Abstract
Proline, Glutamic acid- and Leucine-rich Protein 1 (PELP1) is a proto-oncogene that modulates estrogen receptor (ER) signaling. PELP1 expression is upregulated in breast cancer, contributes to therapy resistance, and is a prognostic marker of poor survival. In a subset of breast tumors, PELP1 is predominantly localized in the cytoplasm and PELP1 participates in extranuclear signaling by facilitating ER interactions with Src and PI3 kinases. However, the mechanism by which PELP1 extranuclear actions contributes to cancer progression and therapy resistance remains unclear. In this study, we discovered that PELP1 crosstalked with the serine/threonine protein kinase mammalian target of rapamycin (mTOR) axis and modulated mTOR signaling. PELP1 knockdown significantly reduced the activation of mTOR downstream signaling components. Conversely, PELP1 overexpression excessively activated mTOR signaling components. We detected the presence of the mTOR signaling complex proteins in PELP1 immunoprecipitates. mTOR targeting drugs (Rapamycin or AZD8055) significantly reduced proliferation of PELP1 over expressed breast cancer cells both in vitro and in vivo xenograft tumor models. MCF7 cells that uniquely retain PELP1 in the cytoplasm showed resistance to hormonal therapy and mTOR inhibitors sensitized PELP1-cyto cells to hormonal therapy in xenograft assays. Notably, IHC studies using xenograft tumors derived from PELP1 overexpression model cells showed increased mTOR signaling and inhibition of mTOR rendered PELP1 driven tumors to be highly sensitive to therapeutic inhibition. Collectively, our data identified the PELP1-mTOR axis as a novel component of PELP1 oncogenic functions and suggest that mTOR inhibitor(s) will be effective chemotherapeutic agents for downregulating PELP1 oncogenic functions.
Keywords: Estrogen Receptor, hormonal therapy, breast cancer, mTOR, ER- coregulators, PELP1, Rapamycin, AZD8055, Tamoxifen
Introduction
Breast cancer is one of the leading causes of deaths among the hormonal cancers (1). Endocrine therapy using tamoxifen, a selective estrogen receptor (ER) modulator and aromatase inhibitor, which ablates peripheral estrogen (E2) synthesis, has been shown to substantially improve disease-free survival (2, 3). Despite the positive effects of hormonal therapy, initial or acquired resistance to endocrine therapies frequently occurs (4).
Emerging evidence suggests that ER action is complex and requires functional interactions with coregulators (5). ER also participates in extra-nuclear signaling events in the cytoplasm, and crosstalk with growth factor signaling is implicated in the development of therapy resistance (6). As modulators of ER functions, coregulators are likely to play a role in breast cancer progression and resistance (7), therefore, the coregulator signaling axis represent a novel therapeutic target for maximizing breast cancer treatment opportunities.
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that belongs to the PI3K-related kinase family (8). mTOR plays an important role in cell growth, proliferation, autophagy, ribosomal biogenesis, development and aging (9–12). mTOR exists as two complexes: the mTOR, mLST8, Raptor containing rapamycin sensitive complex (mTORC1) and mTOR, mLST8, rictor containing rapamycin insensitive complex (mTORC2) (13, 14). mTORC1 phosphorylates and activates downstream signaling components such as S6K and 4E-BP1, both of which are involved in protein translation. mTORC2 associates with ribosomes and facilitates its activation (15). mTORC2 also phosphorylates Akt/PKB and SGK1 (16–18), which are implicated in therapy resistance. Estrogen signaling modulates mTOR signaling (19) and the mTOR/PI3K/Akt pathway is altered in most of the breast cancers (20). These emerging findings suggest that the blockade of the mTOR pathway has potential to modulate pathways activated by growth factor– and ER-dependent pathways.
Proline, Glutamic acid- and Leucine-rich Protein 1 (PELP1) is an ER coregulator that functions in nuclear as well as in extranuclear actions (21, 22). PELP1 couples the ER to several cytosolic signaling axes, such as Src-MAPK and PI3K-Akt (23). PELP1 localizes to the cytoplasm in a subset of breast tumors, and forced PELP1 cytoplasmic localization in model cells promotes excessive activation of AKT, leading to therapy resistance (24). PELP1 is a novel substrate of CDKs, PELP1 overexpression promotes E2-mediated G1-S progression (25). PELP1 signaling participates in rDNA transcription (26), and PELP1 facilitates ribosomal subunit processing (27, 28). Deregulation of PELP1 expression is also reported to occur in several cancers including breast, brain, and ovarian, and PELP1 expression correlates with poor prognosis (29–32). These emerging findings suggest that the proto-oncogene PELP1 functions as a scaffolding protein with no known enzymatic activity, and alternative means of targeting PELP1 oncogenic function are urgently needed.
We show that PELP1 plays a critical role in the optimal activation of mTOR and that PELP1 deregulation contributes to excessive activation of mTOR signaling. Pharmacological inhibition of mTOR significantly reduced PELP1-mediated tumorigenesis and therapy resistance in preclinical models. Our findings suggest that PELP1-mTOR axis is important in breast cancer progression and hormonal therapy resistance, and implicate the mTOR–PELP1 axis as a potential therapeutic target.
Materials and Methods
Cell lines and reagents
Human breast cancer cells MCF7 and ZR75 cells were obtained from American-Type Culture Collection (ATCC, Manassas, VA) and maintained and passaged in our laboratory for less than six months in RPMI-1640 medium supplemented with 10% FBS (Hyclone Laboratories Ltd, Logan, UT). Generation and characterization of MCF7-PELP1#20 and #13 (33), ZR75-PELP1(33), MCF7-PELP1 cyto model cells (34) were earlier described. PELP1 and Raptor antibodies were purchased from Bethyl lab (Montgomery, TX). Ki-67 was from Dako (Carpinteria, CA) and all other antibodies were purchased from Cell Signaling Technology (Boston, MA). β-actin and all secondary antibodies were from Sigma Chemical Co (St. Louis, MO). PELP1 SMARTpool human siRNA was purchased from Dharmacon (Lafayette, CO). Rapamycin and AZD8055 were purchased from Tocris Bioscience (Ellisville, Mo). Captisol was purchased from CyDex (La Jolla, CA).
Cell lysis and Western blotting
Whole cell lysates were prepared by using modified RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 50 mM NaF, 5 mM EDTA, 0.5% [wt/vol] sodium deoxycholate and 1% NP40) containing phosphatase and protease inhibitors as described (35). Total proteins (50 µg) were separated on 7 or 12% SDS-polyacrylamide gels and resolved proteins were transferred onto nitrocellulose membranes. The membranes were blocked with 5% non-fat dry milk powder in TBST solution for 1 hr at room temperature and incubated overnight in the desired primary antibodies at 4°C. Membranes were then washed and incubated with the respective secondary antibodies for 45 min at room temperature, and immunoreactivity was detected by using an ECL kit (GE Health Care, CA). GST pull-down assays were performed using various PELP1-GST deletions and by incubating them with total cellular lysates from MCF7 cells as described previously (25).
