Abstract
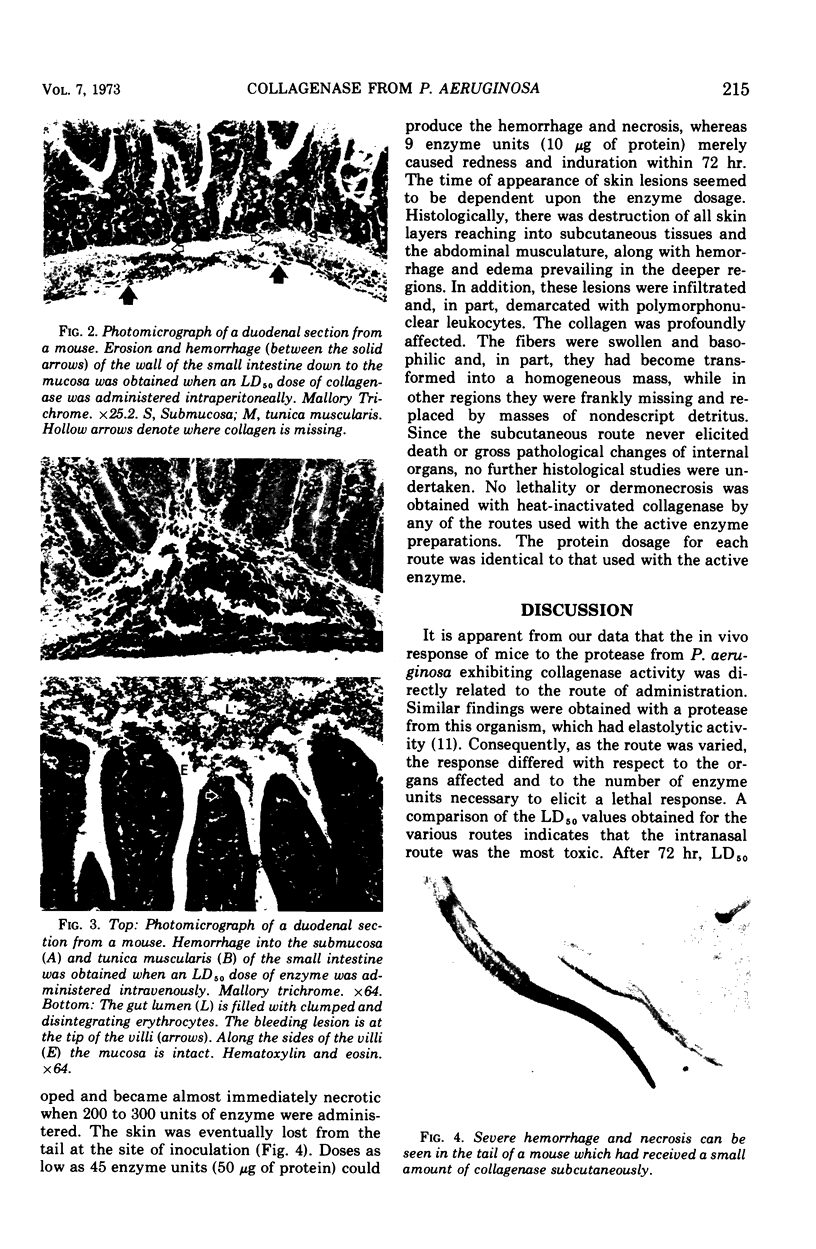
An extracellular protease from Pseudomonas aeruginosa having collagenase activity was assayed in vivo. The lethality of the enzyme for white female mice was determined by use of intravenous, intraperitoneal, intranasal, and subcutaneous routes, respectively. The collagenase exhibited the following 72-hr mean lethal dose values: intranasally, 55 collagenase units; intraperitoneally, 148 collagenase units; and intravenously, 288 collagenase units. In the concentrations tested, no lethality was obtained when the subcutaneous route was employed. Gross and microscopic studies revealed that the collagenase was capable of eliciting a variety of tissue responses in mice depending upon its route of administration. Intranasal instillation resulted in confluent pulmonary hemorrhage, whereas intraperitoneal injections resulted in severe abdominal hemorrhage with foci on the intestinal serosa. Intravenous injections elicited abdominal hemorrhage and petechial hemorrhage with focal necrosis of the lungs, whereas subcutaneous injections resulted in necrotic, ulcerating lesions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERK R. S. PARTIAL PURIFICATION OF THE EXTRACELLULAR HEMOLYSIN OF PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:559–565. doi: 10.1128/jb.88.3.559-565.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINLAND M., JONES W. F., Jr, BARNES M. W. Occurrence of serious bacterial infections since introduction of antibacterial agents. J Am Med Assoc. 1959 Aug 29;170:2188–2197. doi: 10.1001/jama.1959.63010180008012. [DOI] [PubMed] [Google Scholar]
- FISHER E., Jr, ALLEN J. H. Corneal ulcers produced by cell-free extracts of Pseudomonas aeruginosa. Am J Ophthalmol. 1958 Jul;46(1 Pt 2):21–27. doi: 10.1016/0002-9394(58)90030-8. [DOI] [PubMed] [Google Scholar]
- FISHER E., Jr, ALLEN J. H. Mechanism of corneal destruction by pseudomonas proteases. Am J Ophthalmol. 1958 Nov;46(5 Pt 2):249–255. doi: 10.1016/0002-9394(58)90804-3. [DOI] [PubMed] [Google Scholar]
