Abstract
T cells with a CD4+ CD8+ double-positive (DP) phenotype are present in small numbers in the peripheral blood of healthy humans and may have anti-viral capacities. Here we investigate numbers and function of DP T cells in patients with relapsing–remitting multiple sclerosis (MS), either treatment-naive or under therapy with natalizumab. Flow cytometry analysis revealed that frequencies of circulating DP T cells in treatment-naive and natalizumab-treated MS patients are comparable to healthy controls. These cells have a memory phenotype with cytotoxic potential, express high levels of CD49d and are similarly functional in treatment-naive as well as natalizumab-treated MS patients. DP T cells were enriched in the cerebrospinal fluid, but do not invade acutely inflamed MS lesions. In conclusion, DP T cells are functional in MS and may play a role in the immune surveillance of the central nervous system, but do not display functional impairment under natalizumab therapy.
Keywords: cerebrospinal fluid, JC virus, memory T cells, natalizumab
Introduction
Besides mature single-positive (SP) CD4+ or CD8+ lymphocytes, T cells with a CD4+ CD8+ double-positive (DP) phenotype are present in small numbers in the peripheral blood of healthy humans. Increased frequencies of circulating DP T cells have been reported in autoimmune diseases such as myasthenia gravis [1], idiopathic immune thrombocytopenia [2] and multiple sclerosis [3]. Moreover, DP T cells were found to be enriched at the site of inflammation in rheumatoid arthritis and autoimmune thyroiditis [4,5].
The origin and function of T cells co-expressing both the CD4 and CD8 co-receptor is the subject of ongoing debate. While initially it has been suggested that DP T cells result from premature release of CD4+CD8+ thymocytes to the periphery [6,7], others have proposed that these cells are in fact specialized T cells with high anti-viral activity [8]. Expansion of DP cells was observed in human immunodeficiency virus (HIV) and Epstein–Barr virus (EBV) infections [9–11] and was reported as an early event following HTLV-1 exposure [12]. DP T cells were found to have a memory phenotype. Upon exposure to viral antigens they may differentiate into effector cells producing high amounts of interferon (IFN)-γ and tumour necrosis factor (TNF)-α, resulting in a high anti-viral activity that exceeds their SP counterparts [9,13]. The finding that circulating CD4+CD8+ DP T cells correlated with viral kinetics in an animal model of persistent chronic viral diseases (i.e. hepatitis C) [8] suggests that peripheral blood DP T cell expansion may be indicative of virus reactivation. Here we addressed the frequency, phenotype and functional activity of circulating DP T cells in multiple sclerosis (MS) patients and controls. Paired cerebrospinal fluid (CSF) and peripheral blood samples as well as biopsy specimens were analysed to assess a possible involvement of DP T cells in the immune surveillance of the central nervous system (CNS). In addition, we addressed a possible impact of natalizumab treatment on this specialized T cell subset and its anti-viral reactivity.
Methods
Patients and sample collection
The study was approved by the Ethics Committee of the Friedrich-Alexander University Erlangen (no. 4203). Written informed consent was obtained from all participants. To be eligible for this study, patients had to be diagnosed with relapsing–remitting multiple sclerosis (RRMS) according to the McDonald criteria [14]. Patients were either treatment-naive (n = 30) or treated with natalizumab (n = 32) for at least 2 months. Patients treated with glucocorticoids within 4 weeks of the study entry were excluded. All patients were assessed for expanded disability status scale (EDSS) and disease-specific parameters at the Academic MS Centre of the Friedrich-Alexander University of Erlangen. Healthy volunteers (n = 41) served as controls. Peripheral blood was obtained by venipuncture and processed immediately as described below. For CSF analysis consecutive patients with primary diagnosis of RRMS (n = 11) and non-inflammatory neurological diseases that underwent lumbar puncture for diagnostic reasons (NIND, n = 29; e.g. pseudotumour cerebri, normal pressure hydrocephalus, headache, somatoform disorder) were included. In addition, two patients under natalizumab therapy underwent lumbar puncture to rule out/confirm progressive multi-focal leucoencephalopathy (PML).