Cell proliferation and immunofluorescence assays
Cell viability rates were measured by using Cell Titer-Glo Luminescent Cell Viability Assay (Promega) in 96-well, flat, clear-bottom, opaque-wall micro plates. For some of the experiments cell proliferation rates were measured by using MTT Cell Viability Assay in 96-well micro plates. Breast cancer cells were seeded in 96-well plates (1×103 cells/well) in phenol red-free RPMI medium containing 5% DCC serum for E2-related experiments. After an overnight incubation, cells were treated with varying concentrations of Rapamycin, AZD8055, tamoxifen in the presence or absence of E2 (1×10−8 M) for 7 days. Cell proliferation rates were measured by using the MTT Cell Viability Assay. For Ki67 staining, cells were fixed in 3% paraformaldehyde, blocked in 5% goat serum and then stained with Ki67 antibody (1:50) overnight. Cells were then incubated with FITC-conjugated secondary antibody and the images were captured using fluorescence microscope. DAPI staining was used to visualize the nuclei. TUNEL staining was performed on fixed cells using manufacturer’s protocol (Roche, Indianapolis, IN).
Immunohistochemistry
Immunohistochemical (IHC) analysis was performed as described (36). Tumor sections were incubated overnight with IHC-specific antibodies of pS6 and Ki-67 at a dilution of 1:50 and immunoreactivity visualized by using the DAB substrate and counterstained with hematoxylin (Vector Lab, Inc. Burlingame, CA). The proliferative index was calculated as the percentage of Ki-67–positive cells in 5 randomly selected microscopic fields at 40X per slide. TUNEL analysis was done by using the In situ Cell Death Detection Kit (Roche, Indianapolis, IN) as per the manufacturer’s protocol. Five randomly selected microscopic fields in each group were used to calculate the relative ratio of TUNEL-positive cells (37).
Xenograft studies
All animal experiments were performed after obtaining UTHSCSA-IACUC approval and the animals were housed in accordance with UTHSCSA’s protocol for animal experiments. For xenograft tumor assays, 2 × 106 MCF-7 WT PELP1 or Cyto-PELP1 cells were mixed with an equal volume of matrigel and implanted in a mammary fat pad of 6-week-old female athymic nude mice as described (36). Once tumors reached measurable size, mice were divided into control and treatment groups. The control group received vehicles (2.5% Tween 80 and PEG 400 intraperitoneally, 30% Captisol orally and 0.3% hydroxyl propyl cellulose subcutaneously), and the treatment groups received Rapamycin (8 mg/kg/day) in 2.5% Tween 80 and PEG 400 intraperitoneally or AZD8055 (20mg/Kg/day) in 30% Captisol orally and tamoxifen (4mg/Kg/day) subcutaneously once in a day for 28 days. Doses were selected based on previous published studies (38–40). Tumor volumes and body weight were measured at weekly intervals. After the 30th day, the mice were euthanized, and the tumors were isolated and processed for immunohistological studies. Tumor volume was calculated by using a modified ellipsoidal formula: tumor volume = ½ (L×W2), where L is the longitudinal diameter and W is the transverse diameter. Body weight was measured at weekly intervals to rule out the drug toxicity.
Statistical analysis
Prism software was used for all statistical analyses. A Student’s t-test was used to assess statistical differences between control and drug treated groups. P values <0.05 were considered significant. Statistical differences among groups were analyzed with ANOVA.
Results
PELP1 modulates mTOR signaling
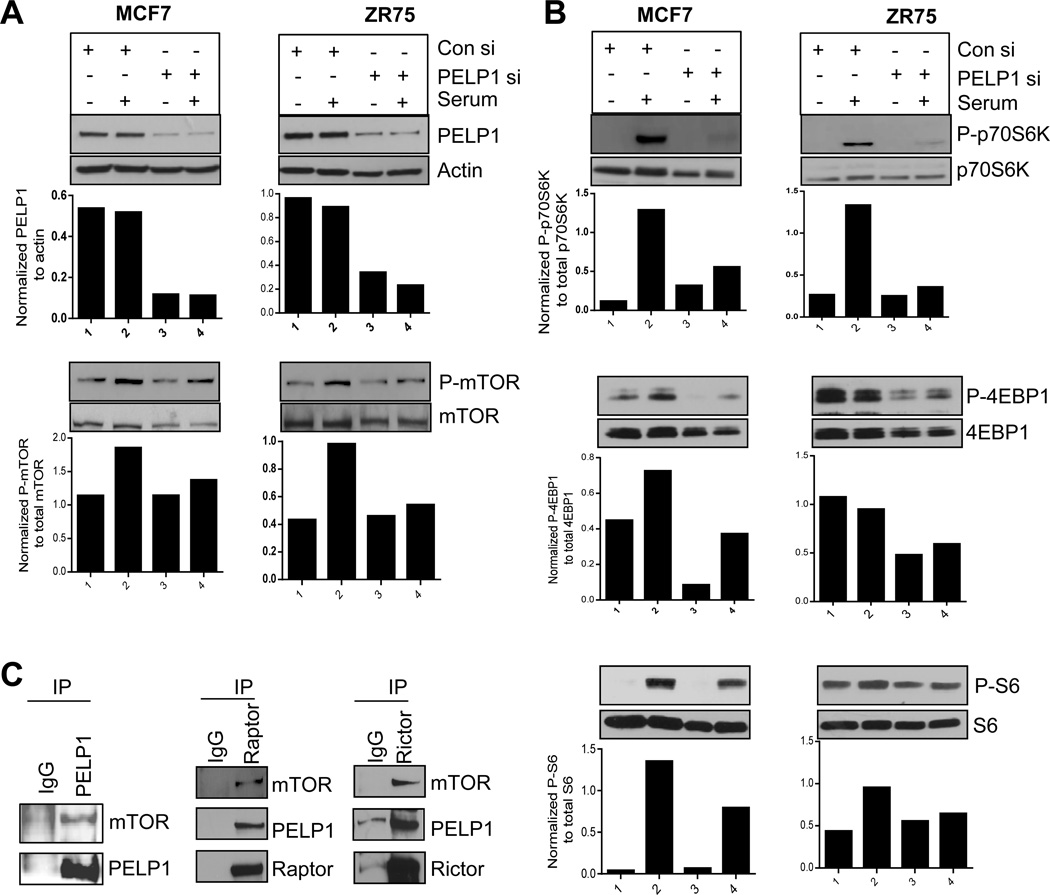
Since PELP1 modulates PI3K-AKT signaling (24) and because its expression is deregulated in breast tumors (41), we hypothesized that PELP1 oncogenic functions in part may involve PELP1 regulation of mTOR pathway. Down regulation of PELP1 expression with small interference RNA (siRNA) in both MCF-7 and ZR75 breast cancer cell lines substantially reduced serum-induced phosphorylation of mTOR signaling components including mTOR, p70S6K, S6K, and 4EBP1 (Fig. 1A, 1B). We confirmed these results using PELP1 shRNA (Supplementary Figure S1A). Overexpression of PELP1 in MCF7 cells further enhanced the serum-induced phosphorylation of S6 protein within 10 min, which is a direct measure of mTOR pathway activation (Supplementary Figure S1B). PELP1 lacks enzymatic activity and evolving evidence suggests that PELP1 functions as a scaffolding protein that modulates function of enzymatic complexes via protein-protein interactions (42). We therefore analyzed whether PELP1 associates with the mTOR complex by immunoprecipitating the total cellular lysates from serum stimulated MCF7 cells. Western analysis of PELP1 immunoprecipitates revealed the presence of mTOR (Fig. 1C, left panel). Similarly, immunoprecipitation of Raptor and Rictor, the critical components of mTORC1 and mTORC2 complexes respectively also revealed the presence of PELP1 in both mTOR complexes (Fig. 1C, middle and right panels). Further, GST pull-down assays using various PELP1 fragments as GST fusions revealed that the mTOR-interacting region in PELP1 is localized in the amino acids 960–1130; however, a weak interaction was also observed in the region containing amino acids 400–600 (Supplementary Figure S1C). Thus, our results suggest that PELP1 interacts with the mTOR complex and has potential to regulate the magnitude of mTOR signaling.
