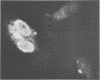
Abstract
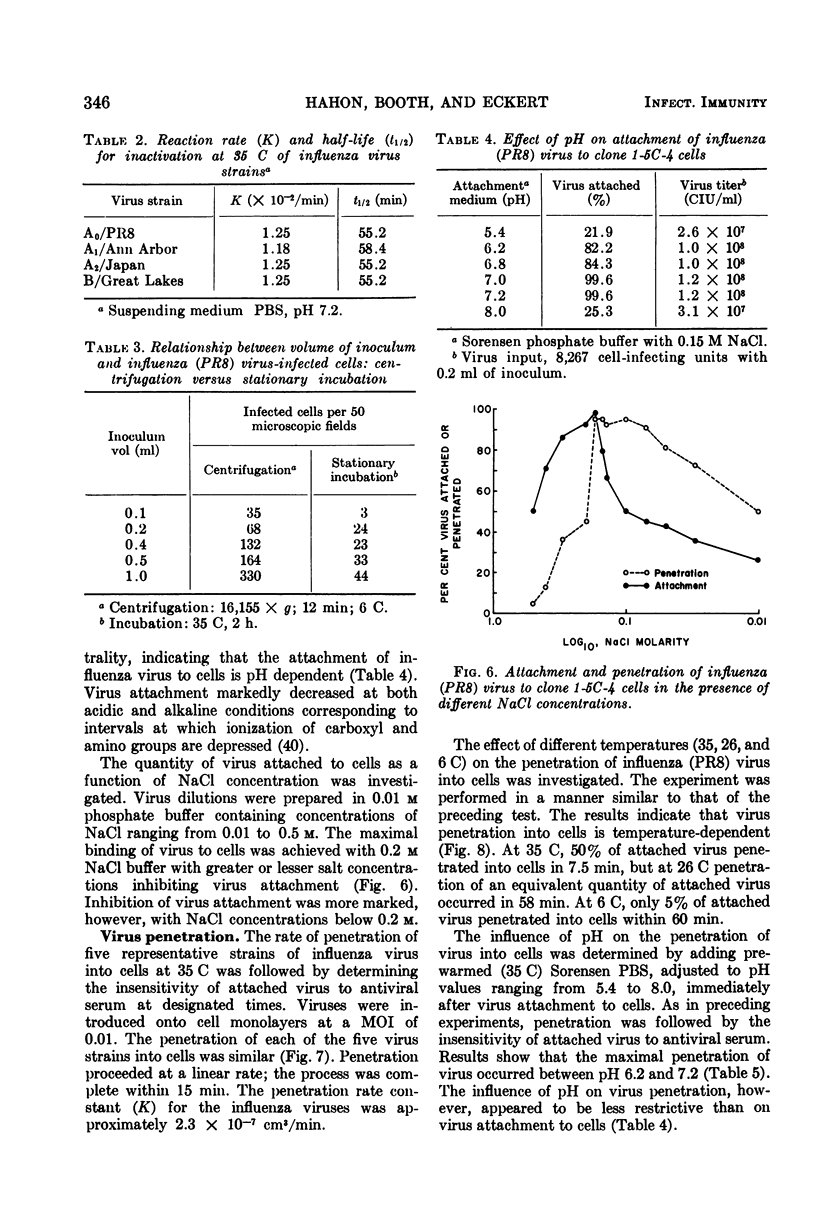
Attachment and penetration of influenza virus into clone 1-5C-4 cells were quantitatively determined by the immunofluorescent cell-counting assay. Aided by centrifugal force, more than 95% of virus inocula of five representative influenza virus strains (A0/PR8, A1/Ann Arbor, A2/Japan, B/Lee, B/Great Lakes) were attached to cells at a linear rate within 10 min, in contrast to approximately 35% after stationary incubation at 35 C for 2 h. By the former procedure, a proportionality between the number of infected cells and volume of inoculum was revealed which was not evident when stationary incubation was employed. Maximal binding of virus to cells occurred at 0.2 M NaCl. The salt requirement, added to evidence of pH dependence and temperature independence, indicated that the initial virus-cell union involved electrostatic forces. Virus penetration into cells, measured by the insensitivity of virus-cell complexes to antiviral serum, was linear and complete within 15 min at 35 C for all five virus strains tested. Maximal virus penetration occurred at 0.1 to 0.2 M NaCl; the process was pH- and temperature-dependent. Both virus attachment and penetration processes were partially inhibited in the presence of diethylaminoethyl-dextran.