Flow cytometry
For DP T cell frequency analysis, 100 μl of ethylenediamine tetraacetic acid (EDTA) containing whole blood were stained in Trucountrrrr™ Tubes (BD Biosciences, San Jose, CA, USA) with anti-CD45 (2D1), anti-CD3 (HIT3a), anti-CD4 (SK3) and anti-CD8 (SK1) antibody or the respective isotype control antibodies in a fluorescence-minus-one control staining for 30 min at 4°C. Following erythrocyte lysis using an ammonium–potassium–chloride buffer, cells were washed twice and analysed on a BD fluorescence activated cell sorter (FACS)Canto II using FacsDiva software. For further characterization of DP T cells, one of the following antibodies was employed in addition to the antibodies named above: anti-granzyme B (GB11), anti-CD49d (9F10), CX3CR1 (2A9-1), anti-CD45RO (UCHL1), anti-CCR7 (3D12) and anti-CD8b (SIDI8BEE). All antibodies were purchased from eBioscience (San Diego, CA, USA) or BD Biosciences. CSF samples were obtained by lumbar puncture and processed immediately for flow cytometry. CSF was centrifuged at 300 g for 10 min to pellet cells. Samples with contaminating red blood cell content were excluded. CSF and paired blood samples were stained as described above. Only samples with > 1000 counts within the lymphocyte gate (acquired by flow cytometry) were included.
Proliferation assay
Peripheral blood mononuclear cells were isolated via Ficoll gradient centrifugation; 106 peripheral blood mononuclear cells (PBMC) were stained with 0·1 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) (Molecular Probes/Invitrogen, Carlsbad, CA, USA) and cultured on a 96-well round-bottomed plate (2·5 × 105) in the presence or absence of CD3/CD28 Dynabeads (at a bead-to-cell ratio of 1:25) for 72 h. To assess antigen-specific proliferation in response to viral stimuli, PBMC were cultured as stated above and exposed to overlapping peptide pools (15-mer) of cytomegalovirus (CMV) antigen pp65 (CMV PepTivator® pp65 human), EBV antigen EBNA-1 (EBV PepTivator® EBNA-1 human), JC virus (JCV) VP-1 (JCV PepTivator® VP1 human) or myelin basic protein (MBP) (MBP PepTivator® Isoform 1 human) in a concentration of 0·6 nmol/l for 7 days (all Miltenyi Biotec, Bergisch Gladbach, Germany). All samples were run in duplicate and pooled for flow cytometric analysis. The mean background proliferation was defined as proliferating fraction in media alone. The mean change in proliferating fraction (ΔPF) was calculated by subtracting the mean background proliferation from the mean proliferating fraction in response to antigen.
IFN-γ secretion
PBMC/well (2 × 106) were cultured for 16 h on a 48-well plate in the presence of CD28 stimulating antibody CD28·2 (2 μg/ml) in addition to CMV PepTivator® pp65, EBV PepTivator® EBNA-1, JCV PepTivator® VP1 human or MBP PepTivator® (Miltenyi Biotec) in a concentration of 0·6 nmol/l. Phorbol myristate acetate (PMA) (50 ng/ml)/ionomycin (750 ng/ml) was used as a positive control. For the last 4 h of culture BD Golgi Plug™ was added. Cells were processed for intracellular cytokine staining using the BD Bioscience intracellular cytokine staining kit in conjunction with anti-CD4 (SK3), anti-CD8 (SK1) and anti-interferon (IFN)-γ (4S.B3) following the manufacturer's instructions.
Transmigration assay
Transmigration was assessed in a well-established assay [15]. Using 3-μm pore-size, fibronectin-coated semi-permeable membranes (Corning Incorporated Costar®, Corning, NY, USA). Membranes were rehydrated with RPMI-1640 for 1 h at 37°C; 106 PBMCs suspended in 1 ml of RPMI-1640 plus 2·5% fetal calf serum (PAA, Pasching, Austria) were added to the upper chamber. The lower compartment was filled with 1·5 ml of RPMI-1640 supplemented with 10% fetal calf serum. After 12 h at 37°C, contents of the lower chamber were collected and processed for flow cytometry.
Immunohistochemistry
Tissue blocks were sampled from the CNS autopsy material of patients diagnosed with MS (n = 4) and CNS vasculitis (n = 2) in accordance with the Ethical Review Board of the Göttingen University Medical Centre (UMG). The age of the four MS patients at the time of death ranged from 36 to 54 years (mean ± standard deviation: 43·5 ± 7·7 years). The vasculitis patients were aged 43 and 49 years. Sections from paraffin-embedded tissue (2–3 μm thick) were deparaffinized, stained and pretreated as described previously [16]. Briefly, the sections were incubated overnight at 4°C with primary antibodies against CD4 [1:50, rabbit monoclonal; dendritic cells (DCs), SP35] and CD8 (1:50, mouse monoclonal; Dako, Glostrup, Denmark; C8/144B) diluted in 10% fetal calf serum in phosphate-buffered saline (PBS). Antibody detection was achieved using fluorescently labelled secondary antibodies (CD4, Alexa-488; Invitrogen; CD8, Cy3; Jackson ImmunoResearch, West Grove, PA, USA). Fluorescent signals were collected with a XM10 camera (Olympus, Tokyo, Japan) mounted on a BX51 epifluorescence microscope (Olympus) using a ×20 objective. All lesions were active demyelinating according to the criteria of Brück et al. [17]. CD4+/CD8–, CD4–/CD8+ and CD4+/CD8+ cells were counted manually for each patient in a minimum of 10 high-power fields (×400) or > 100 lymphocytes.
