Abstract
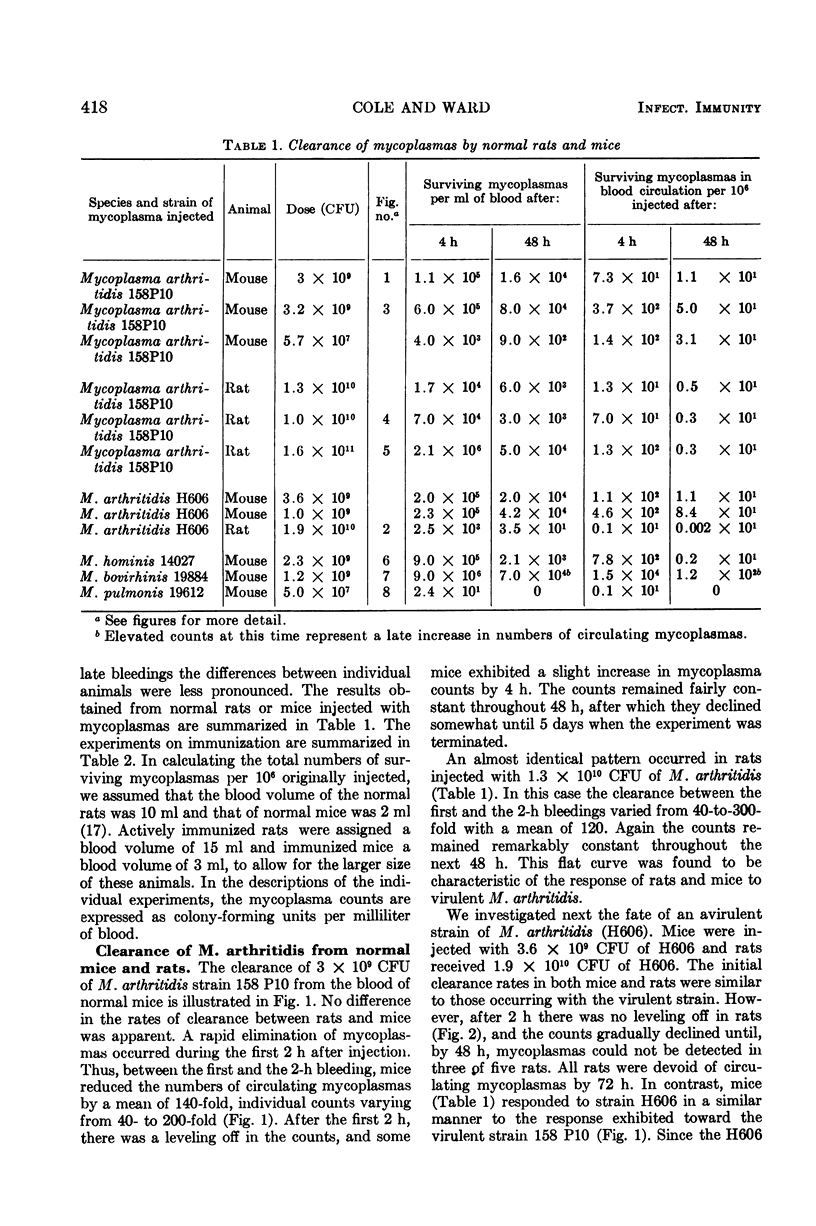
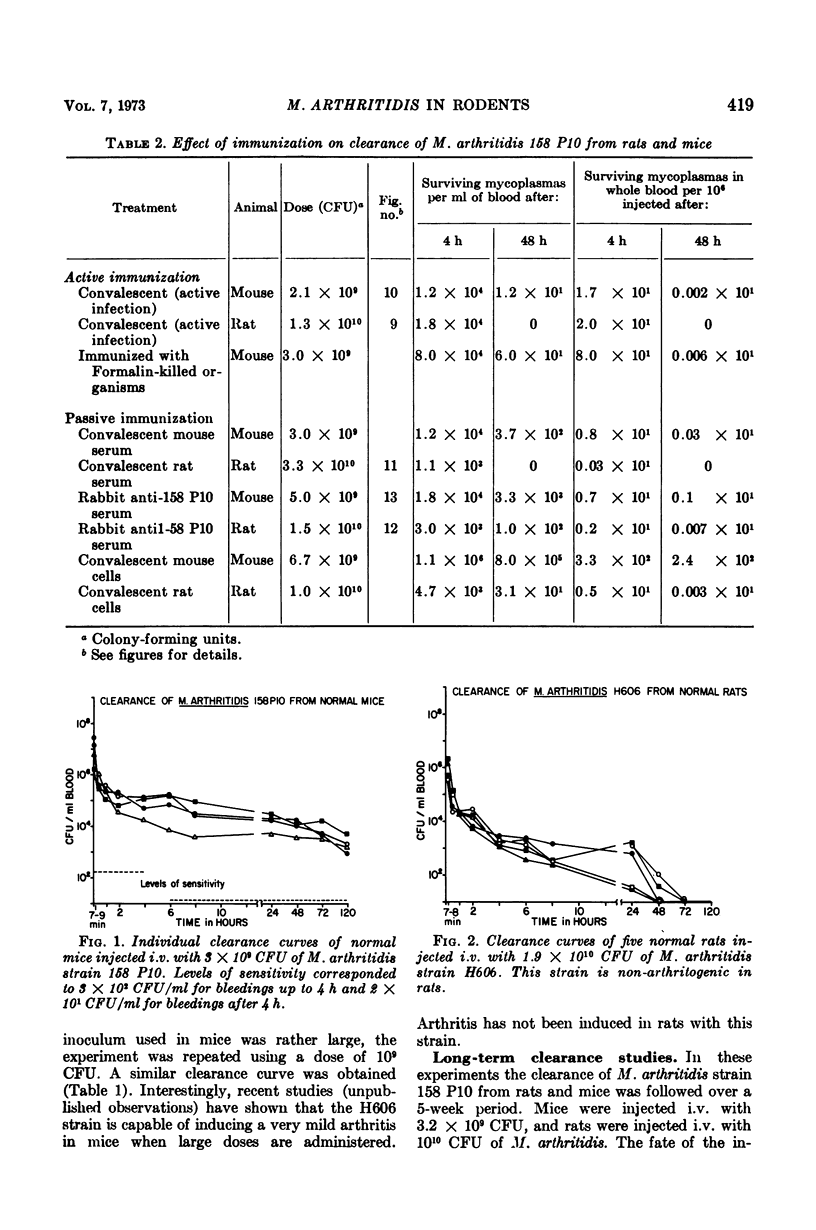
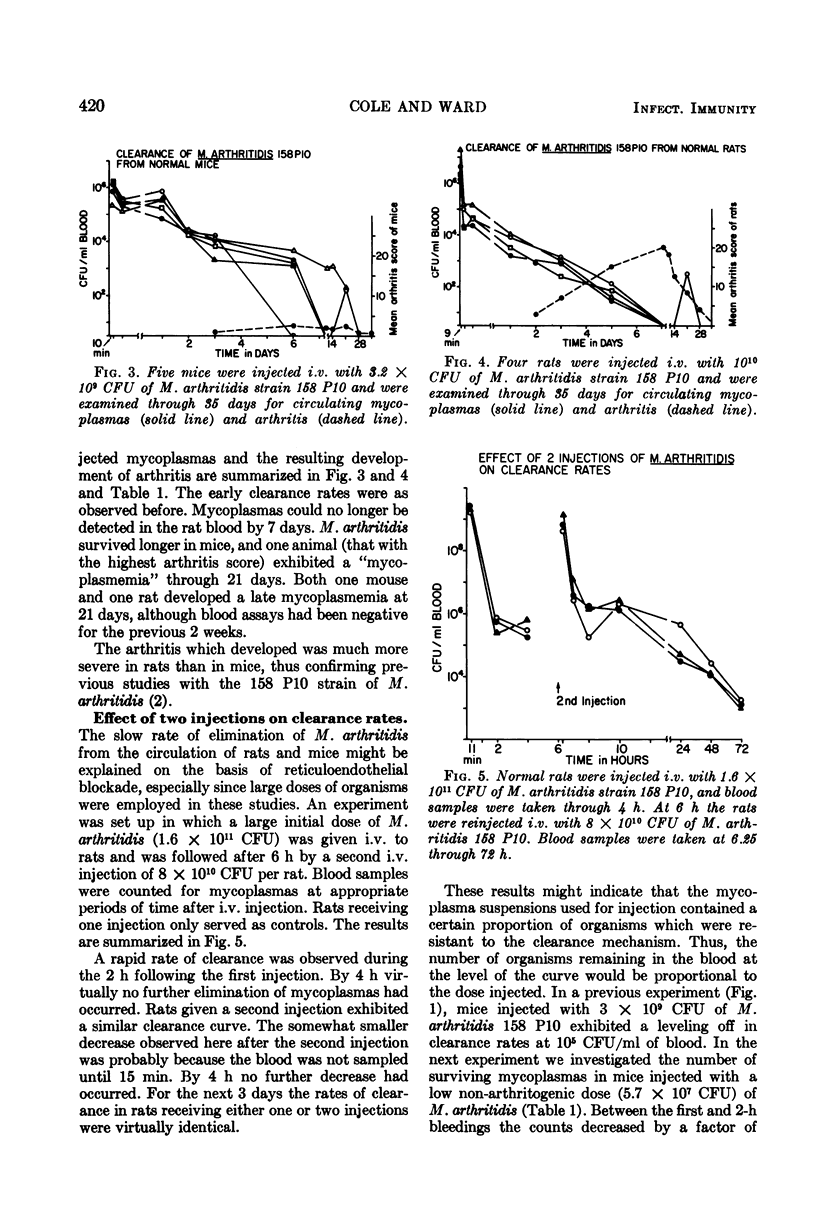
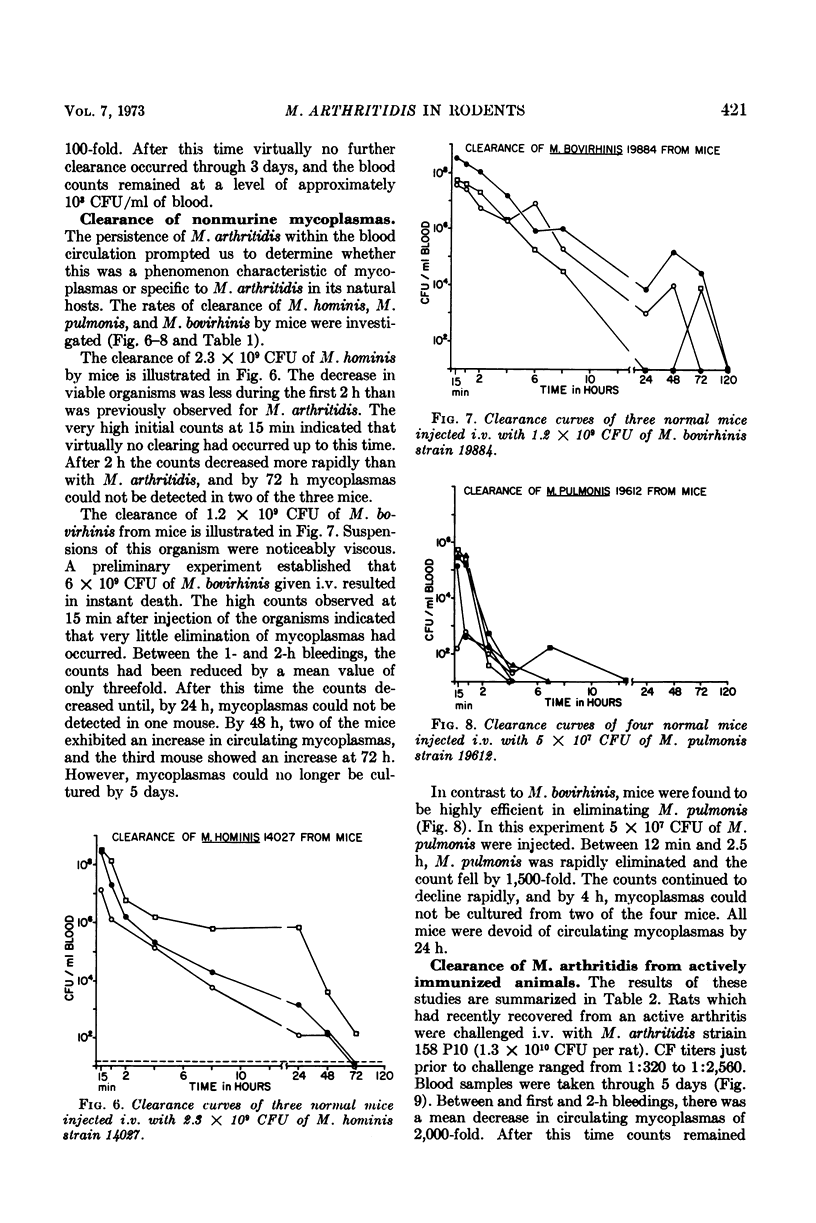
Virulent Mycoplasma arthritidis strain 158 P10 was rapidly eliminated from the peripheral circulation of rats and mice during the first 2 h after intravenous injection of the organisms. Characteristically after this time, the numbers of viable organisms remained remarkably constant through 48 to 72 h. The inability to clear the remaining mycoplasmas was not due to reticuloendothelial blockade but appeared to indicate the presence of a certain number of cells which were resistant to the clearance mechanism. Rat blood was usually devoid of mycoplasmas at 6 days and mouse blood was sterile by 14 days. An occasional transient mycoplasmemia occurred after these times. Avirulent M. arthritidis strain H606 was cleared more rapidly from rats in which it is non-arthritogenic. Different, but characteristic, clearance curves were obtained in mice using M. hominis, M. bovirhinis, and M. pulmonis. Active immunization procedures resulted in enhanced clearance of virulent M. arthritidis