Abstract
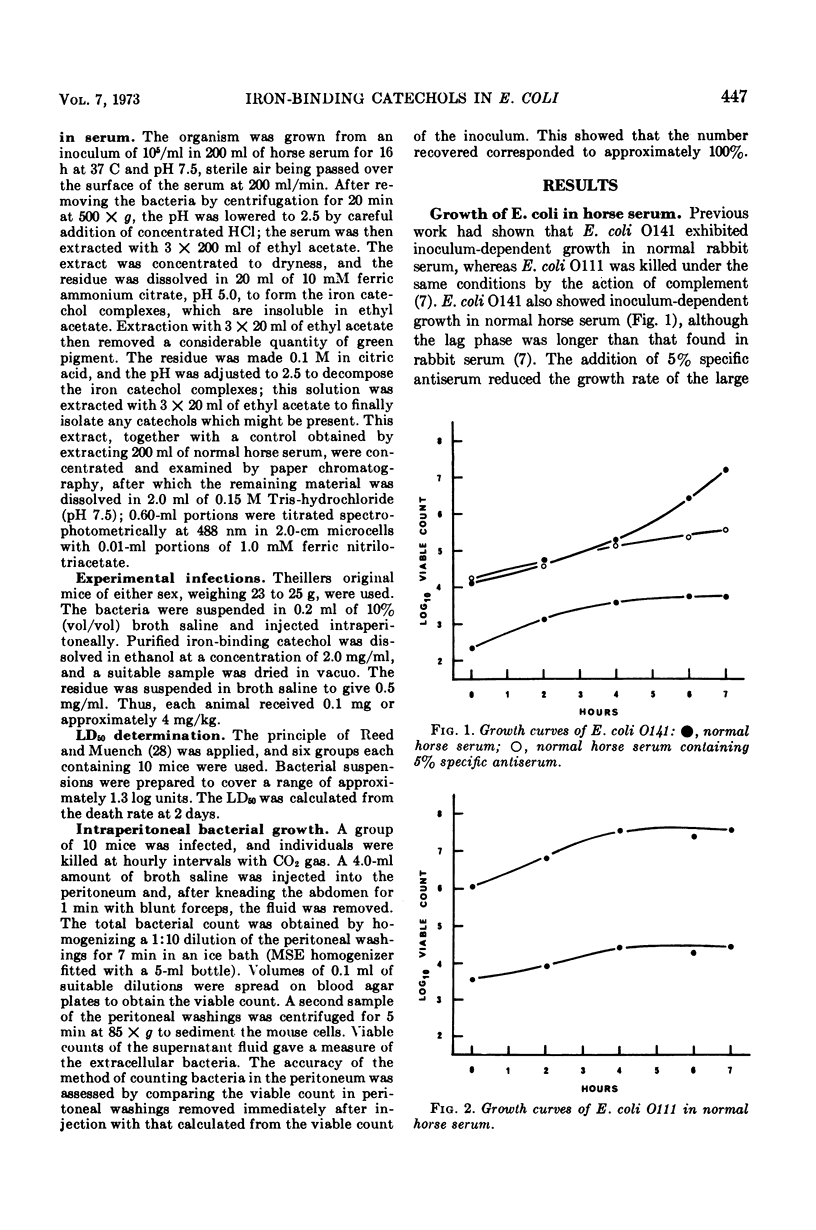
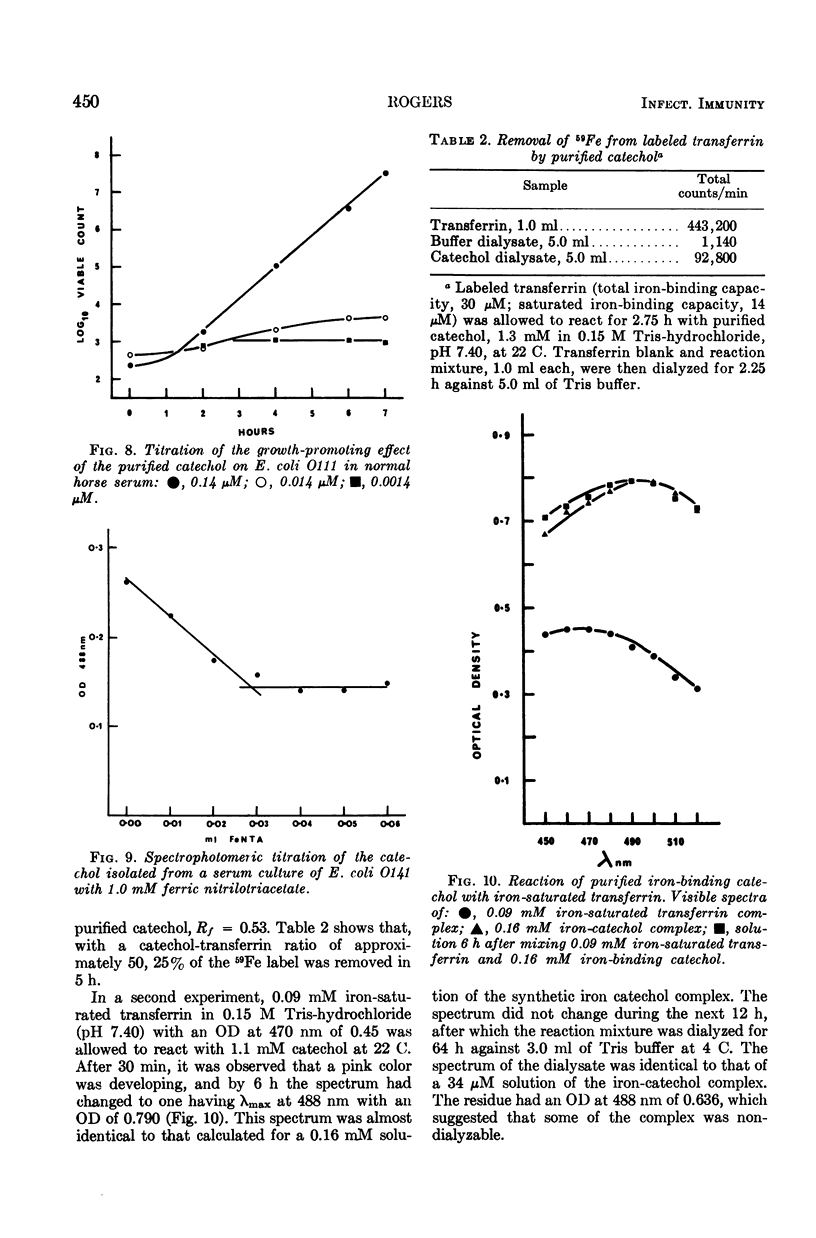
Previous work suggested that virulent bacteria, which can grow rapidly in serum, must possess a specific mechanism for removing iron from its transferrin complex. Two strains of Escherichia coli were examined with this in mind. Strain O141, which showed inoculum-dependent growth in serum and multiplied in the mouse peritoneum, secreted iron-binding catechols into both synthetic medium and serum. One of these compounds has an association constant for iron similar to that of transferrin. Both transferrin and ethylenediamine-di-o-hydroxyphenyl acetic acid (EDDA), which have very high affinities for ferric iron, induced catechol synthesis in growing cultures of strain O111. This organism was inhibited by normal horse serum. Further work showed that traces of specific antibody inhibited catechol synthesis by O111 exposed to EDDA; therefore, the existence of this inhibitory process means that the organism can no longer obtain Fe3+, which all remains bound to transferrin in serum. In vivo, the inhibition of O111 is similar to that produced by serum in vitro. Neither phagocytosis nor killing by complement appeared to be of any significance during the first 4 h of the infections. Significantly, the purified catechol was capable of abolishing bacteriostasis in vivo. Since these results show that the production of iron-binding catechols is essential for rapid bacterial growth both in vitro and in vivo, these compounds should therefore be considered as true virulence factors. Conversely, any interference by the host with the production or activity of these compounds would constitute an important aspect of antibacterial defense.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AASA R., MALMSTROEM B. G., SALTMAN P. THE SPECIFIC BINDING OF IRON(III) AND COPPER(II) TO TRANSFERRIN AND CONALBUMIN. Biochim Biophys Acta. 1963 Sep 24;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- Bornside G. H., Bouis P. J., Jr, Cohn I., Jr Hemoglobin and Escherichia coli, a lethal intraperitoneal combination. J Bacteriol. 1968 May;95(5):1567–1571. doi: 10.1128/jb.95.5.1567-1571.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Goodwin J. Regulation of 2,3-dihydroxybenzoylserine synthetase by iron. J Biol Chem. 1968 Feb 10;243(3):510–513. [PubMed] [Google Scholar]