Figure 1. PELP1 is needed for optimal activation of the mTOR pathway.
(A, B) MCF7 and ZR75 cells were transiently transfected with nonspecific control siRNA or PELP1 specific siRNA for 72 h, serum starved for 24h and stimulated with 10% serum for 10 min. Status of phosphorylation of mTOR signaling components was analyzed by Western blotting. Quantitation of phosphorylation after normalizing to its respective total protein is shown.(C) Lysates of MCF7 breast cancer cells were subjected to immunoprecipitation with PELP1, Raptor, and Rictor antibodies, and the presence of mTOR and PELP1 in the immunoprecipitates was analyzed by Western blotting.
PELP1-enhanced mTOR signaling and cell proliferation can be abrogated by rapamycin and AZD8055
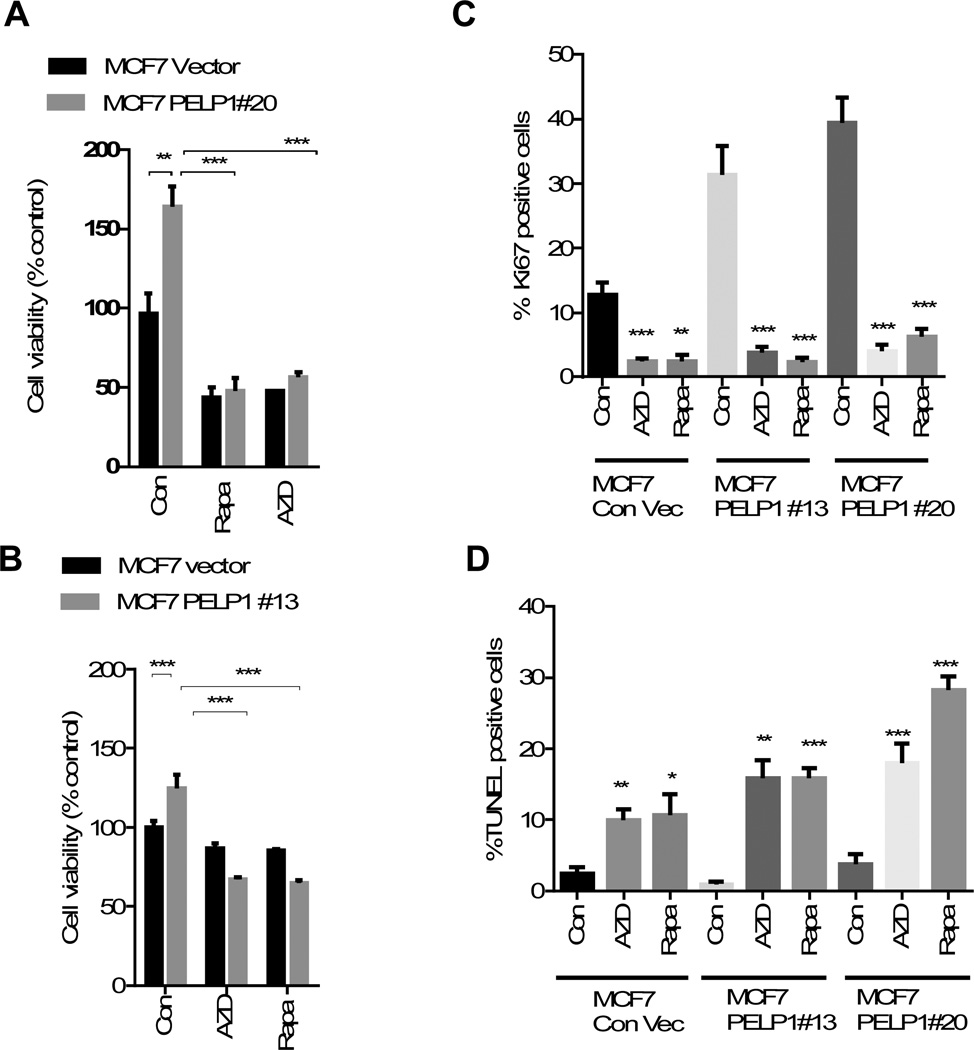
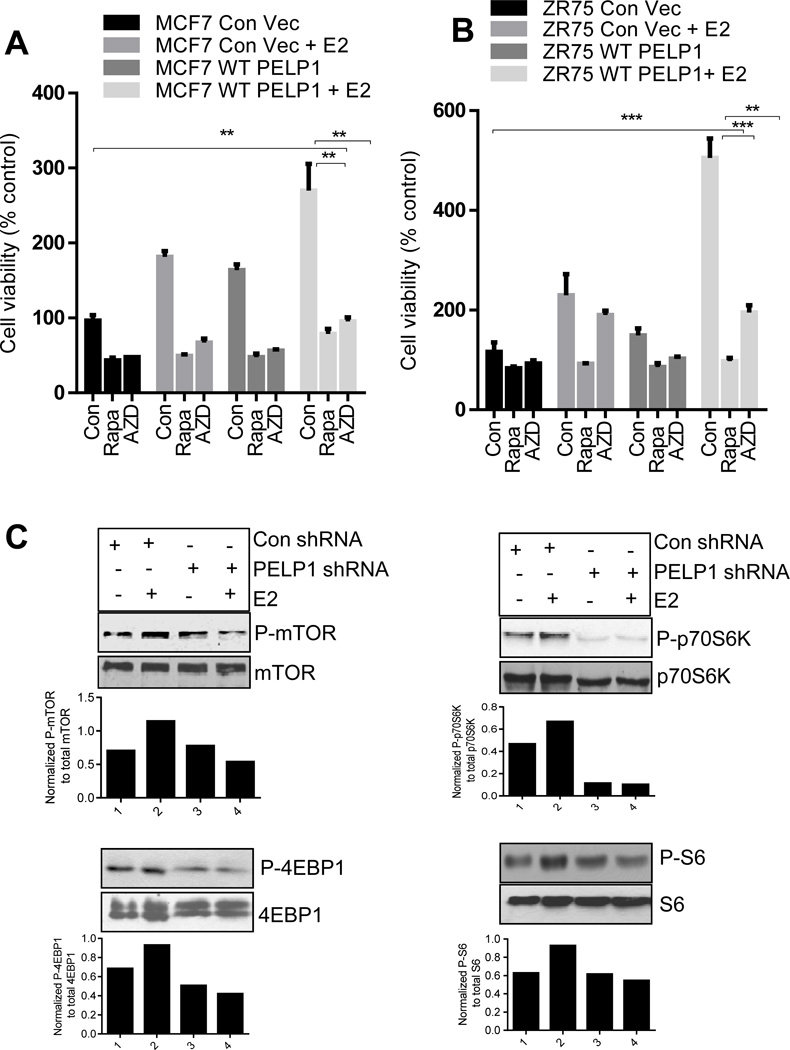
Because PELP1 functions as a proto-oncogene, we investigated whether PELP1 oncogenic functions involve activation of mTOR signaling using two pharmacological inhibitors: Rapamycin, which inhibits mTORC1, and AZD8055, which inhibits both mTORC1 and mTORC2 complexes. For this assays, we have used earlier established MCF7-PELP1 model cells (MCF7-PELP #20 and #13) that stably express 2–3 fold more PELP1over endogenous PELP1 and provide growth advantage compared to MCF7 cells. Interestingly, treatment with either rapamycin or AZD8055 substantially reduced the PELP1-mediated increase in cell viability (Fig. 2A, B). We then examined whether, mTOR inhibitors (rapamycin or AZD8055) reduces PELP1 driven proliferation using Ki67 as a marker of proliferation. Results showed that both rapamycin and AZD8055 significantly reduced the proliferation in both PELP1 clones (Fig. 2C). Further, TUNEL analysis revealed significant induction of apoptosis in rapamycin and AZD8055 treated cells (Fig. 2D). Collectively these results suggest that mTOR inhibitors have potential to reduce oncogenic potential of PELP1 both by reducing proliferation and by promoting apoptosis. Accordingly, both rapamycin and AZD8055 also significantly reduced the estrogen-driven, PELP1-mediated increase in cell viability in both MCF7 and ZR75 breast cancer cells (Fig. 3A, 3B). Further, PELP1 knock down also reduced estrogen-mediated activation of mTOR signaling (Fig. 3C). These results suggest that mTOR activation plays an important role in PELP1-mediated proliferation.
Figure 2. mTOR inhibitors reduced PELP1-mediated signaling and cell proliferation.
(A) Cell viability of MCF7-vec, MCF7-PELP1#20, MCF7-PELP1#13 cells were analyzed after treating the cells with or without 20 nM of rapamycin or AZD8055 in 5% FBS in RPMI medium for 72 h using an MTT assay. Results are the mean value of experiment performed in triplicates. (B) Cell viability of MCF7-vec, and MCF7-PELP1#13 cells were analyzed after treating the cells with or without 40 nM of rapamycin or AZD8055 in 5% DCC in RPMI medium for 72 h using MTT assay. Results are the mean value of experiments performed in triplicates. (C, D) Model cells were plated on cover slips in six well plates, treated with or without 40 nM of Rapamycin or 20 nM AZD8055 in 5% DCC serum in RPMI medium. (C) Ki-67 staining as a marker of proliferation was performed as described in methods section. (D)TUNEL staining was performed as a marker of apoptosis on fixed cells. Quantitation of Ki-67 and TUNEL staining was done as described in the methods section. **, P<0.01; ***, P<0.001; ***, P<0.0001.
