2 mg/ml) and hyaluronidase (2.5 μg/ml, Sigma) Then, individual cells were isolated by digestion of the seminiferous tubules with 2 μg/ml trypsin (Sigma) and 7 mg/ml DNase I. After isolation, the cells were used directly for xenotransplantation or cultivated on coverslips coated with poly-L-lysine in DMEM containing 10% fetal bovine serum at 37°C for various times as indicated in the text, fixed in 4% paraformadelhyde in PBS, and processed for immunocytochemistry. For lithium treatment, the dispersed testis cells were treated with DMEM containing 10% fetal bovine serum and 50 mM lithium chloride as described previously (Ou et al. 2009).
Grafting of testis cell suspensions
The recipient animals were 6- to 8-week-old NCR Nude mice (Taconic, Germantown, NY, USA). A single cell suspension of pig testis cells was transplanted under the dorsal skin of recipient mice as described previously (Honaramooz et al. 2007). Briefly, recipient mice were anesthetized with tribromoethanol (0.03 ml/g) and castrated. Their backs were aseptically prepared and pellets of 50 million pig testis cells were placed under the back skin of each mouse through ~1 cm incisions. Skin incisions were closed with Michel clips and mice were allowed to recover. After a period of time as indicated in the text, mice were sacrificed by CO2 inhalation and xenografts were collected for immunocytochemistry analysis. All animal procedures were approved by and under the oversight of the University of Calgary Animal Care and Use Committee.
Tissue processing for paraffin embedding and staining
Pig testis specimen from donor pig testis were fixed in Bouin’s solution, embedded in paraffin, and processed for staining as described previously (Rodriguez-Sosa et al. 2012). After embedding, the tissues were sectioned into pieces of 5 um in thickness and processed as follows. Briefly, sections were treated with xylene, rehydrated stepwise in 90% ethanol to water, exposed to 3% H2O2 in distilled water for 15 min, and washed in PBS for 5 min. Nonspecific binding was blocked in CAS Block (Invitrogen, Carlsbad, CA, USA) for 30 min at room temperature.
Graft processing for cryosectioning and staining
For cryosectioning, the pig testis tissue or cell xenografts were fixed in 4% paraformaldehyde in PBS, washed in water, infiltrated two times in OCT for at least 5 hours each at room temperature, embedded in fresh OCT, frozen in ethanol-dry ice bath, and stored at −70°C until sectioning. The sections were washed in distilled water for 10 min, followed by treatment in 1% Triton X100 in PBS for 15 min. Nonspecific binding was blocked in 5% BSA in PBS for 30 min at room temperature.
Immunofluorescence microscopy
Indirect immunofluorescence microscopy (IIF) was performed as described previously (Ou et al. 2002; Ou and Rattner 2000). Cells were characterized with the antibodies listed in table 1 and nuclear counterstaining with DAPI. Images were obtained using a Zeiss microscopy Axiovert 200M equipped with a CCD camera and controlled with AxioVision 4.8 software.
Table 1.
Antibodies used for IHC
