Abstract
Calcium is a universal messenger that mediates egg activation at fertilization in all sexually reproducing species studied. However, signaling pathways leading to calcium generation and the mechanisms of calcium-induced exit from meiotic arrest vary substantially among species. Here, we review the pathways of calcium signaling and the mechanisms of meiotic exit at fertilization in the eggs of the established developmental model, African clawed frog, Xenopus laevis. We also discuss calcium involvement in the early fertilization-induced events in Xenopus egg, such as membrane depolarization, the increase in intracellular pH, cortical granule exocytosis, cortical contraction, contraction wave, cortical rotation, reformation of the nuclear envelope, sperm chromatin decondensation and sister chromatid segregation.
Keywords: eggs, fertilization, activation, calcium, Xenopus laevis
1. Introduction
Molecular mechanisms of sperm-egg interaction differ greatly among sexually reproducing biological species. However, some events of fertilization-induced egg activation are highly conserved. One of them, the generation of the calcium wave or calcium oscillations in the egg cytoplasm, was found to be universal. The calcium signal represents a main early event of fertilization-induced egg activation observed in all species studied. It is a prerequisite for subsequent fertilization events, such as the block to polyspermy, exit from meiotic arrest, nuclear reformation, etc. Notably, significant differences exist between the generation pathways, spatiotemporal patterns and downstream effectors of the fertilization calcium signal in different species. This review summarizes in a concise form our current knowledge about different aspects of calcium signaling at fertilization in the eggs of African clawed frog Xenopus laevis.
Frog oocytes and eggs have been widely used in studies of meiotic progression and fertilization. Many of the control mechanisms that operate in maturing oocytes, fertilized eggs and early embryos have been first established in the frog model. A number of pioneering studies, concerning involvement of calcium in egg fertilization, activation and meiotic exit, have been carried out using Xenopus eggs. Here, we also refer occasionally to other biological species; however, a comprehensive discussion of calcium signaling at fertilization in different species is outside the scope of this paper.
2. Xenopus Eggs before Fertilization
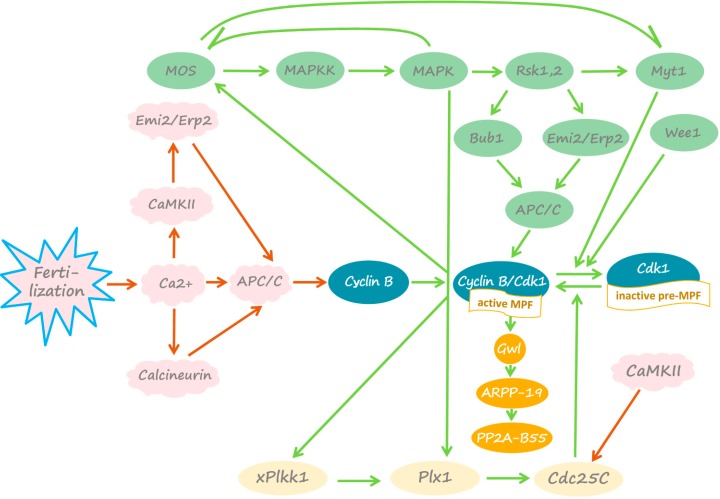
To better understand the physiological significance of the fertilization-induced calcium signal, it is important to characterize the state of eggs prior to fertilization. In all sexually reproducing organisms, including Xenopus, oocytes undergo meiotic reduction divisions to yield a haploid content of chromosomes. Before that, immature fully-grown fertilization-incompetent Xenopus oocytes reside in the frog ovaries arrested in the prophase of the first meiotic division. The steroid hormone, progesterone, released from surrounding follicle cells, induces oocyte transition from prophase I to metaphase II in the process of meiotic maturation. In frogs, the term “egg” is conventionally used for the ovulated mature oocytes arrested in metaphase II. The cytoplasmic activity from eggs that causes complete maturation upon injection into immature oocytes was originally defined by Masui and Markert as a maturation promoting factor (MPF) [1]. It consists of cyclin B and cyclin-dependent protein kinase Cdk1 (Figure 1). The major bulk of Cdk1 in immature oocytes is present in a free inactive monomeric form, and some part of Cdk1 is stored as an inactive complex, called pre-MPF. Catalytic activity of Cdk1 in the oocytes is inhibited by phosphorylation on Thr 14 and Tyr 15 by the inhibitory kinase, Myt1 [2]. Another Cdk1-inhibitory kinase, Wee1, is not expressed in immature Xenopus oocytes and starts to accumulate at meiosis I exit [3,4]. The MPF-activating phosphatase Cdc25C is also inhibited in the immature oocytes by direct phosphorylation on Ser 287 [5]. In addition, the absence of an active cytostatic factor (CSF), defined as an activity that causes metaphase arrest in frog eggs [1], is also crucial for maintaining prophase I arrest. Although the exact molecular composition of CSF is not established, it was found that the proto-oncogenic protein kinase, Mos, which is present only during meiosis and disappears after fertilization [6], and the active MAPK pathway represent its major components [7,8,9].