Figure 3. mTOR inhibitors reduced PELP1-mediated estrogen driven growth in breast cancer cells.
(A) MCF7 or (B) ZR75 cells (2×103) stably expressing control vector or PELP1 WT were seeded in 96-well plates and stimulated with E2 (1×10−8 M) in 5% charcoal-stripped medium for 7 days in the presence or absence of mTOR inhibitors. Cell viability was determined by using an MTT assay. Results are the mean value of experiments performed in triplicates. Student t-test was used for analysis. **, P<0.01; **, P<0.001. (C) ZR75 cells stably expressing control shRNA or PELP1 shRNA were E2 starved for 72h and stimulated with E2 (1×10−8 M) for 15 min in 5% charcoal stripped media. Status of mTOR signaling was analyzed by Western analysis. Quantitation of phosphorylation after normalizing to its respective total protein is shown.
mTOR inhibitors reduce PELP1-mediated tumor growth in vivo
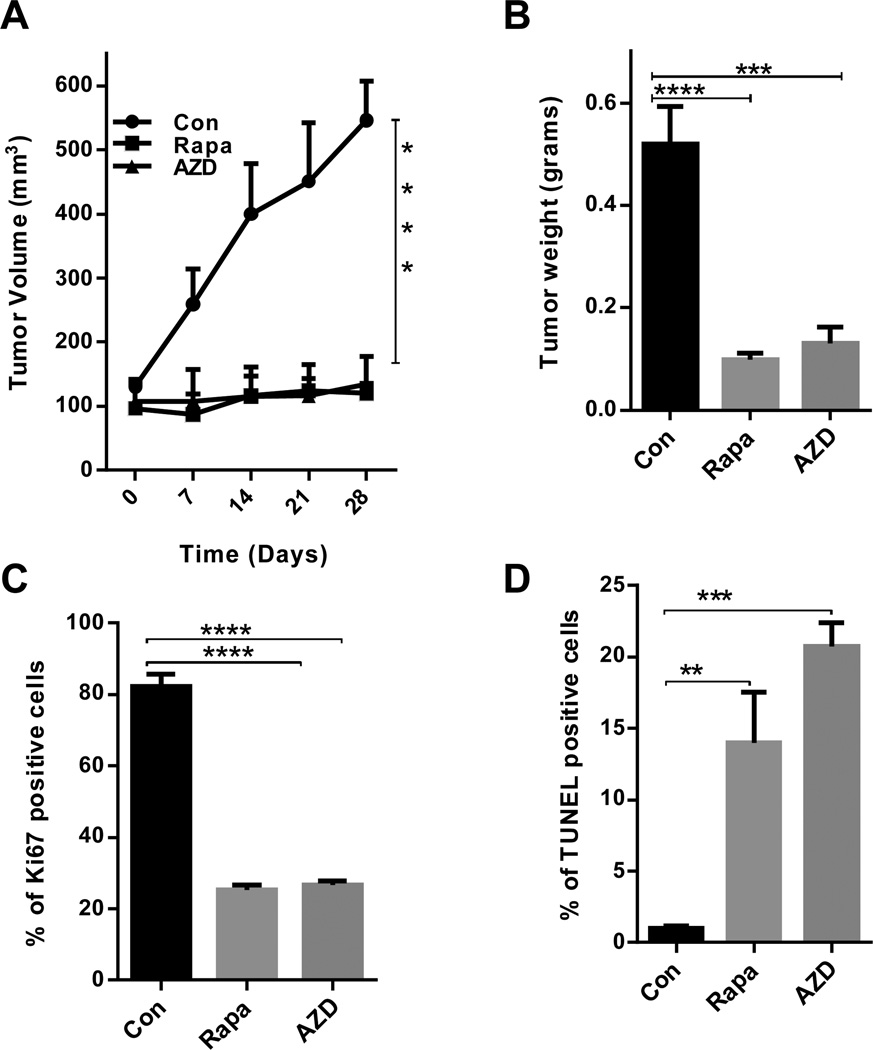
We then tested the therapeutic potential of mTOR inhibitors to block PELP1-driven breast tumorigenesis using a preclinical xenograft model. We used previously established MCF7-PELP1 cells. Normal MCF7 cells do not form tumors in mice in the absence of estrogen supplementation, while MCF7-PELP1 cells form tumors under these conditions, thus this provides a unique model to test a drug’s capability to block PELP1-mediated tumorigeneis (33). The mammary fat pads of nude mice were injected with MCF7-PELP1 cells. After establishment of tumors, 5 mice per group (for a total of 10 tumors) were treated with rapamycin (8 mg/kg/day/i.p.) or AZD8055 (20mg/Kg/day/oral gavage) for a period of 4 weeks, while control mice received daily vehicle injections. Toxicity as assessed by behavioral changes, such as eating habits and mobility, was not detected in the animals treated with rapamycin or AZD8055. Mouse weights were not significantly different among the control, rapamycin- or AZD8055-treated groups (data not shown). Rapamycin- and AZD8055-treated mice had significantly reduced tumor volumes in MCF-7-PELP1 xenografts, respectively, when compared to tumors in the control group (Fig. 4A). Rapamycin and AZD8055 treatments significantly reduced tumor weights (Fig. 4B). IHC analysis of phospho-S6 protein in tumor samples as a marker of mTOR signaling pathway activation revealed that rapamycin- or AZD8055-treated tumors had a substantial reduction in the phosphorylation of S6 when compared to the phosphorylation of S6 in the control group (Supplementary Figure S2A, B). Further, rapamycin- and AZD8055-treated tumors exhibited reduced proliferation as evidenced by decreased Ki67 (Fig. 4C, Supplementary Figure S2C) and exhibited increased apoptosis as seen by TUNEL positivity (Fig. 4D, Supplementary Figure S2D). These results suggest that mTOR inhibitors (rapamycin or AZD8055) have the potential to reduce PELP1-mediated tumor progression.