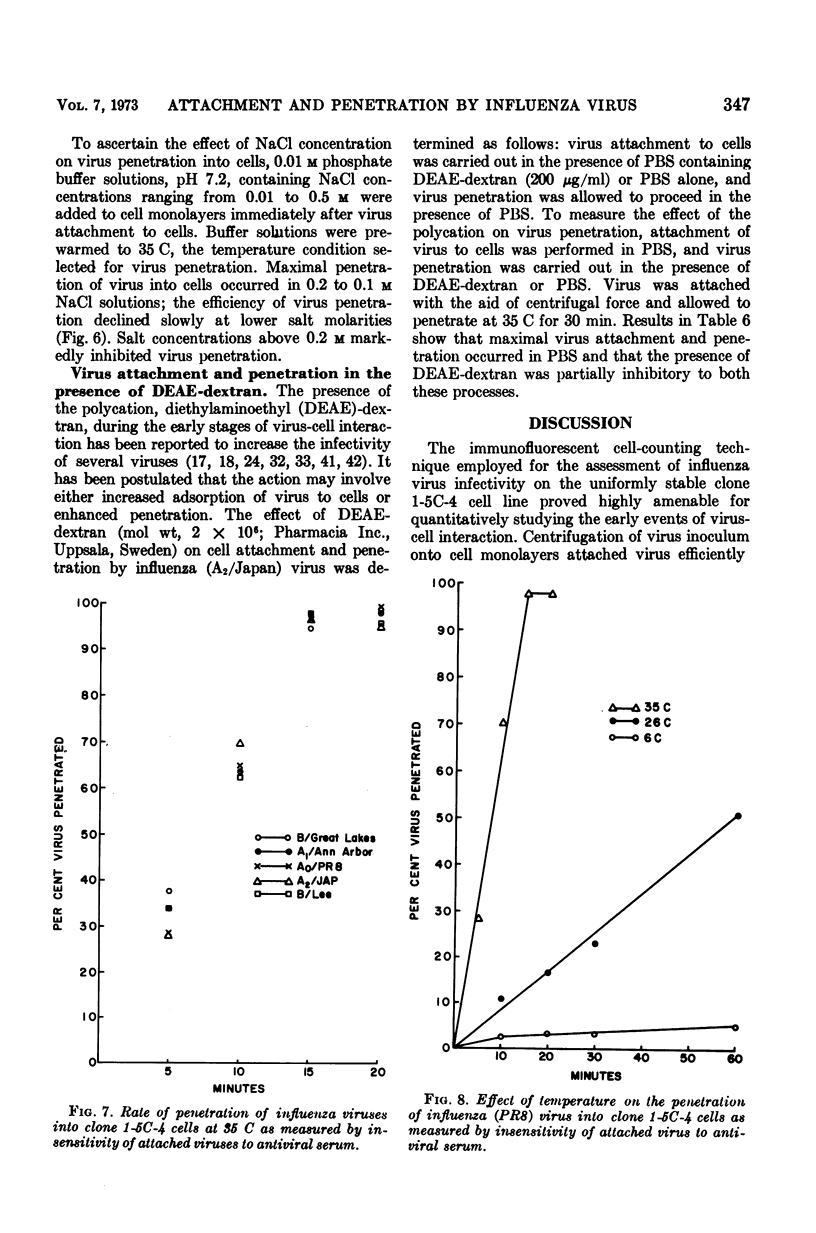
Full text
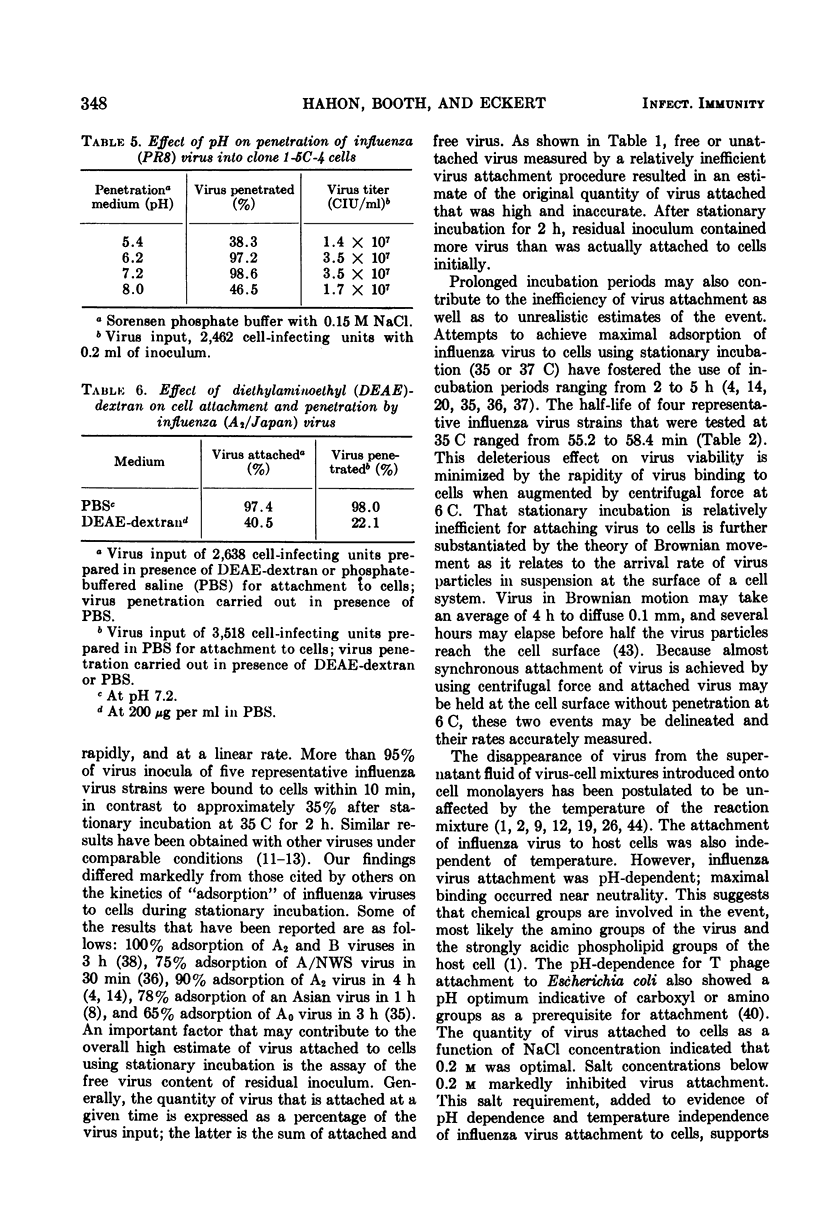
PDF

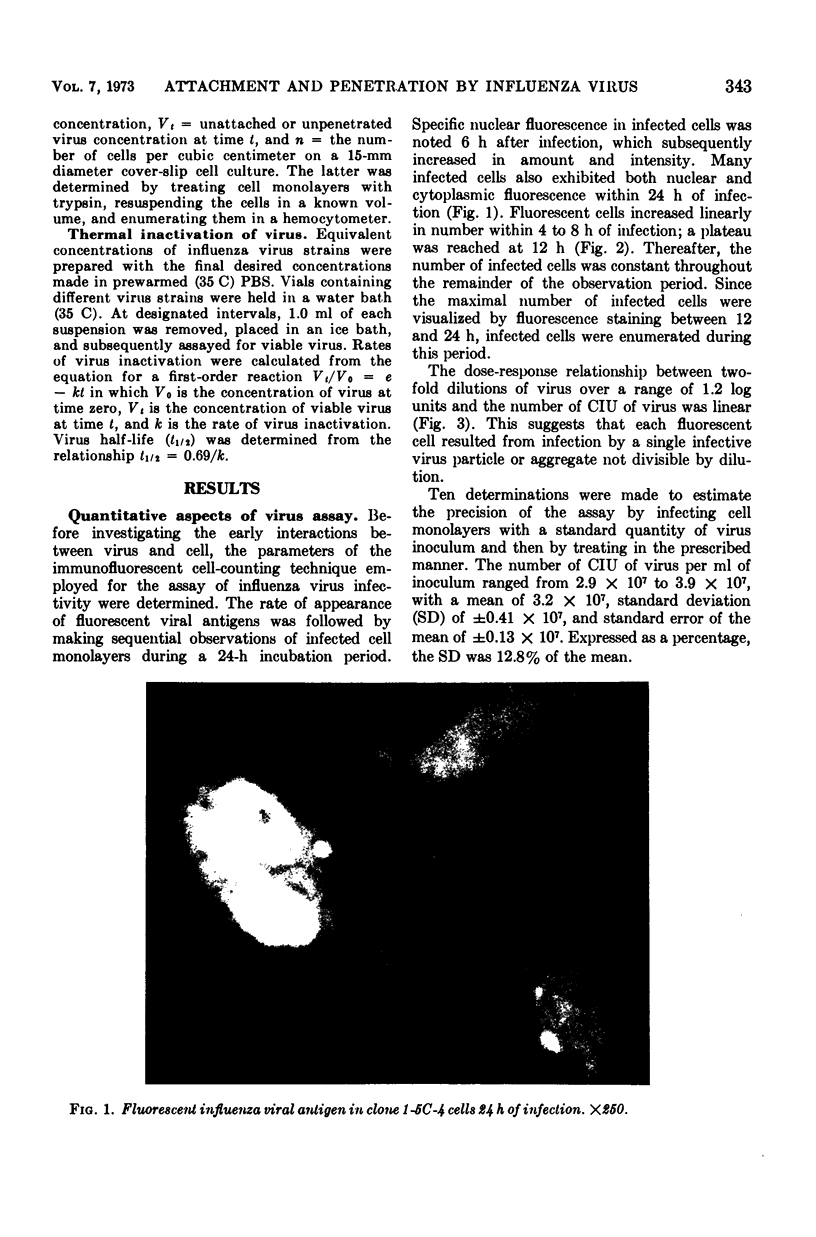
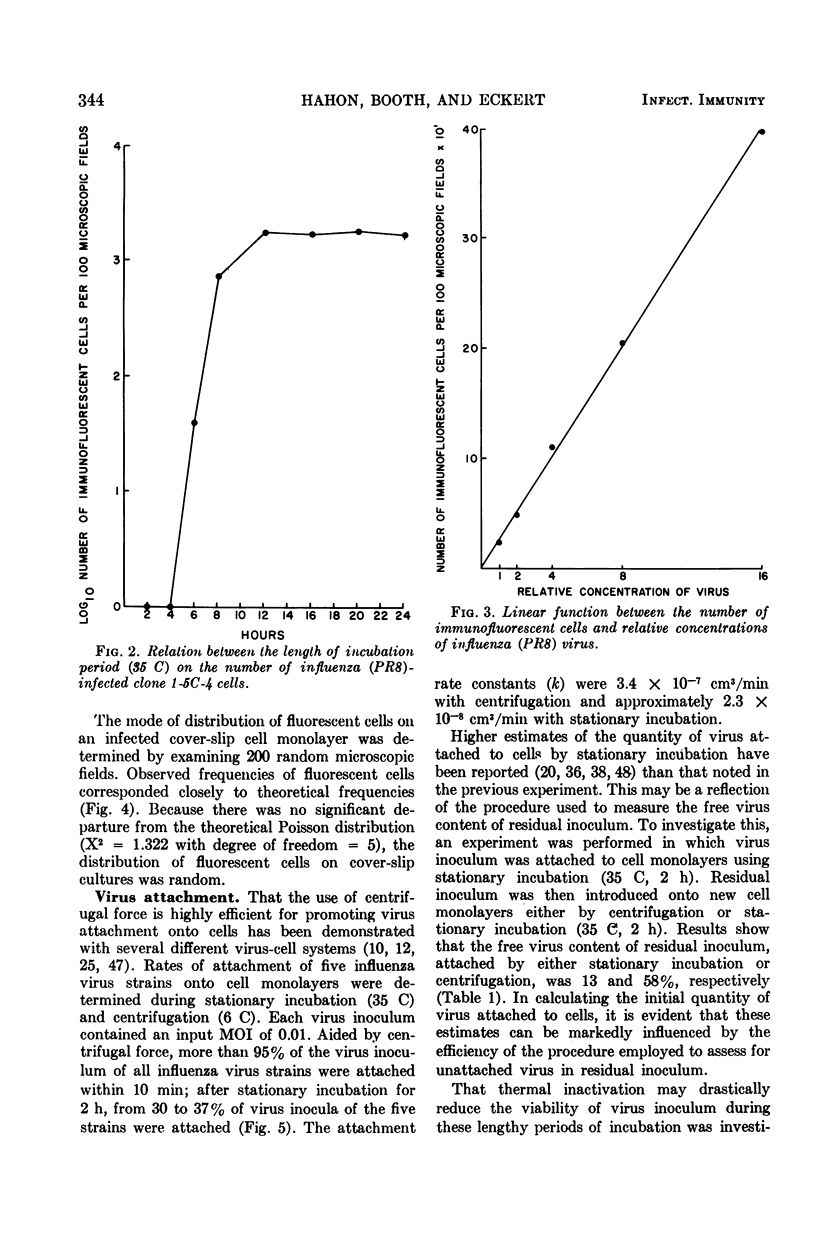
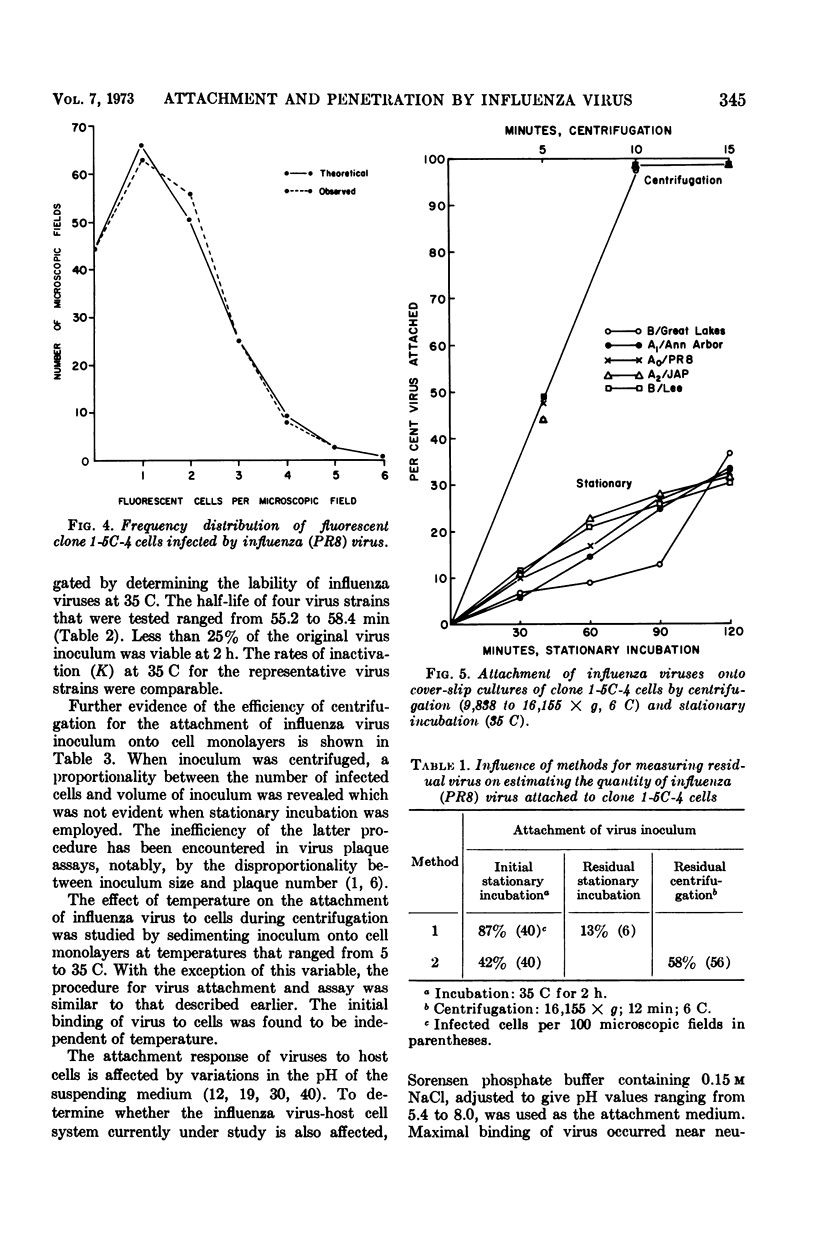



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- BACHTOLD J. G., BUBEL H. C., GEBHARDT L. P. The primary interaction of poliomyelitis virus with host cells of tissue culture origin. Virology. 1957 Dec;4(3):582–589. doi: 10.1016/0042-6822(57)90087-9. [DOI] [PubMed] [Google Scholar]
- CARTER G. B. THE RAPID DETECTION, TITRATION, AND DIFFERENTIATION OF VARIOLA AND VACCINIA VIRUSES BY A FLUORESCENT ANTIBODY-COVERSLIP CELL MONOLAYER SYSTEM. Virology. 1965 Apr;25:659–662. doi: 10.1016/0042-6822(65)90094-2. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W. MULTIPLICATION OF TWO KINDS OF INFLUENZA A2 VIRUS PARTICLES IN MONKEY KIDNEY CELLS. Virology. 1963 Nov;21:342–352. doi: 10.1016/0042-6822(63)90195-8. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- DEIBEL R., HOTCHIN J. E. Quantitative applications of fluorescent antibody technique to influenza-virus-infected cell cultures. Virology. 1959 Jul;8(3):367–380. doi: 10.1016/0042-6822(59)90036-4. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Meléndez L. V. Herpes T virus plaque assay studies. Arch Gesamte Virusforsch. 1968;25(1):8–17. doi: 10.1007/BF01243085. [DOI] [PubMed] [Google Scholar]