Figure 1.
Signaling pathways in metaphase-arrested and fertilized Xenopus eggs. Molecular components of the signaling pathways operating in mature metaphase-arrested eggs and the factors involved in fertilization-induced exit from meiotic metaphase arrest are shown connected by green and red arrows, respectively. Detailed explanations are provided in the text (see Section 2 and Section 4).
After the completion of maturation, ovulated fertilization-competent Xenopus eggs are arrested in the metaphase of the second meiotic division due to the high activity of MPF and CSF. Cyclin B, synthesized during maturation, directly binds and activates Cdk1 kinase, whereas newly synthesized Mos protein supports the high activity of the MAPK cascade. The meiotic arrest allows eggs to await fertilization, preventing parthenogenetic continuation of cell cycles after meiosis. A number of interlocking feedback loops contribute to the stability of metaphase II arrest in mature eggs. Most notably, the MAPK cascades of CSF and MPF are embedded in a loop of positive feedback (Figure 1). Active MPF increases Mos stability by direct phosphorylation on Ser 3 [10]. In addition, MPF also upregulates Mos synthesis by enhancing polyadenylation of maternal mos mRNA [11]. On the other hand, MAPK, activated in the presence of Mos, phosphorylates and activates the downstream target protein kinase, Rsk, which phosphorylates and downregulates the Cdk1-inhibitory kinase, Myt1 (Figure 1) [2,12]. Another Cdk1-inactivating kinase, Wee1, is also inhibited in metaphase-arrested eggs via a phosphorylation-dependent mechanism [13]. Furthermore, Rsk directly phosphorylates and activates Emi2/Erp1 and Bub1 proteins, the inhibitors of the APC/C ubiquitin ligase, controlling cyclin B degradation [14,15,16]. Emi2/Erp1 protein is not expressed in prophase oocytes, but it accumulates in mature metaphase-arrested eggs, due to cytoplasmic polyadenylation and translational unmasking of its mRNA [17]. This protein was identified as a pivotal component of CSF required to maintain meiotic metaphase arrest [18,19]. The phosphorylated inhibitor proteins downregulate the APC/C by sequestering the Cdc20 activator subunit of the ligase [20]. In addition, both Cdk1 and MAPK activate the polo-like protein kinase pathway, including Xenopus polo-like kinase kinase xPlkk1 and polo-like kinase Plx1 (Figure 1) [21,22]. This pathway is linked to upregulation of the MPF-activating phosphatase Cdc25C (Figure 1) [23,24]. Some other mechanisms, such as Mos-stimulated polyadenylation of cyclin mRNA, also contribute to maintaining high MPF activity [25]. Furthermore, a protein synthesis-dependent loop of positive feedback in the MAPK cascade enhances Mos accumulation by promoting cytoplasmic polyadenylation of mos mRNA [11] and increasing the stability of Mos protein through its direct phosphorylation on Ser 3 [26].
Recently, it was found that high MPF activity in eukaryotic meiotic eggs and mitotic somatic cells involves the suppression of the major anti-Cdk1 phosphatase PP2A-B55, which dephosphorylates Cdk1-phosphorylated substrates [27]. This event is mediated by the Greatwall kinase (Gwl) activated downstream of Cdk1/cyclin B [28,29,30]. Activated Gwl directly phosphorylates two small related proteins of around 20 kDa, ARPP-19 and/or a-endosulfine, which then interact with and inhibit PP2A-B55 phosphatase (Figure 1) [31,32]. It was further demonstrated that the Gwl/ARPP19/PP2A-B55 module behaves as a major component of the MPF auto-amplification loop contributing to MPF activation in maturing Xenopus oocytes [33,34].