from rats. These procedures were less successful in mice. The results of passive immunization of normal rats and mice using convalescent serum indicated the presence of a serum factor which greatly enhanced clearance from rats but had only minimal effects in mice. This serum factor could not be correlated with metabolic-inhibiting antibody. Lymphocytes taken from convalescent rats enhanced the clearance rates of recipient rats. Mouse convalescent lymphocytes were without effect. The results indicated that mice are less able to control long-term infection by M. arthritidis than are rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barden J. A., Tully J. G. Experimental arthritis in mice with Mycoplasma pulmonis. J Bacteriol. 1969 Oct;100(1):5–10. doi: 10.1128/jb.100.1.5-10.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J. F., Cole B. C., Wiley B. B., Ward J. R. Role of Biological Mimicry in the Pathogenesis of Rat Arthritis Induced by Mycoplasma arthritidis. Infect Immun. 1971 Jan;3(1):24–35. doi: 10.1128/iai.3.1.24-35.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coke B. C., Ward J. R., Golightly-Rowland C., Trapp G. A. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. II. Serological responses of the lost and effect of vaccines. Infect Immun. 1971 Oct;4(4):431–440. doi: 10.1128/iai.4.4.431-440.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Cahill J. F., Wiley B. B., Ward J. R. Immunological responses of the rat to Mycoplasma arthritidis. J Bacteriol. 1969 Jun;98(3):930–937. doi: 10.1128/jb.98.3.930-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly L., Ward J. R. Characterization of mycoplasma strains from cats. J Bacteriol. 1967 Nov;94(5):1451–1458. doi: 10.1128/jb.94.5.1451-1458.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARD D. G., FREUNDT E. A. The classification and nomenclature of organisms of the pleuropneumonia group. J Gen Microbiol. 1956 Feb;14(1):197–207. doi: 10.1099/00221287-14-1-197. [DOI] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hudson J. R., Buttery S., Cottew G. S. Investigations into the influence of the galactan of Mycoplasma mycoides on experimental infection with that organism. J Pathol Bacteriol. 1967 Oct;94(2):257–273. doi: 10.1002/path.1700940204. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. Rift Valley Fever virus in mice. III. Further quantitative features of the infective process. Br J Exp Pathol. 1956 Apr;37(2):120–128. [PMC free article] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D. C., Chanock R. M. A color test for the measurement of antibody to the non-acid-forming human Mycoplasma species. Am J Epidemiol. 1966 Jul;84(1):51–66. doi: 10.1093/oxfordjournals.aje.a120627. [DOI] [PubMed] [Google Scholar]
- Smith G. R. Mycoplasma mycoides var. mycoides: production of bacteriaemia and demonstration of passive immunity in mice. J Comp Pathol. 1967 Apr;77(2):203–209. doi: 10.1016/0021-9975(67)90012-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., SOMERSON N. L., TURNER H. C., CHANOCK R. M. SEROLOGICAL RELATIONSHIPS AMONG HUMAN MYCOPLASMAS AS SHOWN BY COMPLEMENT-FIXATION AND GEL DIFFUSION. J Bacteriol. 1963 Jun;85:1261–1273. doi: 10.1128/jb.85.6.1261-1273.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZILVERSMIT D. B., BOYD G. A., BRUCER M. The effect of particle size on blood clearance and tissue distribution of radioactive gold colloids. J Lab Clin Med. 1952 Aug;40(2):255–260. [PubMed] [Google Scholar]