Statistical Analysis
Data from multiple experiments are expressed as mean ± standard error of the mean (s.e.m.). Non-parametric tests (Mann–Whitney U-test, Kruskal–Wallis test) were used to compare the data. The GraphPad Prism software was used. A value of P < 0·05 was considered statistically significant and was indicated as *.
Results
The frequency of circulating DP T cells in treatment-naive MS patients is comparable to healthy controls
DP T cell frequencies were assessed by flow cytometry in the peripheral blood of treatment-naive patients with relapsing–remitting disease (RRMS, n = 30) and healthy donors (n = 41; see Supporting information, Table S1 for patient characteristics). The mean percentage of DP T cells was 1·75 ± 0·37% (± s.e.m.) in RRMS patients and did not differ from the ostensibly healthy control cohort (1·74 ± 0·23%, Fig. 1a). The same held true for absolute numbers of DP T cells (HD 59·8 ± 8·95 cells/μl; RRMS 57·3 ± 9·75 cells/μl). Two subsets of CD4+CD8+ DP T cells have been reported that can be differentiated based on the level of CD4 and CD8 expression and may account for different DP T cell functions [18]. Therefore, we further analysed our data by gating on CD4highCD8low and CD4lowCD8high CD3+ T cells. Despite large interindividual variations (Fig. 1b), the frequency of CD4highCD8low and CD4lowCD8high DP T cell subsets was comparable in MS patients and controls (Fig. 1c,d). Moreover, the CD4highCD8low to CD4lowCD8high ratio did not differ between both groups. Our gating strategy was confirmed by analysing the composition of the CD8 receptor (αα homodimer in 89·9 ± 2·5% of CD4highCD8low, αβ heterodimer in 90·4 ± 2·2% CD4lowCD8high) in a subgroup of patients (n = 10, data not shown).
Fig. 1.
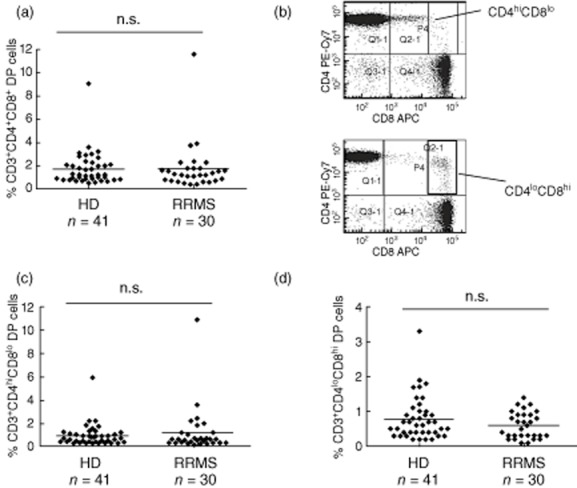
Double-positive (DP) T cell frequency in the peripheral blood of multiple sclerosis (MS) patients and controls. (a) The percentage of CD4+CD8+ DP T cells within the peripheral blood CD45+CD3+ T cell population was assessed by flow cytometry in treatment-naive MS patients with relapsing–remitting disease (RRMS, n = 30) and healthy donors (control, n = 41). (b) Exemplary dot plot of DP T cell staining in two different donors demonstrating the high interindividual variation concerning the proportions of CD4hiCD8lo and CD4loCD8hi cells within the DP T cell population. (c) Percentages of CD4hiCD8lo DP T cells within the peripheral blood CD3+ T cell population as assessed by flow cytometry. (d) Percentage of CD4loCD8hi DP T cells within the peripheral blood CD3+ T cell population as assessed by flow cytometry.