Thus, multiple interlocking loops of positive feedback promote and stabilize meiotic metaphase arrest in Xenopus eggs before fertilization (Figure 1). Fertilization triggers the disruption of the positive feedback between the two major determinants of the meiotic arrest, CSF and MPF, via calcium-dependent mechanisms, as explained further. Notably, eggs from different species are arrested at different stages of the meiotic cell cycle before fertilization (reviewed in [35,36]). However, the sperm-triggered calcium transient universally activates eggs and releases them from the meiotic arrest. The elevation of intracellular calcium is necessary and sufficient for egg activation.
3. Generation of Calcium Transient in Xenopus Egg at Fertilization
Although calcium universally mediates egg activation in different species, the upstream pathways by which sperm triggers the calcium signal within egg vary substantially among species. Currently, two opposing mechanisms of fertilization-induced elevation of intracellular calcium concentration have been elucidated. The sperm factor-mediated mechanism was found to trigger the calcium signal via a soluble factor released after gamete fusion from sperm into egg in mammals. For instance, a series of calcium oscillations is initiated by the sperm-specific phospholipase C, PLCζ, which is introduced by sperm into mouse eggs [37,38,39]. In some other species, the membrane receptor-mediated mechanism was proposed to account for the initiation of intracellular signaling after interaction between sperm ligand(s) and egg receptor(s). Fertilization in Xenopus laevis, as well as in sea urchin, ascidians, starfish, zebrafish, etc., was found to involve membrane-originated tyrosine kinase-mediated generation of the calcium signal in eggs (reviewed in [40,41]). Notably, the two opposing mechanisms may not be necessarily mutually exclusive, and it has been suggested that Xenopus sperm may contain some biologically active compounds that contribute to egg activation. As a result of intracellular calcium release, its concentration in fertilized Xenopus eggs increases several fold from ~200 nM to above 1 µM within 5 min and returns to the pre-activation level within ~20 min after fertilization [42,43]. The calcium transient in fertilized Xenopus eggs, as well as that in the eggs of fish, sea urchin, jellyfish, etc., represents a single calcium wave, propagating from the sperm entry point, within several minutes after fertilization. However, multiple calcium oscillations sustained for a much longer time were observed in the fertilized eggs of some other species, including mammals, ascidians, mollusks, etc. (reviewed in [36]). The calcium wave in fertilized Xenopus eggs travels from the animal to vegetal hemisphere of the egg, propagating at a rate of approximately 10 microns/s [42]. The apparent delay between the initial sperm-induced membrane depolarization, which can be considered as the start of fertilization, and initiation of the calcium wave at the sperm entry site was estimated to be approximately 1 min [42]. A delay of this magnitude strongly suggests that the fertilization-induced calcium signal is originated in the egg after a series of time-consuming intracellular events. These events were successfully delineated in recent studies.
The initial steps of sperm-egg interaction still remain obscure, and putative sperm ligand(s) and egg receptor(s) in the plasma membrane are yet to be identified. A plausible candidate for the egg receptor at fertilization in Xenopus can be the uroplakin III/uroplakin 1b complex [44,45]; however, surface-mediated activation of multiple receptor proteins in the egg membrane also cannot be ruled out. Involvement of the GTP-binding proteins, such as heterotrimeric G-proteins, was implicated by the fact that egg activation can be induced by the injection of the non-hydrolyzable GTP analog, GTPγS [46,47]. However, this was challenged later by the finding that an antibody against Gαq family G-proteins did not inhibit a calcium rise at fertilization, suggesting nonspecific effects of GTPγS [48]. Considering that no other type of G-protein α subunit can activate G-protein-sensitive PLCβ [49,50,51], this finding argues against a role for G-protein α subunits in the calcium rise at fertilization. In addition, it was shown that calcium release at fertilization of frog eggs is not inhibited by pertussis toxin, suggesting that βγ subunits from Gi or Go are not required for this process [46]. Still, involvement of the pertussis toxin-insensitive βγ subunits was not investigated in detail, and it cannot be ruled out completely.