| Antibody | Dilution | Description | Source | Catalog number |
|---|---|---|---|---|
| anti Acetylated alpha-tubulin | 1:200 | Mouse monoclonal | Sigma Aldrich | T7451 |
| anti Adenylate cyclase III | 1:200 | Mouse monoclonal | Santa Cruz | SC588 |
| anti Arl13b | 1:500 | Rabbit polyclonal | Proteintech | 17711-1 |
| anti Ddx4/MVH | 1:400 | Mouse monoclonal | Abcam | AB27591 |
| anti Gamma-tubulin | 1:200 | Mouse monoclonal | Sigma Aldrich | T6557 |
| anti Gata4 | 1:40 | Mouse monoclonal | Santa Cruz | SC25310 |
| anti MIS | 1:200 | Mouse monoclonal | Santa Cruz | SC-6886 |
| Alexafluor 555 | 1:400 | Anti mouse IgG produced in mouse | Sigma Aldrich | A21422 |
| Alexafluor 488 | 1:400 | Anti goat IgG produced in rabbit | Sigma Aldrich | SAB4600053 |
Identification and characterization of primary cilia
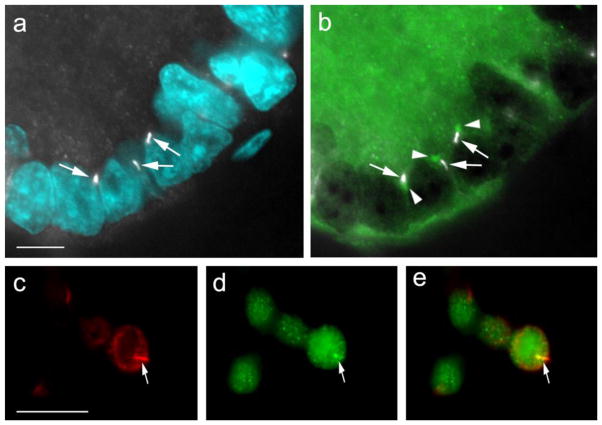
Testis sections were stained with an antibody against acetylated tubulin, a commonly-used marker for primary cilia. We reported previously that acetylated alpha tubulins are present in spermatogonia [(Luo et al. 2010); see also Figure 1, germ cells indicated by arrows]. To distinguish these cytosolic microtubules from primary cilia we used two strategies. First, we co-stained tissue sections with an antibody against γ-tubulin to visualize the basal body, the organelle that produces the primary cilium (Ou and Rattner 2004). We then stained tissue sections with an antibody against adenylate cyclase III (Bishop et al. 2007). We had shown previously that adenylate cyclase III is present in primary cilia (Ou et al. 2009). To avoid overlap with acetylated alpha tubulin staining in germ cells (Luo et al. 2010) we also labeled cilia with an antibody against ARL13B. ARL13B is a GTPase expressed in primary cilia. ARL13B deficiency is linked to Joubert syndrome, a human ciliopathy (Cantagrel et al. 2008). Knockout of Arl13b in mice lead to impairment of Sonic hedgehog signalling and deformation of primary cilia leading to defects in neuronal tube development (Horner and Caspary 2011).
Fig. 1.
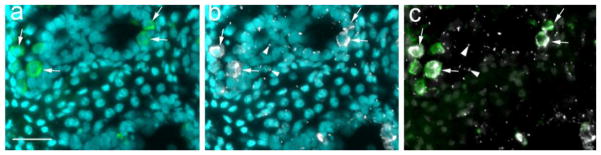
Appearance of primary cilia in testes from 6-day old piglets. Testis tissue was cryo-sectioned and stained with two different antibodies. (a), stained with anti-VASA antibody to mark germ cells (arrows) and DAPI to show nuclei; (b): stained with anti-acetylated tubulin antibody to indicate primary cilia (arrowheads show examples) and DAPI to stain nuclei; (c): merged image showing VASA stain and acetylated tubulin stain. The bar indicates 50 μm
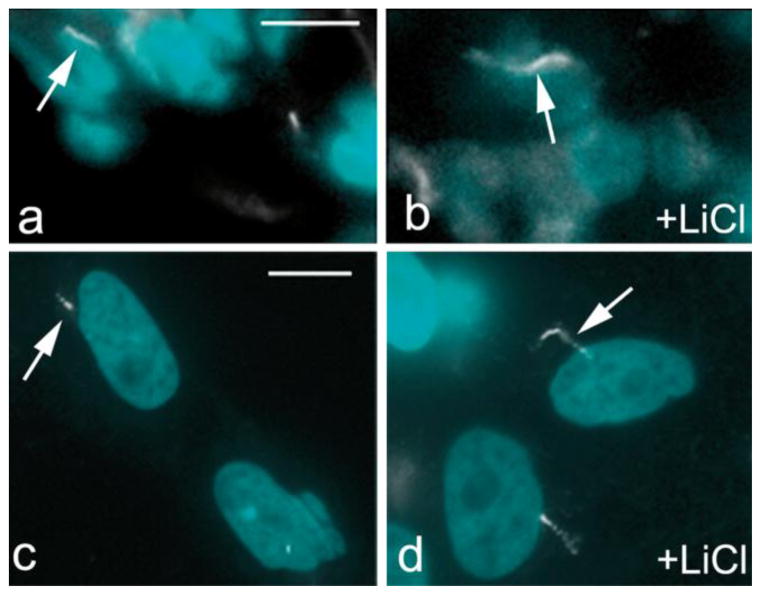
In our previous study, we found that primary cilia respond to lithium treatment by elongation (Ou et al. 2009). Therefore, we tested whether primary cilia in testis can respond to lithium. We incubated a small piece of testis tissue (approximately 1 mm3) in DMEM medium containing 10% foetal bovine serum and lithium chloride. To further study this elongation, individual testicular cells were isolated and cultivated in DMEM containing 10% foetal bovine serum in the presence or absence of 50 mM lithium.