DP T cells in the peripheral blood of MS patients and healthy controls are memory cells with cytotoxic potential and high anti-viral activity
The majority of DP T cells displayed a memory phenotype. Fifty-four per cent of DP T cells stained CD45RO+CCR7– and were considered T effector memory cells (Fig. 2a). Approximately 8% of the DP T cells displayed a CD45RO+CCR7+ central memory phenotype (Fig. 2b). To assess the cytotoxic potential of DP T cells, we analysed the expression of the chemokine receptor CX3CR1 for fractalkine that has been reported to correlate with the cytotoxic activity of the carrying cell [19]. CD4+CD8+CD3+ T cells expressed high levels of CX3CR1 that were comparable to CD8 SP T cells (Fig. 2c). No differences were observed between untreated MS patients (n = 14) and controls (n = 10) with respect to CD45RO, CCR7, CD62L and CX3CR1 expression. Stimulation of DP T cells with anti-CD3/anti-CD28 coated beads in increasing concentrations led to a robust proliferation of both SP as well as DP T cells (n = 6, data not shown). When exposed to viral peptide pools containing overlapping 15-mer of CMV pp65 or EBV EBNA-1, antigen-specific T cell proliferation of SP and DP cells could be observed with large interindividual variations (Fig. 2d,e). The proliferating fraction of DP T cells was significantly higher compared to CD4 and CD8 SP T cells. No differences were observed between MS patients and controls (Fig. 2d,e). Besides myelin-reactive SP T cells, DP T cells that proliferated in response to myelin basic peptide pools were detected in HD and RRMS patients (Fig. 2f). No differences concerning the percentage of myelin-reactive SP or DP T cells could be identified between MS patients and healthy controls. To further assess functionality of DP T cells in MS patients, we analysed antigen-induced IFN-γ production in SP and DP T cells. In line with our findings on antigen-induced cell proliferation, DP T cells exposed to viral peptide pools were strong IFN-γ secretors that exceeded their SP counterparts in both HD and RRMS patients (Supporting information, Fig. S1).
Fig. 2.
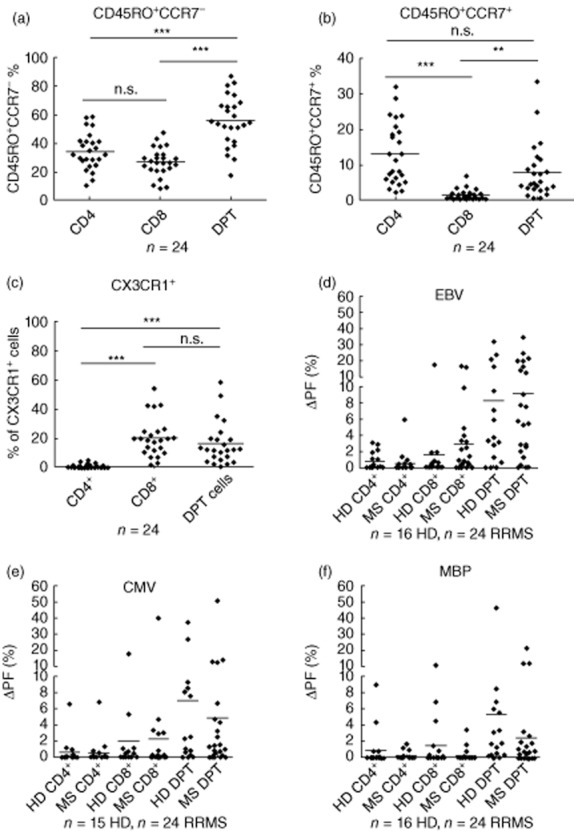
Double-positive (DP) T cells have a memory phenotype and display high anti-viral activity. (a) Percentage of CD45RO+CCR7– effector memory cells within the single-positive (CD4+, CD8+) and double-positive CD3+ T cell populations (DPT) as assessed by flow cytometry. (b) Percentage of CD45RO+CCR7+ central memory cells within the single-positive (SP) (CD4+, CD8+) and double-positive CD3+ T cell populations (DPT) as assessed by flow cytometry. (c) Percentage of CX3CR1+ cells with single-positive (CD4+, CD8+) and double-positive CD3+ T cell populations (DPT) as assessed by flow cytometry. (d,e) carboxyfluorescein diacetate succinimidyl ester (CFDA-SE)-based proliferation assays were performed to assess the proliferative response of DP and single-positive T cells to viral antigens [cytomegalovirus (CMV) pp65 or Epstein–Barr virus (EBV) EBNA-1 peptide pools]. The proliferating fraction (ΔPF) was calculated as: proliferating fraction in response to antigen minus background proliferation. (f) DP and SP T cell proliferation in response to myelin basic protein (MBP) peptide pools.
DP T cells in the cerebrospinal fluid of MS patients and controls
The choroid plexus has been proposed to constitute an important route for T cell trafficking to and from the CNS during physiological neuroimmune surveillance. Lymphocytes found in cerebrospinal fluid (CSF) were described to display an activated memory phenotype and attributed important functions in the immune surveillance of the CNS [20,21]. Having established that DP T cells are memory cells with a high anti-viral potency, we were interested whether DP T cells can be found within the CSF of patients with non-inflammatory neurological diseases (NIND, n = 29) or MS (n = 11). Paired samples revealed an enrichment of DP T cells in CSF (2·40 ± 0·20% of CD3+ T cells) compared to peripheral blood (1·68 ± 0·14%; P = 0·0015; Fig. 3a). However, no differences were observed between MS patients and controls (MS 2·44 ± 0·42%; controls 2·38 ± 0·23%; P = 0·39; Fig. 3b).