- FARNHAM A. E., NEWTON A. A. The effect of some environmental factors on herpes virus grown in HeLa cells. Virology. 1959 Apr;7(4):449–461. doi: 10.1016/0042-6822(59)90073-x. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N., Cooke K. O. Primary virus-cell interactions in the immunofluorescence assay of Venezuelan equine encephalomyelitis virus. J Virol. 1967 Apr;1(2):317–326. doi: 10.1128/jvi.1.2.317-326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N. Fluorescent cell-counting assay of yellow fever virus. J Infect Dis. 1966 Feb;116(1):33–40. doi: 10.1093/infdis/116.1.33. [DOI] [PubMed] [Google Scholar]
- Hahon N., Hankins W. A. Assay of Chikungunya virus in cell monolayers by immunofluorescence. Appl Microbiol. 1970 Feb;19(2):224–231. doi: 10.1128/am.19.2.224-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N. Immunofluorescent cell-counting assay of Rift Valley fever virus. Am J Vet Res. 1969 Jun;30(6):1007–1014. [PubMed] [Google Scholar]
- Hatano M., Morita O. Multiplication and plaque assay of influenza viruses in a continuous cell line (G2) of human origin. Arch Gesamte Virusforsch. 1967;20(3):305–313. doi: 10.1007/BF01241950. [DOI] [PubMed] [Google Scholar]
- Kan T., Koyama K., Takaku K., Takahashi M., Kunita N. Improvement of the method of plaque formation of influenza virus and its application to neutralization tests. Biken J. 1970 Sep;13(3):185–192. [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sharp D. G. The influence of DEAE-dextran on plain and synergistic plaque formation by two poxviruses. J Gen Virol. 1969 Jun;4(4):505–512. doi: 10.1099/0022-1317-4-4-505. [DOI] [PubMed] [Google Scholar]