In other cell types, serum starvation during culture has been shown to induce expression of primary cilia [(Ou et al. 2009) and references therein]. Dispersed pig testis cells were cultured in the absence of serum for 2 days, and compared for cilia expression to those cultivated under control conditions (DMEM with 10 % FBS)
Identification of Sertoli cells and germ cells
GATA-4 and MIS are specifically expressed in Sertoli cells. GATA-4 is a transcription factor expressed abundantly in the nuclei of Sertoli cells throughout fetal and prepubertal stages of testis development (Ketola et al. 2000; McCoard et al. 2001). Similarly, MIS-positive cells within testicular cords are Sertoli cells (McCoard et al. 2001). VASA (mouse VASA homologue) is an RNA-binding protein specifically expressed in fetal and adult gonadal germ cells (Castrillon et al. 2000).
Statistical analysis
Data are presented as mean +/− s.d. (standard deviation). ANOVA followed by Tukey’s multiple comparisons test was used to determine significance (Prism 6, GraphPad Software, La Jolla, CA, USA). P values less than 0.05 are considered to be significant.
RESULTS
Functionally competent primary cilia are expressed in the developing pig testis
To explore primary cilia during testis development, we investigated the expression pattern of primary cilia in testis from pigs in the infantile/juvenile stages. Indirect immunofluorescence (IIF) microscopy revealed that the primary cilium microtubule bundles could be seen in the testis (Figure 1, arrowheads). They were located both in testis cords and in interstitial spaces (Figure 1). Acetylated tubulin antibody-stained primary cilia microtubules emerged directly from the basal body labeled with anti-γ-tubulin, not from other sites in the cytosol, as expected (Figure 2, panels a and b: arrowheads indicate basal bodies). The acetylated tubulin antibody-stained primary cilia are also labelled by anti-adenylate cyclase III antibody (Figure 2, panels c – e). Therefore, primary cilia are indeed present in developing testicular somatic cells.
Fig. 2.
Primary cilia originate from basal bodies in pig testis cells and express adenylate cyclase III. (a) anti-acetylated tubulin antibody was used to detect primary cilia (white; arrows point to cilia) and DAPI to stain nuclei. (b) staining with anti-gamma tubulin antibody to visualize the basal body (green; arrowheads point to basal bodies) and anti-acetylated tubulin antibody to detect primary cilia (white; arrows). The bar indicates 10 mm. Pig testis was stained with anti-adenylate cyclase (c; arrow) and anti-acetylated tubulin (d; arrow). (e), shows an overlay of images in (c) and (d). The bar indicates 20 μm
As a sensory organelle, a major function of primary cilia is to detect changes in the extracellular environment. Figure 3 shows that after treatment with lithium, primary cilia in the testis tissue (panels a and b) and on isolated testis cells (panels c and d) elongated significantly in comparison to untreated controls. The extent of elongation was approximately 3 times between treated and untreated, comparable to other cell types tested previously (Ou et al. 2009). Conversely, when dispersed pig testis cells were cultured in the absence of serum for 2 days, we found a 2-fold increase in the number of cells presenting a primary cilium for cells that were serum-depleted for 2 days compared to those cultivated under control conditions (data not shown), indicating that testicular somatic cells respond to serum starvation in a manner similar to what has been reported for non-testis cells. Taken together, these results suggest that the primary cilia observed in testis are functionally competent.
Fig. 3.