Fig. 3.
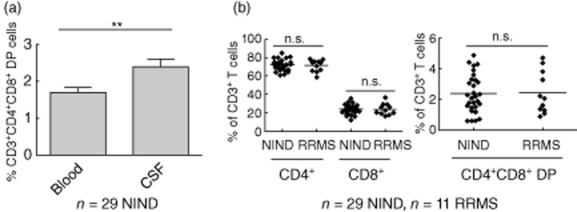
Double-positive (DP) T cells are present in cerebrospinal fluid (CSF). (a) The percentage of CD3+CD4+CD8+ T cells was assessed within paired CSF and peripheral blood samples derived from patients with non-inflammatory neurological diseases (NIND). (b) Comparative analysis of DP T cell and single-positive T cell frequency in the CSF derived from relapsing–remitting disease (RRMS) patients (n = 11) and patients with NIND (n = 29).
DP T cells were not detected at the site of inflammation in MS
We analysed the distribution of DP T cells in human MS lesions. For this purpose, we quantified CD4 and CD8 immunoreactive cells in tissue sections of active lesions from four RRMS patients as well as two patients with CNS vasculitis. While CD4 and CD8 single positive T cells were readily detectable at the site of inflammation in all patients irrespective of the underlying inflammatory condition (at a CD4 : CD8 ratio of 0·4:1·44), no double-positive cells could be identified (Fig. 4a,b).
Fig. 4.
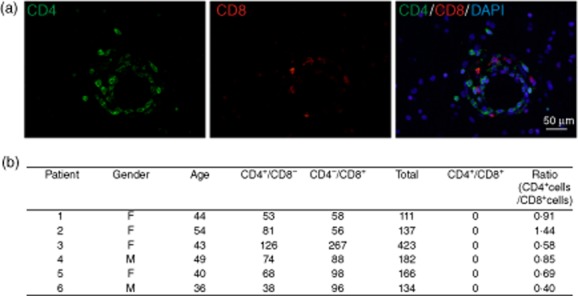
CD4+ and CD8+ lymphocytes in human central nervous system (CNS) lesions – no evidence of double-positive (DP) T cells at the site of inflammation. (a) Representative image from a human multiple sclerosis lesion from patient no. 2 showing fluorescently labelled CD4+ (green, left panel) and CD8+ (red, middle panel) perivascular lymphocytes. No overlap between the CD4 and CD8 signals could be observed (right panel). (b) Quantification of the number of CD4+/CD8–, CD4–/CD8+ and CD4+/CD8+ cells in each patient. A minimum of 10 high-power fields (×400) or > 100 lymphocytes were analysed. The CD4+/CD8+ ratio shows a significant predominance of CD8+ positive cells in the studied lesions (χ2 = 43·817, df = 5, P < 0·001).
Natalizumab treatment neither alters the frequency nor the functional activity of JC virus-reactive double-positive T cells
The natalizumab target molecule CD49d was found to be expressed highly on DP T cells (Fig. 5a). No significant differences concerning the peripheral blood frequency of DP T cells were identified between natalizumab treated (n = 32, 1·38 ± 0·18%; for patient characteristics see Supporting information, Table S1) and untreated RRMS patients (1·75 ± 0·37%, see above). When exposed to JCV VP1 antigen peptide pools, robust DP T cell responses, exceeding SP responses, were identified in both HD (=16) and RRMS patients (n = 25, Fig. 5b). No differences were detected between untreated (n = 11) and natalizumab-treated RRMS patients (n = 15, Supporting information, Fig. S2). DP T cell proliferation to JCV antigen in individual donors correlated well with the proliferative response of SP T cells (CD4+: Spearman's r 0·6655; P < 0·0001; CD8+: Spearman's r 0·815; P < 0·0001; Fig. 5c). In addition, DP T cells were found to be strong IFN-γ secretors when exposed to JCV peptides (Supporting information, data 1). Of note, cellular responses to JCV peptides were observed in both JCV antibody seropositive and seronegative donors/patients (Fig. 5d). As a proof-of-principle experiment, DP T cell frequency was assessed in two natalizumab patients undergoing CSF analysis for suspected PML. Although the CD4/CD8 ratio was remarkably decreased, as can be expected in natalizumab treated patients [22], the percentage of DP T cells in the CSF was comparable to our findings in all other MS patients and controls. One of these patients (depicted in Fig. 5e) was finally diagnosed with PML. Differential effects of natalizumab on SP and DP T cell trafficking were assessed in modified Boyden chambers coated with fibronectin, as described previously by Niino et al. [15]. In this experimental set-up, CD49d on immune cells is known to contribute to migration by interacting with the CS-1 fragment of fibronectin, implicated as one of the functional binding partners of VLA-4 in migration across human brain endothelial cells [15]. Although natalizumab pretreatment significantly inhibited cellular migration in this model, no differential effects on DP T cells versis SP T cells were observed (n = 6; data not shown).