- Hessburg P. C. Management of pseudomonas keratitis. Surv Ophthalmol. 1969 Jul;14(1):43–54. [PubMed] [Google Scholar]
- IACOCCA V. F., SIBINGA M., BARBERO G. J. RESPIRATORY TRACT BACTERIOLOGY IN CYSTIC FIBROSIS. Am J Dis Child. 1963 Sep;106:315–324. doi: 10.1001/archpedi.1963.02080050317012. [DOI] [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- Lin Y., Means G. E., Feeney R. E. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969 Feb 10;244(3):789–793. [PubMed] [Google Scholar]
- MARGARETTEN W., NAKAI H., LANDING B. H. Significance of selective vasculitis and the "bone-marrow" syndrome in Pseudomonas septicemia. N Engl J Med. 1961 Oct 19;265:773–776. doi: 10.1056/NEJM196110192651603. [DOI] [PubMed] [Google Scholar]
- Meinke G., Barum J., Rosenberg B., Berk R. In Vivo Studies with the Partially Purified Protease (Elastase) from Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):583–589. doi: 10.1128/iai.2.5.583-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke G., Berk R. S. In vivo studies with a toxic fraction of Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1970 Nov;135(2):360–363. doi: 10.3181/00379727-135-35051. [DOI] [PubMed] [Google Scholar]
- Mull J. D., Callahan W. S. The role of the elastase of Pseudomonas aeruginosa in experimental infection. Exp Mol Pathol. 1965 Dec;4(6):567–575. doi: 10.1016/0014-4800(65)90037-7. [DOI] [PubMed] [Google Scholar]
- NAOI M., EGAMI F., HAMAMURA N., HOMMA J. Y. Das toxische Lipopolysaccharid von Pseudomonas aeruginosa. Biochem Z. 1958;330(5):421–427. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- TEPLITZ C. PATHOGENESIS OF PSEUDOMONAS VASCULITIS AND SEPTIC LEGIONS. Arch Pathol. 1965 Sep;80:297–307. [PubMed] [Google Scholar]
- Waldvogel F. A., Swartz M. N. Collagenolytic activity of bacteria. J Bacteriol. 1969 May;98(2):662–667. doi: 10.1128/jb.98.2.662-667.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAK B., COHEN J. Automatic analysis of tissue culture proteins with stable Folin reagents. Clin Chim Acta. 1961 Sep;6:665–670. doi: 10.1016/0009-8981(61)90112-7. [DOI] [PubMed] [Google Scholar]