Pig testis primary cilia elongate in response to lithium treatment. (a and b): control and lithium treated (+LiCl) pig testis tissue; (c and d): control and lithium treated (+LiCl) Sertoli cell in vitro. Cilia are stained with anti-acetylated tubulin antibody (white; arrows); nuclei are stained with DAPI. The bar indicates 50 μm
Porcine Sertoli cells express primary cilia
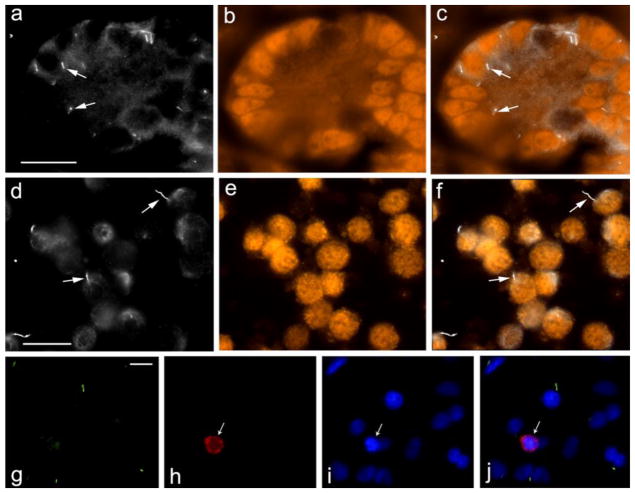
The results presented in Figure 1 suggested that primary cilia do not appear to be expressed in germ cells and instead appear expressed from somatic cells. To conclusively identify whether germ cells or Sertoli cells within the testis cords express primary cilia, we used IHC with cell-type specific markers
The results shown in Fig. 4 (panels a – c, arrows) indicate that GATA-4 positive Sertoli cells within testis cords express primary cilia. Similar results were obtained when tissue sections were stained with the antibody against MIS (not shown).
Fig. 4.
Pig Sertoli cells express primary cilia. Pig testis sections (a – c) and cultured Sertoli cells (d – f) were stained with anti-acetylated tubulin antibody to detect primary cilia (a, d; arrows point to examples) and anti-GATA4 antibody to identify Sertoli cells (b, e). c and f show a merged images of a, b and d, e, respectively. The bar indicates 20 μm
Six day pig testis cells, cultured in vitro, were stained with anti-Arl13b antibody to detect primary cilia (g), anti-VASA to detect germ cells (h), DAPI (i). j shows a merged image of g, h, i. The bar indicates 10 μm
To further verify that Sertoli cells (and not germ cells) express primary cilia, testicular cells were isolated from 6-day old pig testes. The cells were then analyzed for expression of primary cilia (acetylated alpha tubulin) and GATA-4 (Fig. 4, panels d – f) and for expression of primary cilia (ARL13B positive) and the germ cell marker VASA (Fig. 4, panels g – j). The results demonstrate that in 6-day old pig testis primary cilia are expressed from GATA-4 positive Sertoli cells and not from VASA-positive germ cells.
Dynamic pattern of primary cilia formation on Sertoli cells during pig testis development
We next investigated the temporal expression pattern of primary cilia on Sertoli cells during pig testis development. To address this, we counted primary cilia in testicular cords of pig testes collected at different time points (two weeks to 8 weeks after birth). Primary cilia were expressed by Sertoli cells and other somatic cell types at all times. However, compared to testes of 2-week old piglets, the number of primary cilia per Sertoli cell decreased 2–3-fold between week 2 and 4 (Figure 6a). Our results show that expression of primary cilia on Sertoli cells in developing pig testis is dynamic, suggesting a possible role of primary cilia in pig testis development.
Fig. 6.