Fig. 5.
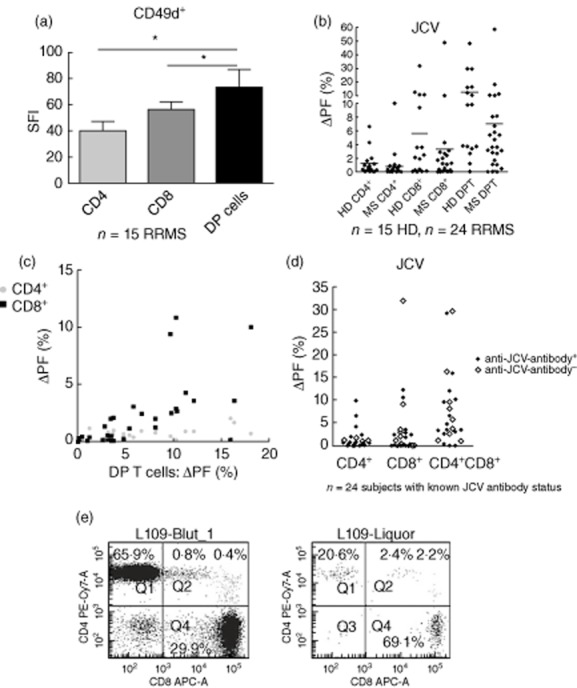
Natalizumab treatment does not affect double-positive (DP) T cell frequency or function in relapsing–remitting multiple sclerosis (RRMS) patients. (a) CD49d surface expression on CD4+ and CD8+ single-positive or CD4+CD8+ double-positive cells (DPT). The specific fluorescent index (MFI CD49d/MFI isotypic control) is depicted (n = 15, *P < 0·05; Kruskal–Wallis/Dunn's multiple comparison). (b) Carboxyfluorescein diacetate succinimidyl ester (CFDA-SE)-based proliferation assays were performed to assess the proliferative response of DP and single-positive T cells to JC virus (JVC) VP-1 peptide pools. (c) The percentage of proliferating cells within the DP T cell subset correlated well with the proliferating fraction within the CD4+ or CD8+ T cell subset. (d) JCV-induced T cell proliferation in anti-JCV antibody-positive and seronegative donors. (e) DP T cells in paired peripheral blood and cerebrospinal fluid (CSF) samples derived from a natalizumab-treated RRMS patient with progressive multi-focal leucoencephalopathy (PML).
Discussion
CD4 and CD8 SP T cell compartments have been studied extensively in MS. However, little is known on circulating DP T cells and if they contribute to the pathogenesis of the disease.
Virus infections have long been implicated in the pathogenesis of multiple sclerosis. However, despite large efforts no virus has been associated unequivocally with the CNS immunopathology. DP T cells have been reported to represent a population of effector cells with high anti-viral activity. Chronic viral infections (i.e. HCV) can be associated with a profound increase in the percentage of circulating DP T cells [8]. Increased frequencies of circulating DP T cells have also been reported in different autoimmune diseases. Therefore, we decided to study DP T cell frequency in MS patients and controls. The percentage of DP T cells found in our study was consistent with previous reports on DP T cell frequency in healthy donors (2–3% [18,23]). In contrast to a study by Munschauer et al. [3], we failed to detect differences concerning the frequency of circulating DP T cells in MS versus healthy controls. A possible explanation for this discrepancy is patient selection. All patients in our analysis presented a relapsing–remitting disease course with early MS [14]. In contrast, patients in the other study had clinically definite MS according to the Poser criteria [24] and 45% suffered a progressive disease course, thus probably also representing higher age and higher disease severity in advanced MS. Accordingly all hypotheses deduced from our study are valid for RRMS only and may not be transferable to patients with secondary progressive disease. Because no quantitative changes in the DP T cell population were observed, we next addressed a potential dysfunction of DP T cells in MS. However, the proliferative response of DP T cells or secretion of effector cytokines was comparable in both groups. Myelin-specific CD4+ and CD8+ T cells have been reported repeatedly to occur in the circulation of MS patients and healthy controls. We add to these findings by demonstrating that besides CD4 and CD8 SP cells, DP T cells with a myelin-specificity can be observed.