- LEVINE S., SAGIK B. P. The interactions of Newcastle disease virus (NDV) with chick embryo tissue culture cells: attachment and growth. Virology. 1956 Feb;2(1):57–68. doi: 10.1016/0042-6822(56)90076-9. [DOI] [PubMed] [Google Scholar]
- LIPPELT H., MANNWEILER E. [The behavior of influenza viruses in tissue cultures of chick embryo kidneys. I. Virus adsorption]. Arch Gesamte Virusforsch. 1961;10:636–646. [PubMed] [Google Scholar]
- MANDEL B. Studies on the interactions of poliomyelitis virus, antibody, and host cells in tissue culture system. Virology. 1958 Oct;6(2):424–447. doi: 10.1016/0042-6822(58)90092-8. [DOI] [PubMed] [Google Scholar]
- Nomura S. Interaction of respiratory syncytial virus with polyions: enhancement of infectivity with diethylaminoethyl dextran. Proc Soc Exp Biol Med. 1968 May;128(1):163–166. doi: 10.3181/00379727-128-32969. [DOI] [PubMed] [Google Scholar]
- PADGETT B. L., WALKER D. L. Use of centrifugal force to promote adsorption of myxoma virus to cell monolayers. Proc Soc Exp Biol Med. 1962 Nov;111:364–367. doi: 10.3181/00379727-111-27793. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- POSTLETHWAITE R. A plaque technique for the titration of vaccinia virus in chick embryo cells and some features of vaccinial infection in this system. Virology. 