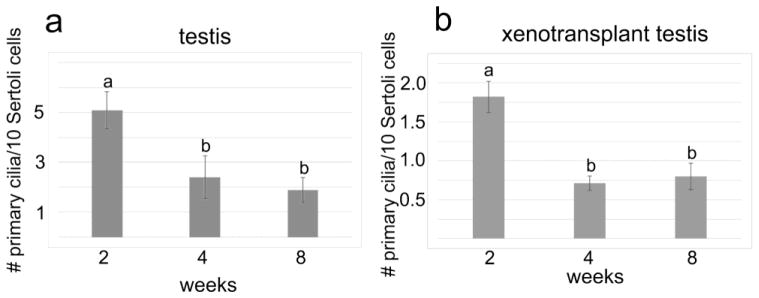
Quantitation of pig testis primary cilia during development. (a) Average number of primary cilia in pig testis (of indicated age) per 10 Sertoli cells. Error bars represent standard deviation; a and b indicate statistically significant differences (P<0.01). (b) Average number of primary cilia per 10 Sertoli cells in pig testicular cell xenotransplants. Primary cilia on 250 – 600 Sertoli cells were counted per sample. Error bars represent standard deviation; a and b indicate statistically significant differences (P<0.01)
Expression of primary cilia in de novo formed testis tissue is similar to expression in situ
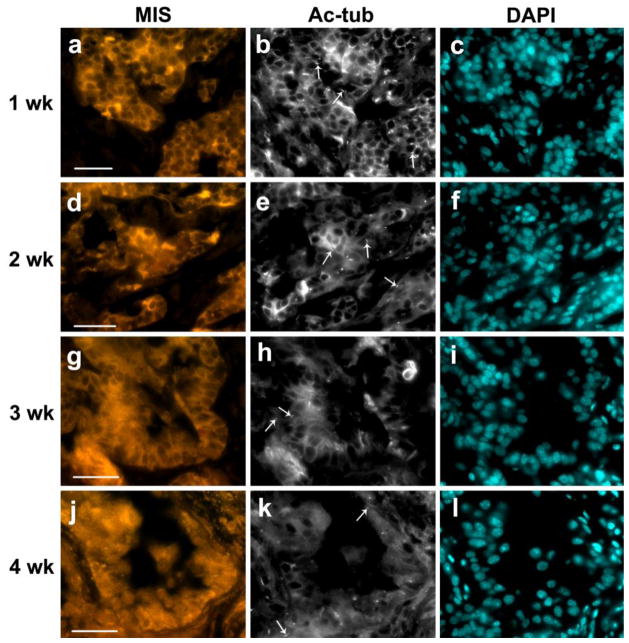
Our previous work showed that pig testis cells transplanted under the skin of immuno-deficient mice can re-organize, form testis cords, and support spermatogenesis (Honaramooz et al. 2007). Here, we used this model to explore the relationship between primary cilia expression and testis development. After transplantation, xenografts were collected every week and analyzed by IIF. After 1 week of cell grafting, primordial cord-like structures were found formed by Sertoli cells, which were labeled by anti-MIS antibody (Figure 5a). Many primary cilia were present both inside and outside of the cord-like structures (Figure 5b).
Fig. 5.
Primary cilium expression in pig testis xenotransplants decreases during development. Pig testicular cells were inoculated subcutaneously into immune-deficient mice, and grafts were collected at 1, 2, 3 and 4 weeks post inoculation (a–c: 1wk, d–f: 2wk, g–i: 3wk and j–l: 4wk, respectively). Grafts were analyzed using anti-MIS antibody (a, d, g, j: MIS) to detect Sertoli cells and anti-acetylated tubulin antibody (b, e, h, k: Ac-tub) to detect primary cilia. Cell nuclei were stained with DAPI (c, f, i, l). Examples of primary cilia expressed by pig testis xenotransplants are indicated by arrows. The bar indicates 50 μm
At 2 weeks after cell grafting, there were still many primary cilia present both inside and outside of the cord-like structures (Figure 5d, e). At week 3 after cell grafting, we noted a significant reduction in numbers of primary cilia in comparison with the number of primary cilia observed in 1 and 2 week grafts (Figure 5g, h). These results suggest a correlation between testis cord formation and primary cilium expression in the transplanted pig testicular cells which resembles the decrease we had observed in pig testis in situ. After 4 weeks of grafting, primary cilia were found in low numbers inside and outside of the testis cords (Figure 5j, k). The lower numbers of primary cilia were maintained thereafter. Quantification of primary cilia on Sertoli cells in de novo formed tissue grafts is shown in Fig. 6b.
DISCUSSION
In this study we report the first data on the expression of primary cilia during testis development using porcine testis as a model. The pig is a useful model to study testis development, with an infantile/juvenile stage of a few months, a window suitable for dissecting events that occur during testis development (Franca et al. 2000). We show that Sertoli cells express primary cilia and that the number of primary cilia appears to undergo a significant reduction during development. This finding was observed both in sections of pig testis, and in a xenotransplantation reconstitution model. Unexpectedly, while mature germ cells express a motile cilium, the sperm flagellum, we did not observe expression of primary cilia on male germ cells during the developmental stages analyzed.