- Bullen J. J., Cushnie G. H., Rogers H. J. The abolition of the protective effect of Clostridium welchii type A antiserum by ferric iron. Immunology. 1967 Mar;12(3):303–312. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Leigh L. C., Rogers H. J. The effect of iron compounds on the virulence of Escherichia coli for guinea-pigs. Immunology. 1968 Oct;15(4):581–588. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J. Bacterial iron metabolism and immunity to Pasteurella septica and Escherichia coli. Nature. 1969 Oct 25;224(5217):380–382. doi: 10.1038/224380a0. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Wilson A. B., Cushnie G. H., Rogers H. J. The abolition of the protective effect of Pasteurella septica antiserum by iron compounds. Immunology. 1968 Jun;14(6):889–898. [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Heppel L. A. Metallo-enzymes released from Escherichia coli by osmotic shock. II. Evidence that 5'-nucleotidase and cyclic phosphodiesterase are zinc metallo-enzymes. J Biol Chem. 1968 May 25;243(10):2647–2653. [PubMed] [Google Scholar]
- Griffiths E. Mechanism of action of specific antiserum on Pasteurella septica. Selective inhibition of net macromolecular synthesis and its reversal by iron compounds. Eur J Biochem. 1971 Nov 11;23(1):69–76. doi: 10.1111/j.1432-1033.1971.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Pellis N. R., Golden C. A. Mechanism of Tuberculostasis in Mammalian Serum III. Neutralization of Serum Tuberculostasis by Mycobactin. Infect Immun. 1971 Apr;3(4):553–558. doi: 10.1128/iai.3.4.553-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford C. E., Walker J. R., Reeves J. B., Nabbut N. H., Byers B. R., Jones R. J. Inoculum-dependent division lag of Bacillus cultures and its relation to an endogenous factor(s) ("schizokinen"). J Bacteriol. 1966 Mar;91(3):1070–1079. doi: 10.1128/jb.91.3.1070-1079.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS S., ESPLIN D. W., DONALDSON D. M. Lack of bactericidal effect of mouse serum on a number of common microorganisms. Science. 1954 Jun 18;119(3103):877–877. doi: 10.1126/science.119.3103.877. [DOI] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Kenny J. F. Observations concerning the pathogenesis of E. coli infections in mice. J Immunol. 1968 Sep;101(3):534–540. [PubMed] [Google Scholar]