Figure 4. Rapamycin or AZD8055 treatment reduced PELP1-mediated xenograft tumor growth.
(A) Six-week-old nude female mice were subcutaneously implanted with MCF7-PELP1 WT cells. After the tumors reached a measurable size, the mice were treated daily with vehicle, Rapamycin or AZD8055 for 4 weeks. Tumor volumes were measured at weekly intervals. (B) Tumor weights are shown in the histogram. (C) Ki-67 expression was analyzed by using IHC and quantitation of Ki-67 staining was done as described in the methods section. (D) TUNEL staining was performed as a marker of apoptosis on tumors that were treated with Rapamycin or AZD8055. Quantitation was done as described in methods. **, P<0.01; ***, P<0.001; ***, P<0.0001.
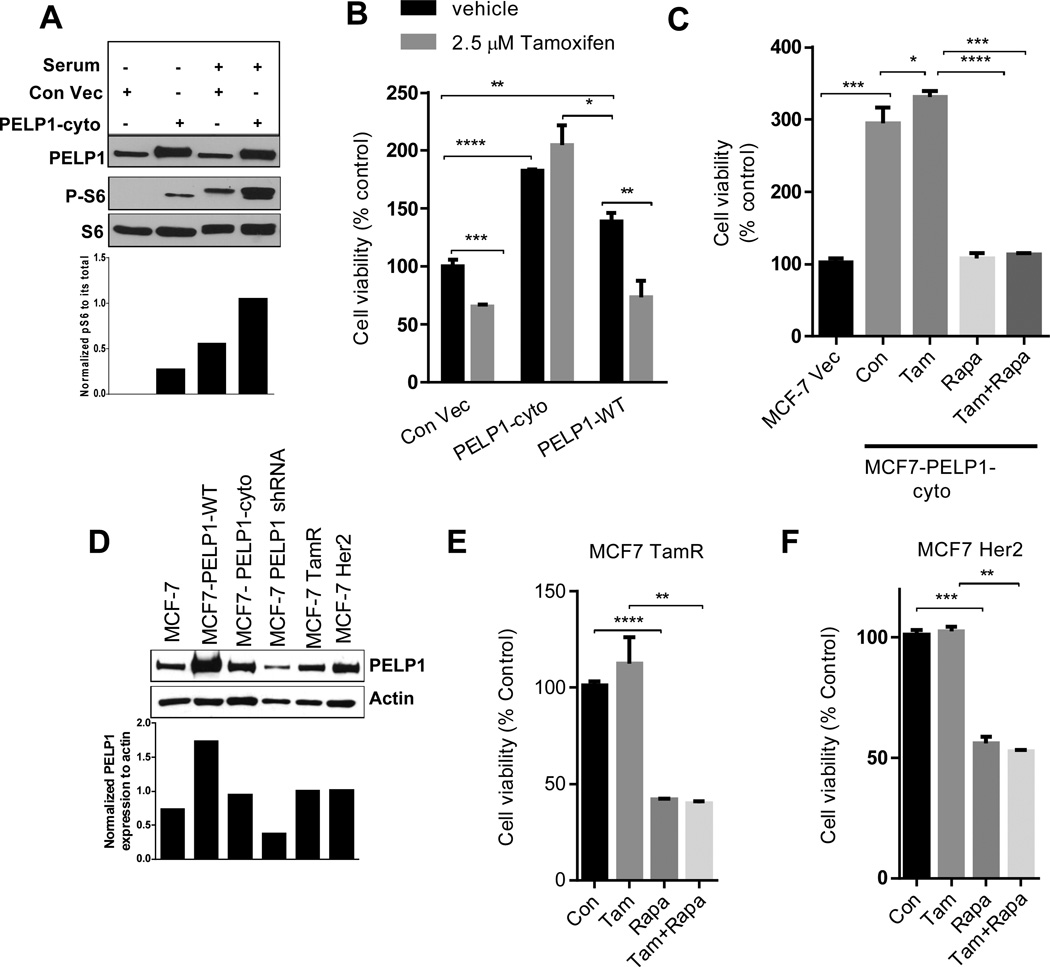
Cytoplasmic retention of PELP1 promotes mTOR signaling and tamoxifen resistance
Earlier studies suggested that PELP1 is predominantly localized in the cytoplasm in a subset of breast tumors (34) and that PELP1 localization could be used as a determinant of hormone sensitivity or vulnerability (43). We examined whether PELP1 localization in the cytoplasm constitutively activates mTOR signaling. We used the previously established MCF7 model cells that overexpress a PELP1cyto mutant lacking a nuclear localization sequence, thus PELP1 predominantly localizes to the cytoplasm in these cells. Overexpression of PELP1cyto in MCF7 cells constitutively activated the mTOR pathway signaling as shown by more phosphorylation of S6 protein in these cells than in control MCF7 cells (Fig. 5A). Compared with the control cells, PELP1cyto cells exhibited substantial increase in the phosphorylation of S6 protein upon serum stimulation (Fig. 5A). In agreement with previously published data, MCF7-PELP1cyto cells had resistance to tamoxifen (Fig. 5B) and the mTOR inhibitors rapamycin and AZD8055 significantly reduced PELP1cyto cell viability (Fig. 5C,). AZD8055 treatment of tamoxifen resistant MCF7-TamR, MCF7-Her2 and PELP1 cyto cells sensitized them to tamoxifen (Supplementary Figure S3A, B, C) Earlier studies showed PELP1 expression deregulated in therapy-resistant tumors (43) and PELP1 knockdown reduced tamoxifen therapy resistance (36). We next examined whether mTOR inhibitors also reduce viability of other tamoxifen-resistant model cells such as MCF7-HER2 and MCF7-Tam. The expression of PELP1 in the Tam and HER2 model cells correlated well with resistance to tamoxifen (Fig. 5D). Similar to the data that were reported in previous studies, both model cells exhibited resistance to tamoxifen treatment while rapamycin treatment significantly reduced the viability of tamoxifen-resistant MCF7-Tam (Fig. 5E) and MCF7-HER2 (Fig. 5F) cells. Collectively, these results suggest that PELP1 deregulation has the potential to constitutively activate the mTOR signaling axis, contributing to therapy resistance and that mTOR inhibitors could be used to reduce PELP1 mediated oncogenic potential leading to therapy resistance.
