Abstract
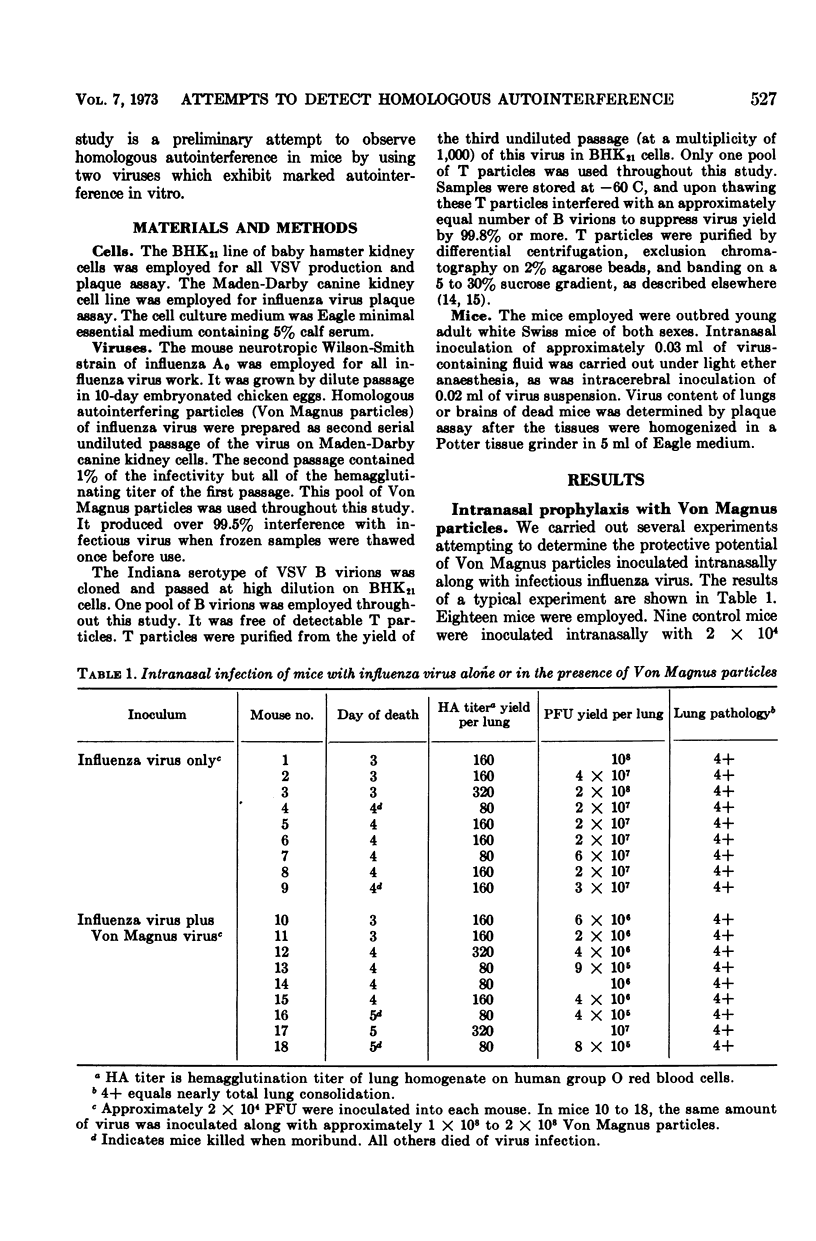
Von Magnus particles of influenza virus and defective interfering T particles of vesicular stomatitis virus were unable to provide significant protection of mice from disease or death when inoculated intranasally or intracerebrally along with moderate or high doses of homologous infectious challenge virus. However, yields of infectious virus from the affected organs were reduced as compared to controls inoculated with infectious virus alone. Serial intracerebral passage of vesicular stomatitis virus in mouse brain at high doses failed to produce T particles detectable by in vitro autointerference assays on BHK21 cells, whether or not T particles were introduced along with B virions at the first passage. When very low challenge doses of infectious B virions were inoculated intracerebrally along with high doses of homologous defective particles, there was significant prolongation of life, although most mice died eventually of slowly progressing disease. Also, the virus yields in the brains of these mice were significantly reduced, and virus was no longer detectable in the brains of “protected” mice surviving for 10 days or more. Our results suggest that although homologous autointerference does occur in vivo, it is a more complex phenomenon than in vitro cell culture experiments might indicate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackstein M. E., Stanners C. P., Farmilo A. J. Heterogeneity of polyoma virus DNA: isolation and characterization of non-infectious small supercoiled molecules. J Mol Biol. 1969 Jun 14;42(2):301–313. doi: 10.1016/0022-2836(69)90045-x. [DOI] [PubMed] [Google Scholar]
- COOPER P. D., BELLETT A. J. A transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:485–497. doi: 10.1099/00221287-21-3-485. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACKETT A. J. A POSSIBLE MORPHOLOGIC BASIS FOR THE AUTOINTERFERENCE PHENOMENON IN VESICULAR STOMATITIS VIRUS. Virology. 1964 Sep;24:51–59. doi: 10.1016/0042-6822(64)90147-3. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. Rift Valley Fever virus in mice. IV. Incomplete virus; its production and properties. Br J Exp Pathol. 1956 Apr;37(2):129–143. [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Dec;42(4):958–968. doi: 10.1016/0042-6822(70)90344-2. [DOI] [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Perrault J., Holland J. Variability of vesicular stomatitis virus autointerference with different host cells and virus serotypes. Virology. 1972 Oct;50(1):148–158. doi: 10.1016/0042-6822(72)90355-8. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Homologous interference by incomplete Sendai virus particles: changes in virus-specific ribonucleic acid synthesis. J Virol. 1971 Oct;8(4):388–394. doi: 10.1128/jvi.8.4.388-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Genome homology of vesicular stomatitis virus and defective T particles and evidence for the sequential transcription of the virion ribonucleic acid. J Virol. 1972 Jun;9(6):946–955. doi: 10.1128/jvi.9.6.946-955.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA's from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968 Jun 10;31(5):685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Kato M. Incomplete growth of simian virus 40 in African green monkey kidney culture induced by serial undiluted passages. Virology. 1966 Jan;28(1):135–141. doi: 10.1016/0042-6822(66)90314-x. [DOI] [PubMed] [Google Scholar]
- VON MAGNUS P. Propagation of the PR8 strain of influenza A virus in chick embryos. III. Properties of the incomplete virus produced in serial passages of undiluted virus. Acta Pathol Microbiol Scand. 1951;29(2):157–181. doi: 10.1111/j.1699-0463.1951.tb00114.x. [DOI] [PubMed] [Google Scholar]