Previous studies in other cell and organ systems have shown that functional primary cilia elongate in response to exposure to lithium (Ou et al., 2009). While the functional significance of increased cilia length in the testis remains unclear, elongation in response to exposure to lithium was used here to demonstrate that primary cilia in the testis have a similar ability to dynamically respond to environmental clues as has been described in other somatic cells. Together with the observed increase in the number of Sertoli cells expressing primary cilia after serum starvation, these results indicate that primary cilia on Sertoli cells are functionally competent and can respond to changes in environmental conditions.
It will be important to consider the implications of the observed reduction in the number of primary cilia expressed by Sertoli cells in both normal developing pig tissue and in pig testis cell-derived xenotransplants and what this observation suggests about possible functions for primary cilia in early testis development. The decrease in primary cilia was observed in pig testes during weeks 2 to 4 after birth and in tissue formed de novo from grafted cells between 2 and 4 weeks after grafting. One possibility is that the function of primary cilia in Sertoli cells and other cell types is to provide cells with sensory function to engage in monitoring environmental changes and involve cells in cell-cell communication. Sertoli cell primary cilia could thus be involved in the coordination and timing of the process of formation of the germ cell niche. Once the testicular microenvironment is fully formed and distinct cell associations are established, there may no longer be a need for primary cilia in large numbers. Disrupting or suppressing the expression of primary cilia on Sertoli cells prior to reconstituting testis tissue from grafted cells could serve to test this hypothesis.
If primary cilia are important for formation of the testicular microenvironment, then the question is why does not every Sertoli cell express a primary cilium at a given time point? Studies from other cell types and tissues have indicated that primary cilium formation is tightly associated with certain stages of the cell cycle and state of differentiation [(Rieder et al. 1979; Ho and Tucker 1989); for a recent review, see (Irigoin and Badano 2011)]. Here we analyzed primary cilium formation in pig testis cells dispersed and cultivated in monolayer during serum starvation, a commonly-used method to promote primary cilia production in non-testis cell types (Ou et al. 2009). We found a 2-fold increase in in primary cilium number under serum-free conditions, indicating that expression of primary cilia on testicular somatic cells is cell cycle dependent as reported for other cell types. Possibly, cells not expressing a primary cilium at the time of analysis in the models we used are thus in a stage of the cell cycle where cilia are not expressed. In this context, our results suggest that primary cilia may be involved in cell-cell communications and sensing the environment, and after the germ cell niche has been established, these functions are no longer required and primary cilia in Sertoli cells may become largely dispensable.
De novo formation of testis tissue from individual cells is a powerful model to study and manipulate testicular morphogenesis and germ cell differentiation [see (Dores et al. 2012)]. We now report that pattern and timing of expression of primary cilia on testicular somatic cells faithfully replicates the in situ situation in this assay. This will allow study of ciliary function in the testis by targeted disruption or enhancement of cilium expression. It also opens the opportunity to use the testicular morphogenesis assay to elucidate pathways implied in ciliopathies with manifestations in other organ systems.
In summary, we report for the first time the existence of primary cilia in mammalian testis and that their expression is dynamic during testis development. The primary cilia expressed on testicular somatic cells appear morphologically normal and react to the environment comparable to what has been described in other cell types. Further, we characterized the cell types that display a primary cilium and identified Sertoli cells as a sole type within the testis cords whereas germ cells did not express primary cilia during postnatal testis development. Most importantly, by studying testis tissue reconstituted in cell xenografts, we found that primary cilia expression is reduced coincidently with the de novo formation of testis cords. Also, our results show that the xenograft model faithfully recapitulates the behavior of primary cilia during development, thus validating the graft model for investigation of primary cilia biology in the testis and as a model system other cell types previously implicated in ciliopathies.
Acknowledgments
This work was supported by NIH/ORIP 9 R01 OD016575-12 (ID) and MOP 111008 from the Canadian Institutes of Health Research (FVDH). CBD was supported by a fellowship from AI-HS.