Figure 5. PELP1 cytoplasmic localization promotes mTOR signaling and tamoxifen resistance.
(A) MCF7 cells stably expressing control vector or PELP1-cyto were serum starved for 72 h and stimulated with 10% serum for 10 min. The status of PELP1 expression and S6 phosphorylation was analyzed by Western blotting. Quantitation of S6 phosphorylation after normalizing to its respective total protein is shown. (B) MCF7 control vector, MCF7-PELP1-cyto or MCF7-PELP1-WT cells were treated with vehicle or 2.5µM tamoxifen for 72 h in 5% charcoal-stripped medium and cell viability was assayed by using an MTT assay. (C) MCF7 control vector or MCF7-PELP1-cyto cells were treated with vehicle, 2.5 µM tamoxifen, 20 nM rapamycin or in combination for 7 days in 5% charcoal-stripped medium. After the treatment, cell viability was determined by using an MTT assay. (D) Total lysates from various model cells were analyzed for the expression of PELP1 by Western blotting. (E) MCF7-TamR cells were treated with vehicle, 2.5 µM tamoxifen, 20 nM rapamycin or in combination for 7 days, and cell viability was determined by using an MTT assay. (F) MCF7-HER2 cells were treated with or without 2.5 µM tamoxifen, 20 nM rapamycin or in combination for 7 days, and cell viability was determined by using an MTT assay. Student t-test was used to analyze the data. **P<0.01; ***, P<0.001; ****, P<0.0001.
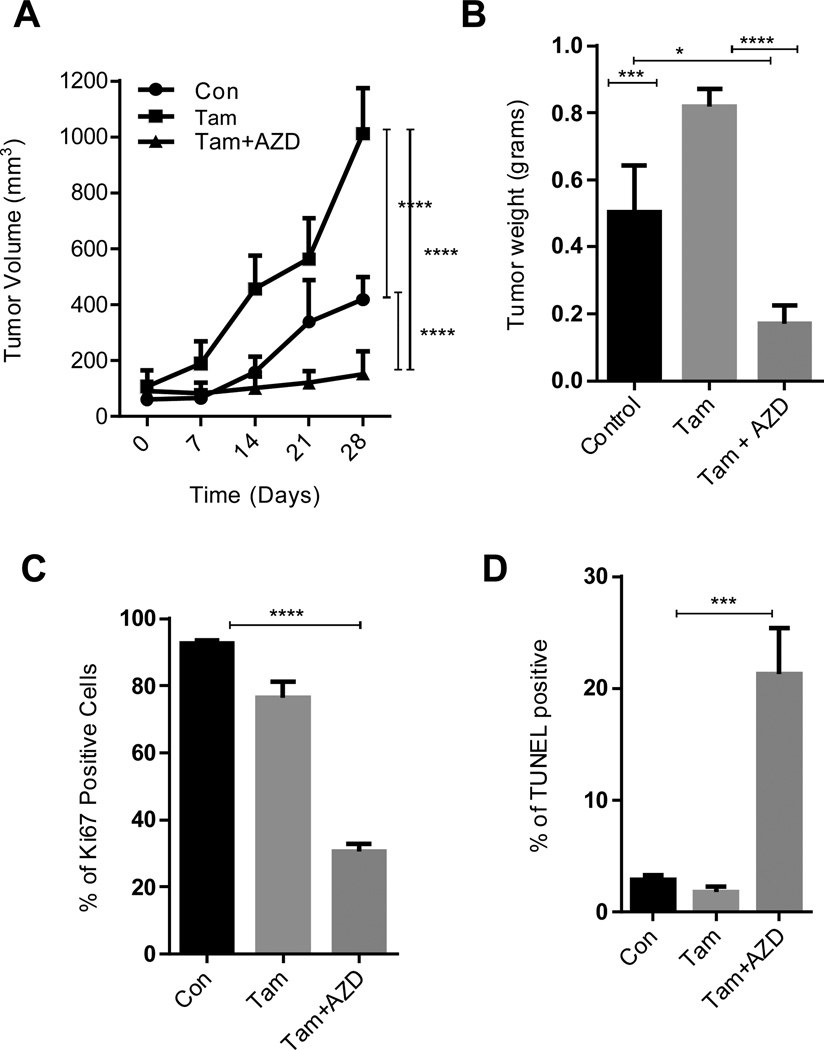
mTOR axis inhibition reduces the PELP1cyto-tamoxifen–mediated xenograft tumor growth
Since PELP1cyto cells exhibited resistance to tamoxifen and had increased activation of mTOR signaling, we tested whether AZD8055, which is currently in clinical trials, is useful in reducing the growth of tamoxifen-resistant PELP1cyto model cells using an in vivo xenograft model. PELP1cyto-overexpressing MCF-7 breast cancer cells were implanted in the mammary fat pad of nude mice. When the tumors reached a measurable size, the mice were treated with tamoxifen or tamoxifen in combination with AZD8055. Tamoxifen treatment further enhanced the PELP1cyto-mediated tumor progression while AZD8055 treatment significantly reduced the tamoxifen-driven tumor progression of PELP1cyto cells (Fig. 6A) and reduced the tumor weight (Fig. 6B). IHC analysis of phosphorylation of S6 as a marker of activated mTOR pathway revealed significant activation of the S6 phosphorylation in the control PELP1cyto tumors, and tamoxifen treatment alone failed to reduce the mTOR signaling (Supplementary Figure S4A, B). Interestingly, AZD8055 treatment in combination with tamoxifen substantially reduced the phosphorylation status of S6 protein (Supplementary Figure S4B). Further analysis of tumor tissues revealed that AZD8055-treated tumor tissues had decreased Ki67 tumor antigen expression (Fig. 6C, Supplementary Figure S4C) with increased apoptosis as seen by TUNEL-positive cells (Fig. 6D, Supplementary Figure S4D). These results suggest that AZD8055 has the potential to reduce the PELP1 cyto-mediated tumor progression by reducing mTOR signaling and promoting apoptosis.