- Medhurst F. A., Glynn A. A. In vivo bactericidal activity of mouse complement against Escherichia coli. Br J Exp Pathol. 1970 Oct;51(5):498–506. [PMC free article] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. 2,3-dihydroxy-N-benzoylserine: chemical synthesis and comparison with the natural product. Biochim Biophys Acta. 1969 Apr 1;177(2):321–328. doi: 10.1016/0304-4165(69)90142-1. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):453–460. doi: 10.1016/0304-4165(70)90165-0. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. 1970 Aug 14;215(2):393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- Pollack J. R., Neilands J. B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 1970 Mar 12;38(5):989–992. doi: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- ROANTREE R. J., COLLIS L. R. Effect of the peritoneal fluid of the guinea pig on strains of enteric bacilli. Nature. 1960 Sep 17;187:1045–1046. doi: 10.1038/1871045a0. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. The virulence of strains of Bacterium coli for mice. Br J Exp Pathol. 1954 Dec;35(6):528–538. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Bullen J. J., Cushnie G. H. Iron compounds and resistance to infection. Further experiments with Clostridium welchii type A in vivo and in vitro. Immunology. 1970 Oct;19(4):521–538. [PMC free article] [PubMed] [Google Scholar]
- STEWARD J. P., ROANTREE R. J. Effect of mouse peritoneal fluid on strains of enteric bacilli. Proc Soc Exp Biol Med. 1961 Dec;108:654–658. doi: 10.3181/00379727-108-27025. [DOI] [PubMed] [Google Scholar]
- Snow G. A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966 Sep;92(3):536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Lankford C. E. Production by Salmonella typhimurium of 2,3-dihydroxybenzoylserine, and its stimulation of growth in human serum. J Infect Dis. 1970 Feb;121(2):129–136. doi: 10.1093/infdis/121.2.129. [DOI] [PubMed] [Google Scholar]
- Young I. G., Gibson F. Regulation of the enzymes involved in the biosynthesis of 2,3-dihydroxybenzoic acid in Aerobacter aerogenes and Escherichia coli. Biochim Biophys Acta. 1969 May 6;177(3):401–411. doi: 10.1016/0304-4165(69)90302-x. [DOI] [PubMed] [Google Scholar]