1960 Apr;10:466–482. doi: 10.1016/0042-6822(60)90130-6. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., GAREN A., CLINE J. The mechanism of virus attachment to host cells. I. The role of ions in the primary reaction. J Exp Med. 1951 Jan;93(1):65–88. doi: 10.1084/jem.93.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., TOLMACH L. J. The mechanism of virus attachment to host cells. IV. Physicochemical studies on virus and cell surface groups. Arch Biochem Biophys. 1954 Jul;51(1):229–245. doi: 10.1016/0003-9861(54)90470-1. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- Rossi C. R. Studies of laryngotracheitis virus in avian tissue cultures. IV. Effect of diethylaminoethyl-dextran on adsorption and elution. Arch Gesamte Virusforsch. 1971;35(2):223–231. doi: 10.1007/BF01249714. [DOI] [PubMed] [Google Scholar]
- Rossi C. R., Watrach A. M. Studies of laryngotracheitis virus in avian tissue cultures. 3. Enhancement of infectivity by diethylaminoethyl-dextran. Appl Microbiol. 1970 Jun;19(6):932–936. doi: 10.1128/am.19.6.932-936.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser H. J. Studies on protein uptake by isolated tumor cells. 3. Apparent stimulations due to pH, hypertonicity, polycations, or dehydration and their relation to the enhanced penetration of infectious nucleic acids. J Cell Biol. 1967 Mar;32(3):737–750. doi: 10.1083/jcb.32.