Figure 6. mTOR inhibitors reduce the PELP1–tamoxifen-mediated subcutaneous xenograft tumor growth.
(A) Six-week-old nude female mice were subcutaneously implanted with MCF7-PELP1-cyto cells. After the tumors reached a measurable size, the mice were treated daily with vehicle, tamoxifen alone or tamoxifen and AZD8055 together for 4 weeks. Tumor volumes were measured at weekly intervals. (B) Tumor weights are shown in the histogram. (C) Ki-67 expression was analyzed by using IHC, and quantitation was done as described in methods section. (D) TUNEL staining was performed as a marker of apoptosis on tumors. Quantitation was done as described in methods. **, P<0.01; ***, P<0.001; ****, P<0.0001
Discussion
mTOR is a central regulatory pathway involved in cell proliferation, growth and survival, and deregulation of the mTOR pathway is associated to the development of endocrine resistance (44, 45). The mTOR pathway plays a critical role in ER-mediated transcription. In addition recent studies revealed a novel link between growth factor pathways and the ER (46). In this study, we examined whether the proto-oncogene PELP1 plays a critical role in the ER–growth factor signaling crosstalk through the mTOR axis and whether mTOR inhibitor(s) will be effective as a chemotherapeutic agent for downregulating PELP1 oncogenic functions. We found that (1) PELP1 knockdown reduced the magnitude of mTOR signaling, (2) PELP1 overexpression promoted activation of the mTOR signaling, (3) PELP1 interacts with mTOR, and (4) mTOR inhibitors significantly reduced PELP1-mediated proliferation in vitro and in vivo. Thus, our results suggest that deregulation of PELP1 has the potential to enhance mTOR signaling, leading to increased mammary tumorigenesis and hormonal therapy resistance.
Estrogen signaling modulates mTOR signaling (19), and the PI3K/Akt/mTOR pathway is altered in majority of the breast cancers (20). PELP1 is an oncogene that functions as ER coregulator and participates in both genomic and extranuclear actions of the ER. Previous investigations indicate that PELP1 couples the ER to several cytosolic signaling axes, such as Src-MAPK, PI3K-Akt and EGFR/HER2 (21) and PELP1 expression is upregulated 2–3 fold in breast cancer (33). To mimic this scenario, in previous studies, we have established model cells that exhibit two to three fold over-expression of PELP1 in MCF7 and ZR75 background(33)(25). Use of PELP1 overexpressing model cells with two different genetic backgrounds clearly argue that the oncogenic phenotype seen in these models is due to PELP1 oncogenic potential. Our results using both models further indicate that PELP1 also has the potential to associate with and regulate mTOR signaling. Accordingly, PELP1-overexpressing breast cancer cells had increased activation of the mTOR pathway. In addition, the mTOR inhibitors substantially reduced PELP1-mediated activation of the mTOR pathway, and significantly reduced E2-dependent, PELP1-mediated proliferation in breast cancer cells. Further PELP1 knockdown substantially reduced activation of various components of the mTOR signaling axis. Collectively, our data indicate that the mTOR pathway played a critical role in PELP1-mediated oncogenic functions.
PELP1 is a novel substrate of cyclin dependent kinases (CDKs) (25). PELP1 overexpression promotes E2-mediated G1-S progression (47) and plays a critical role in ribosomal biogenesis (48). Our results further suggest that PELP1 has the potential to modulate mTOR signaling via direct interactions, and PELP1 association was found in both the mTORC1 and mTORC2 complexes. Such results indicate that the PELP1–mTORC2 complex may have more functions than the PELP1–mTORC1 complex. Recent studies showed that active mTORC2 physically associates with the ribosome and that the mTORC2 complex plays a critical role in connecting PI3K signaling to the ribosome (15). Since PELP1 plays an important role in ribosomal signaling, the association of PELP1 with mTORC2 may have additional functions in connecting hormonal crosstalk to ribosomal signaling, leading to cell proliferation. However, our ongoing studies are examining this possibility.
Emerging data suggest that altered levels of ER regulatory proteins contribute to breast cancer progression. PELP1 functions as a scaffolding protein and its expression is commonly deregulated in breast cancer (42). Thus, PELP1 status is reported as an independent prognostic marker of breast cancer progression (29); however, no drug that specifically targets PELP1 is currently available. Thus, drugs that can affect the PELP1 axis will have potential use in reducing progression of breast cancer. In our studies, both of the mTOR inhibitors rapamycin and AZD8055 inhibited PELP1-mediated proliferation, suggesting PELP1 oncogenic function could be targeted in part by mTOR inhibitors. Our data using rapamycin and AZD8055 in preclinical models provided important information and suggest that the disruption of the PELP1–mTOR axis can be exploited to develop new therapeutic strategies against breast cancer progression.
Altered availability or recruitment of co-regulators to the tamoxifen–ER complex is suggested to contribute to the tissue dependence of tamoxifen effects and may contribute to tamoxifen resistance (49). The mTOR/S6K pathway regulates ERα ser167 phosphorylation and such modification of ERα is implicated in therapy resistance (50). PELP1 expression appears to be predominantly in the cytoplasm in a subset of breast tumors and model cells, and forced PELP1 cytoplasmic localization promoted excessive activation of AKT and resistance to tamoxifen therapy (24). Recent studies also implicated PELP1 deregulation in growth factor–driven therapy resistance (24, 51, 52). We found that PELP1cyto cells exhibit increased activation of the mTOR signaling axis, and mTOR-targeting drugs (Rapamycin and AZD8055) produced therapeutic effects in both in vitro and in vivo xenograft-based assays. Future studies using tumor samples are needed to examine whether the cytoplasmic localization of PELP1 correlates with the activation of mTOR.
In summary, our data demonstrated that PELP1 deregulation has the potential to aberrantly activate the mTOR signaling and establish a signaling pathway that connects the ER with the pathological mTOR signaling axis and thus may provide a novel target for combination therapies to treat advanced therapy-resistant breast tumors. Since mTOR inhibitors with good safety profile are currently in clinical trials, they can be potentially used for downregulating PELP1 oncogenic functions and for blocking PELP1-mediated therapy resistance.
Supplementary Material
Acknowledgements
We thank the members of Vadlamudi lab for critical reading of the manuscript.