To our knowledge, we here provide the first systematic analysis of CD4+CD8+ DP T cells in cerebrospinal fluid and at the site of inflammation in multiple sclerosis. Previous studies addressing the phenotype of CSF (single-positive) T cells have demonstrated that CD4+ central memory cells represent the vast majority of CSF lymphocytes [20] and that the CSF CD4 : CD8 ratio is increased in RRMS, which is considered to be due to a higher propensity of activated CD4+ T cells to migrate into the CSF [21,25]. Given the fact that circulating CD4+CD8+ DP T cells display a memory phenotype, we addressed the presence of DP T cells within the CSF. Our data provide evidence that DP T cells are indeed present within the CSF. Furthermore, paired analysis demonstrated an enrichment of this highly potent T cell subset within the CSF, suggesting a possible role of DP T cells in the anti-viral immune surveillance of CSF-filled spaces. However, the lack of DP T cell accumulation within active MS lesions argues against a major role of this T cell subset in the pathogenesis of MS.
Long-term natalizumab treatment is associated with an increased risk of PML [26]. Given the high anti-viral capacity of DP T cells that bear high levels of the natalizumab target molecule, CD49d, we hypothesized that natalizumab treatment may impair DP T cell frequency or function. However, we did not observe differences between untreated and natalizumab treated MS patients. The fact that JCV-reactive DP and SP T cells were found in both JCV antibody-positive and -negative donors is not surprising. JCV antibody testing was reported to underestimate JCV infection rates, as 37% of JCV antibody-negative patients were found to have JC viruria in a recent study [27]. Similarly, JCV-specific T cells were repeatedly reported in JCV-seronegative patients. The frequency of JCV-reactive T effector memory cells was found to increase with long-term natalizumab treatment, which has been discussed to be indicative of JC virus replication [28]. The question of whether prominent DP T cell responses may serve as indicators of JCV replication in natalizumab-treated patients needs to be evaluated in longitudinal studies.
Summarizing, we did not find evidence of DP T cell impairment in multiple sclerosis. However, the presence of DP T cells within the CSF supports a possible role of this infrequent but highly potent T cell subset in the immune surveillance of CSF-filled spaces.
Acknowledgments
We wish to thank Katrin Bitterer and Brigitte Maruschak for expert technical assistance. This investigator-initiated study was supported in part by a grant from Biogen Idec GmbH and the Else Kröner-Fresenius Stiftung (2011_A123).
Author contributions
For conception and design, A. W. and R. L.; for acquisition of data, A. W., L. S., S. S. and A. B. F.; for analysis and interpretation of data, A. W., D. H. L., C. S. and R. L.; for the drafting of the manuscript, A. W., L. S. and R. L.; and for critical revision of the manuscript, S. S., A. B. F. and C. S.
Disclosure
A. W. has received travel grants or speaking honoraria from Merck Serono GmbH, TEVA GmbH, Sanofi-Aventis, Novartis Pharma GmbH and Biogen Idec GmbH and research funding from Merck Serono GmbH and Biogen Idec GmbH. L. S. and S. S. have nothing to disclose. D. H. L. and R. L. have received travel grants or speaking honoraria from Merck Serono GmbH, TEVA GmbH, Sanofi-Aventis, Novartis Pharma GmbH, Biogen Idec GmbH and Genzyme and research funding from Merck Serono GmbH, Biogen Idec GmbH and Genzyme. A. B. F. has received speaking honoraria from Novartis Pharma GmbH. C. S. has received travel grants and/or speaking honoraria from Merck Serono GmbH, TEVA GmbH, Sanofi-Aventis, Novartis Pharma GmbH and Biogen Idec GmbH.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Antigen-induced interferon (IFN)-γ production by single- and double-positive T cells.
Fig. S2. Single- and double-positive T cell proliferation induced by JC virus peptide pools.
Table S1. Clinical characteristics of patients.