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGIURA A., KILBOURNE E. D. GENETIC STUDIES OF INFLUENZA VIRUSES. II. PLAQUE FORMATION BY INFLUENZA VIRUSES IN A CLONE OF A VARIANT HUMAN HETEROPLOID CELL LINE. Virology. 1965 Jul;26:478–488. doi: 10.1016/0042-6822(65)90010-3. [DOI] [PubMed] [Google Scholar]
- Sattar S. A., Westwood J. C. Immunofluorescence in the study of viruses in tissue culture. II. Development of an immunofluorescent cell assay for influenza (A/PR8) virus. Can J Microbiol. 1968 May;14(5):533–536. [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INFLUENCE OF ACID POLYSACCHARIDES ON PLAQUE FORMATION BY INFLUENZA A2 AND B VIRUSES. Proc Soc Exp Biol Med. 1963 Dec;114:811–814. doi: 10.3181/00379727-114-28806. [DOI] [PubMed] [Google Scholar]
- TOLMACH L. J. Attachment and penetration of cells by viruses. Adv Virus Res. 1957;4:63–110. doi: 10.1016/s0065-3527(08)60596-5. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., ALLISON A. C. Virus particle adsorption. I. Theory of adsorption and experiments on the attachment of particles to non-biological surfaces. Biochim Biophys Acta. 1959 Jul;34:10–23. doi: 10.1016/0006-3002(59)90228-8. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A. Growth of rubella virus in BHK21 cells. II. Enhancing effect of DEAE-dextran, semicarbazide and low doses of metabolic inhibitors. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1092–1098. doi: 10.3181/00379727-125-32284. [DOI] [PubMed] [Google Scholar]