This study was supported by NIH-CA0095681 (R.K. Vadlamudi) and Cancer Center Support Grant P30CA054174 (R.K. Vadlamudi and R.R. Tekmal)
Footnotes
Disclosure of Potential Conflicts of Interest: Authors have no potential conflict of interests
Reference List
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Zhao H, Fuqua SA. Selective estrogen receptor modulators: structure, function, and clinical use. J Clin Oncol. 2000;18:3172–3186. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- 3.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 4.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 5.O'Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–8222. doi: 10.1158/0008-5472.CAN-09-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 7.Lonard DM, Lanz RB, O'Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–587. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- 8.Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- 9.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 11.Jewell JL, Guan KL. Nutrient signaling to mTOR and cell growth. Trends Biochem Sci. 2013;38:233–242. doi: 10.1016/j.tibs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MN. mTOR-what does it do? Transplant Proc. 2008;40:S5–S8. doi: 10.1016/j.transproceed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 15.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacinto E, Lorberg A. TOR regulation of AGC kinases in yeast and mammals. Biochem J. 2008;410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 19.Chang SB, Miron P, Miron A, Iglehart JD. Rapamycin inhibits proliferation of estrogen-receptor-positive breast cancer cells. J Surg Res. 2007;138:37–44. doi: 10.1016/j.jss.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 20.O'Regan R, Hawk NN. mTOR inhibition in breast cancer: unraveling the complex mechanisms of mTOR signal transduction and its clinical implications in therapy. Expert Opin Ther Targets. 2011;15:859–872. doi: 10.1517/14728222.2011.575362. [DOI] [PubMed] [Google Scholar]
- 21.Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard BJ, Daniel AR, Lange CA, Ostrander JH. PELP1: A review of PELP1 interactions, signaling, and biology. Mol Cell Endocrinol. 2013;10 doi: 10.1016/j.mce.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, et al. Extranuclear Functions of ER Impact Invasive Migration and Metastasis by Breast Cancer Cells. Cancer Res. 2010;70:4092–4101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–7732. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair BC, Nair SS, Chakravarty D, Challa R, Manavathi B, Yew PR, et al. Cyclin-dependent kinase-mediated phosphorylation plays a critical role in the oncogenic functions of PELP1. Cancer Res. 2010;70:7166–7175. doi: 10.1158/0008-5472.CAN-10-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonugunta VK, Nair BC, Rajhans R, Sareddy GR, Nair SS, Vadlamudi RK. Regulation of rDNA transcription by proto-oncogene PELP1. PLoS One. 2011;6:e21095. doi: 10.1371/journal.pone.0021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkbeiner E, Haindl M, Muller S. The SUMO system controls nucleolar partitioning of a novel mammalian ribosome biogenesis complex. EMBO J. 2011;30:1067–1078. doi: 10.1038/emboj.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castle CD, Cassimere EK, Denicourt C. LAS1L interacts with the mammalian Rix1 complex to regulate ribosome biogenesis. Mol Biol Cell. 2012;23:716–728. doi: 10.1091/mbc.E11-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, et al. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat. 2009;120:603–612. doi: 10.1007/s10549-009-0419-9. [DOI] [PubMed] [Google Scholar]
- 30.Kefalopoulou Z, Tzelepi V, Zolota V, Grivas PD, Christopoulos C, Kalofonos H, et al. Prognostic value of novel biomarkers in astrocytic brain tumors: nuclear receptor co-regulators AIB1, TIF2, and PELP1 are associated with high tumor grade and worse patient prognosis. J Neurooncol. 2012;106:23–31. doi: 10.1007/s11060-011-0637-y. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarty D, Tekmal RR, Vadlamudi RK. PELP1: A novel therapeutic target for hormonal cancers. IUBMB Life. 2010;62:162–169. doi: 10.1002/iub.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, Balasenthil S, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–4909. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- 33.Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic Potential of the Nuclear Receptor Coregulator Proline-, Glutamic Acid-, Leucine-Rich Protein 1/Modulator of the Nongenomic Actions of the Estrogen Receptor. Cancer Res. 2007;67:5505–5512. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–7732. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sareddy GR, Nair BC, Gonugunta VK, Zhang QG, Brenner A, Brann DW, et al. Therapeutic significance of estrogen receptor beta agonists in gliomas. Mol Cancer Ther. 2012;11:1174–1182. doi: 10.1158/1535-7163.MCT-11-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortez V, Mann M, Tekmal S, Suzuki T, Miyata N, Rodriguez-Aguayo C, et al. Targeting PELP1-KDM1 axis as a potential therapeutic strategy for breast cancer. Breast Cancer Res. 2012;14:R108. doi: 10.1186/bcr3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sareddy GR, Nair BC, Krishnan SK, Gonugunta VK, Zhang QG, Suzuki T, et al. KDM1 is a novel therapeutic target for the treatment of gliomas. Oncotarget. 2013;4:18–28. doi: 10.18632/oncotarget.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee N, Woodrum CL, Nobil AM, Rauktys AE, Messina MP, Dabora SL. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 2009;9:8. doi: 10.1186/1471-2210-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu M, Howes A, Lesperance J, Stallcup WB, Hauser CA, Kadoya K, et al. Antitumor activity of rapamycin in a transgenic mouse model of ErbB2-dependent human breast cancer. Cancer Res. 2005;65:5325–5336. doi: 10.1158/0008-5472.CAN-04-4589. [DOI] [PubMed] [Google Scholar]
- 40.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarty D, Tekmal RR, Vadlamudi RK. PELP1: A novel therapeutic target for hormonal cancers. IUBMB Life. 2010;62:162–169. doi: 10.1002/iub.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar R, Zhang H, Holm C, Vadlamudi RK, Landberg G, Rayala SK. Extranuclear coactivator signaling confers insensitivity to tamoxifen. Clin Cancer Res. 2009;15:4123–4130. doi: 10.1158/1078-0432.CCR-08-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavez-MacGregor M, Gonzalez-Angulo AM. Everolimus in the treatment of hormone receptor-positive breast cancer. Expert Opin Investig Drugs. 2012;21:1835–1843. doi: 10.1517/13543784.2012.726218. [DOI] [PubMed] [Google Scholar]
- 45.Yardley DA. Combining mTOR Inhibitors with Chemotherapy and Other Targeted Therapies in Advanced Breast Cancer: Rationale, Clinical Experience, and Future Directions. Breast Cancer (Auckl) 2013;7:7–22. doi: 10.4137/BCBCR.S10071. Epub;%2013 Feb 13.:7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker MA, Ibrahim YH, Cui X, Lee AV, Yee D. The IGF pathway regulates ERalpha through a S6K1-dependent mechanism in breast cancer cells. Mol Endocrinol. 2011;25:516–528. doi: 10.1210/me.2010-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balasenthil S, Vadlamudi RK. Functional interactions between the estrogen receptor coactivator PELP1/MNAR and retinoblastoma protein. J Biol Chem. 2003;278:22119–22127. doi: 10.1074/jbc.M212822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonugunta VK, Nair BC, Rajhans R, Sareddy GR, Nair SS, Vadlamudi RK. Regulation of rDNA transcription by proto-oncogene PELP1. PLoS One. 2011;6:e21095. doi: 10.1371/journal.pone.0021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 50.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–128. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar R, Zhang H, Holm C, Vadlamudi RK, Landberg G, Rayala SK. Extranuclear coactivator signaling confers insensitivity to tamoxifen. Clin Cancer Res. 2009;15:4123–4130. doi: 10.1158/1078-0432.CCR-08-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallabhaneni S, Nair BC, Cortez V, Challa R, Chakravarty D, Tekmal RR, et al. Significance of ER-Src axis in hormonal therapy resistance. Breast Cancer Res Treat. 2010;130:377–385. doi: 10.1007/s10549-010-1312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.