References
- 1.Berrih S, Gaud C, Bach MA, Le Brigand H, Binet JP, Bach JF. Evaluation of T cell subsets in myasthenia gravis using anti-T cell monoclonal antibodies. Clin Exp Immunol. 1981;45:1–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Mizutani H, Katagiri S, Uejima K, et al. T-cell abnormalities in patients with idiopathic thrombocytopenic purpura: the presence of OKT4+8+ cells. Scand J Haematol. 1985;35:233–239. doi: 10.1111/j.1600-0609.1985.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 3.Munschauer FE, Stewart C, Jacobs L, et al. Circulating CD3+ CD4+ CD8+ T lymphocytes in multiple sclerosis. J Clin Immunol. 1993;13:113–118. doi: 10.1007/BF00919267. [DOI] [PubMed] [Google Scholar]
- 4.Iwatani Y, Hidaka Y, Matsuzuka F, Kuma K, Amino N. Intrathyroidal lymphocyte subsets, including unusual CD4+ CD8+ cells and CD3loTCR alpha beta lo/–CD4–CD8– cells, in autoimmune thyroid disease. Clin Exp Immunol. 1993;93:430–436. doi: 10.1111/j.1365-2249.1993.tb08196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Maria A, Malnati M, Moretta A, et al. CD3+4-8-WT31-(T cell receptor gamma+) cells and other unusual phenotypes are frequently detected among spontaneously interleukin 2-responsive T lymphocytes present in the joint fluid in juvenile rheumatoid arthritis. A clonal analysis. Eur J Immunol. 1987;17:1815–1819. doi: 10.1002/eji.1830171221. [DOI] [PubMed] [Google Scholar]
- 6.Perez AR, Morrot A, Berbert LR, Terra-Granado E, Savino W. Extrathymic CD4+CD8+ lymphocytes in Chagas disease: possible relationship with an immunoendocrine imbalance. Ann NY Acad Sci. 2012;1262:27–36. doi: 10.1111/j.1749-6632.2012.06627.x. [DOI] [PubMed] [Google Scholar]
- 7.Bonomo A, Kehn PJ, Shevach EM. Premature escape of double-positive thymocytes to the periphery of young mice. Possible role in autoimmunity. J Immunol. 1994;152:1509–1514. [PubMed] [Google Scholar]
- 8.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–486. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 9.Frahm MA, Picking RA, Kuruc JD, et al. CD4+CD8+ T cells represent a significant portion of the anti-HIV T cell response to acute HIV infection. J Immunol. 2012;188:4289–4296. doi: 10.4049/jimmunol.1103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss L, Roux A, Garcia S, et al. Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J Infect Dis. 1998;178:1158–1162. doi: 10.1086/515674. [DOI] [PubMed] [Google Scholar]
- 11.Ortolani C, Forti E, Radin E, Cibin R, Cossarizza A. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem Biophys Res Comm. 1993;191:601–609. doi: 10.1006/bbrc.1993.1260. [DOI] [PubMed] [Google Scholar]
- 12.Macchi B, Graziani G, Zhang J, Mastino A. Emergence of double-positive CD4/CD8 cells from adult peripheral blood mononuclear cells infected with human T cell leukemia virus type I (HTLV-I) Cell Immunol. 1993;149:376–389. doi: 10.1006/cimm.1993.1163. [DOI] [PubMed] [Google Scholar]
- 13.Kitchen SG, Whitmire JK, Jones NR, et al. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc Natl Acad Sci USA. 2005;102:3794–3799. doi: 10.1073/pnas.0406603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niino M, Bodner C, Simard ML, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006;59:748–754. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- 16.Schirmer L, Merkler D, Konig FB, Bruck W, Stadelmann C. Neuroaxonal regeneration is more pronounced in early multiple sclerosis than in traumatic brain injury lesions. Brain Pathol. 2013;23:2–12. doi: 10.1111/j.1750-3639.2012.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruck W, Schmied M, Suchanek G, et al. Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol. 1994;35:65–73. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- 18.Nascimbeni M, Pol S, Saunier B. Distinct CD4+ CD8+ double-positive T cells in the blood and liver of patients during chronic hepatitis B and C. PLOS ONE. 2011;6:e20145. doi: 10.1371/journal.pone.0020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura M, Umehara H, Nakayama T, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 20.Kivisakk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen KM, Gocke AR, Allie R, et al. Expression of CCR7 and CD45RA in CD4+ and CD8+ subsets in cerebrospinal fluid of 134 patients with inflammatory and non-inflammatory neurological diseases. J Neuroimmunol. 2012;249:86–92. doi: 10.1016/j.jneuroim.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuve O, Marra CM, Bar-Or A, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol. 2006;63:1383–1387. doi: 10.1001/archneur.63.10.1383. [DOI] [PubMed] [Google Scholar]
- 23.Blue ML, Daley JF, Levine H, Schlossman SF. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985;134:2281–2286. [PubMed] [Google Scholar]
- 24.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 25.Hafler DA, Weiner HL. In vivo labeling of blood T cells: rapid traffic into cerebrospinal fluid in multiple sclerosis. Ann Neurol. 1987;22:89–93. doi: 10.1002/ana.410220121. [DOI] [PubMed] [Google Scholar]
- 26.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 27.Berger JR, Houff SA, Gurwell J, Vega N, Miller CS, Danaher RJ. JC virus antibody status underestimates infection rates. Ann Neurol. 2013;74:84–90. doi: 10.1002/ana.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendel-Chavez H, de Herve MG, Giannesini C, et al. Immunological hallmarks of JC virus replication in multiple sclerosis patients on long-term natalizumab therapy. J Virol. 2013;87:6055–6059. doi: 10.1128/JVI.00131-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Antigen-induced interferon (IFN)-γ production by single- and double-positive T cells.
Fig. S2. Single- and double-positive T cell proliferation induced by JC virus peptide pools.
Table S1. Clinical characteristics of patients.