- Vonka V., Benyesh-Melnick M. Interactions of human cytomegalovirus with human fibroblasts. J Bacteriol. 1966 Jan;91(1):213–220. doi: 10.1128/jb.91.1.213-220.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS J. F. The adsorption, penetration and eclipse of herpes simplex virus in chorio-allantoic membrane ectoderm. J Gen Microbiol. 1960 Feb;22:40–56. doi: 10.1099/00221287-22-1-40. [DOI] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Centrifugation and Rickettsiae and viruses onto cells and its effect on infection. Proc Soc Exp Biol Med. 1960 Apr;103:691–695. doi: 10.3181/00379727-103-25637. [DOI] [PubMed] [Google Scholar]
- WONG S. C., KILBOURNE E. D. Changing viral susceptibility of a human cell line in continuous cultivation. I. Production of infective virus in a variant of the Chang conjunctival cell following infection with swine or N-WS influenza viruses. J Exp Med. 1961 Jan 1;113:95–110. doi: 10.1084/jem.113.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Roles of metallic ions in host-parasite interactions. Bacteriol Rev. 1966 Mar;30(1):136–151. doi: 10.1128/br.30.1.136-151.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis N. G., Corey P. N., Campbell J. B., Macpherson L. W. Development and statistic evaluation of a plaque assay for the virus of epizootic hemorrhagic disease of deer. Can J Microbiol. 1970 Dec;16(12):1293–1301. doi: 10.1139/m70-215. [DOI] [PubMed] [Google Scholar]