References
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, Valente EM, Woods CG, Gleeson JG. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores C, Alpaugh W, Dobrinski I. From in vitro culture to in vivo models to study testis development and spermatogenesis. Cell Tissue Res. 2012;349:691–702. doi: 10.1007/s00441-012-1457-x. [DOI] [PubMed] [Google Scholar]
- Franca LR, Silva VA, Jr, Chiarini-Garcia H, Garcia SK, Debeljuk L. Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol Reprod. 2000;63:1629–1636. doi: 10.1095/biolreprod63.6.1629. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD, Solari A, Tung PS, Fritz IB. Stimulation by follicle-stimulating hormone of DNA synthesis and of mitosis in cultured Sertoli cells prepared from testes of immature rats. Mol Cell Endocrinol. 1977;7:151–165. doi: 10.1016/0303-7207(77)90064-8. [DOI] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Ho PT, Tucker RW. Centriole ciliation and cell cycle variability during G1 phase of BALB/c 3T3 cells. J Cell Physiol. 1989;139:398–406. doi: 10.1002/jcp.1041390224. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Rathi R, Dobrinski I. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol Reprod. 2007;76:43–47. doi: 10.1095/biolreprod.106.054999. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- Horner VL, Caspary T. Disrupted dorsal neural tube BMP signaling in the cilia mutant Arl13b hnn stems from abnormal Shh signaling. Dev Biol. 2011;355:43–54. doi: 10.1016/j.ydbio.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IA, Coleman N, Faisal AS, Ng KL, Cheng A, Lim HN, Hawkins JR. Sexual dimorphism in the neonatal gonad. Acta Paediatr Suppl. 1999;88:23–30. doi: 10.1111/j.1651-2227.1999.tb14347.x. [DOI] [PubMed] [Google Scholar]
- Irigoin F, Badano JL. Keeping the balance between proliferation and differentiation: the primary cilium. Curr Genomics. 2011;12:285–297. doi: 10.2174/138920211795860134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola I, Pentikainen V, Vaskivuo T, Ilvesmaki V, Herva R, Dunkel L, Tapanainen JS, Toppari J, Heikinheimo M. Expression of transcription factor GATA-4 during human testicular development and disease. J Clin Endocrinol Metab. 2000;85:3925–3931. doi: 10.1210/jcem.85.10.6900. [DOI] [PubMed] [Google Scholar]
- Luo J, Rodriguez-Sosa JR, Tang L, Bondareva A, Megee S, Dobrinski I. Expression pattern of acetylated alpha-tubulin in porcine spermatogonia. Mol Reprod Dev. 2010;77:348–352. doi: 10.1002/mrd.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoard SA, Lunstra DD, Wise TH, Ford JJ. Specific staining of Sertoli cell nuclei and evaluation of Sertoli cell number and proliferative activity in Meishan and White Composite boars during the neonatal period. Biol Reprod. 2001;64:689–695. doi: 10.1095/biolreprod64.2.689. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Cluett EC, Jensen CG, Poole CA. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn. 2008;237:2013–2020. doi: 10.1002/dvdy.21501. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Orth JM. The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinol. 1984;115:1248–1255. doi: 10.1210/endo-115-4-1248. [DOI] [PubMed] [Google Scholar]
- Ou Y, Rattner JB. A subset of centrosomal proteins are arranged in a tubular conformation that is reproduced during centrosome duplication. Cell Motil Cytoskeleton. 2000;47:13–24. doi: 10.1002/1097-0169(200009)47:1<13::AID-CM2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ou Y, Rattner JB. The centrosome in higher organisms: structure, composition, and duplication. Int Rev Cytol. 2004;238:119–182. doi: 10.1016/S0074-7696(04)38003-4. [DOI] [PubMed] [Google Scholar]
- Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou YY, Mack GJ, Zhang M, Rattner JB. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J Cell Sci. 2002;115:1825–1835. doi: 10.1242/jcs.115.9.1825. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Petersen C, Soder O. The sertoli cell--a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res. 2006;66:153–161. doi: 10.1159/000094142. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Jensen CG, Jensen LC. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–185. doi: 10.1016/s0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Costa GM, Rathi R, Franca LR, Dobrinski I. Endocrine modulation of the recipient environment affects development of bovine testis tissue ectopically grafted in mice. Reproduction. 2012;144:37–51. doi: 10.1530/REP-12-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Tasouri E, Tucker KL. Primary cilia and organogenesis: is Hedgehog the only sculptor? Cell Tissue Res. 2011;345:21–40. doi: 10.1007/s00441-011-1192-8. [DOI] [PubMed] [Google Scholar]
- Willaredt MA, Tasouri E, Tucker KL. Primary cilia and forebrain development. Mech Dev. 2013;130:373–380. doi: 10.1016/j.mod.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]