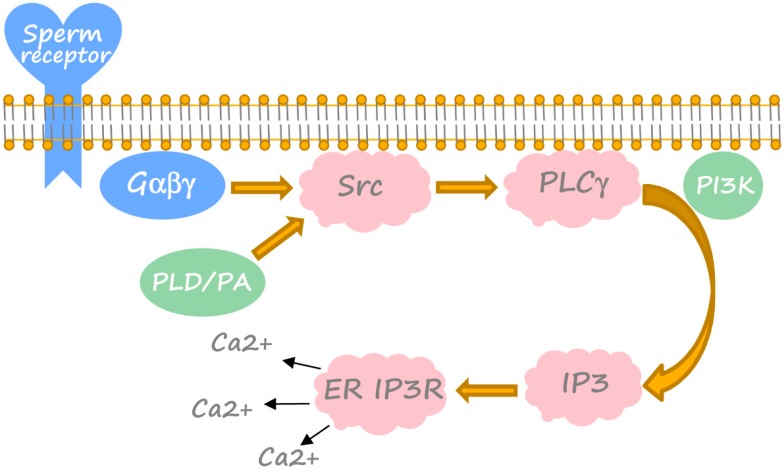
A number of studies established the sequential activation of Src family kinases, PLCγ and IP3 receptor of the endoplasmic reticulum as the early universal events of fertilization-induced egg activation (Figure 2) (reviewed in [52,53,54,55]). These events precede the calcium transient. The signaling pathway leading to the calcium rise was delineated in Xenopus eggs and egg extracts using mainly an inhibitor-based approach [48,56,57,58,59]. It was shown that, although Src kinase does not play a significant role in Xenopus oocyte maturation and meiotic arrest [60], it is indispensable for fertilization-induced egg activation, as demonstrated with the use of pharmacological inhibitors of Src-family kinases [56,57]. Activation of Src kinase can be detected in Xenopus eggs within 1 min after fertilization [61]. In parallel, PLCγ becomes tyrosine-phosphorylated, translocated from the cytoplasm to the membrane, associated with Src and several-fold activated [57,58,62]. Then, activated PLCγ produces IP3 and DAG by hydrolysis of PIP2. A role for SH2-domain mediated activation of PLCγ in Xenopus egg activation is unlikely, since injecting PLCγ SH2 domains does not inhibit the calcium rise at fertilization [63]. Instead, the PH domain binding to the membrane PIP2/PIP3 may be involved in the process [54]. This suggests the engagement of the PIP3-generating enzyme, PI3 kinase (Figure 2). Its pharmacological inhibition was found to suppress fertilization-induced PLCγ activation and calcium release [59].
Figure 2.
Fertilization-induced cascade of intracellular calcium release in Xenopus eggs. Major established constituents, crucial accessory factors and unknown putative components of the cascade are shown in pink, green and blue, correspondingly. Detailed explanations are provided in the text (see Section 3).
The IP3 mass increase in Xenopus eggs starts 1 min after fertilization and reaches more than four-fold excess over the basal state in about 5 min [57,64]. IP3 triggers initial calcium release from the IP3-receptor-operated calcium ER stores at the sperm binding site: an antibody against the type I IP3 receptor blocks calcium release in fertilized Xenopus eggs [48]. However, following self-propagation of the calcium wave in the egg involves, most probably, two processes: IP3-induced calcium release (IICR) and calcium-induced calcium release (CICR) [65,66,67]. Notably, Xenopus eggs acquire the capacity to generate the fertilization-specific calcium transient during oocyte maturation; injection of IP3 in eggs results in a slower and more prolonged calcium wave than that in oocytes [68]. The major events of calcium signaling differentiation during maturation include internalization of the plasma membrane calcium ATPase, loss of store-operated calcium entry and spatial reorganization of IP3 receptors in the ER membrane [68,69,70,71]. Recently, computational modeling demonstrated that jointly, these factors can account for the observed changes in calcium signaling wave propagation [72].
Notably, evidence has been provided that phospholipase D (PLD) and phosphatidic acid (PA) may act as upstream regulators of Src and PLCγ in Xenopus eggs fertilization (Figure 2) [73]. Indeed, fertilization elevates PA levels within 1 min of fertilization, and PA addition to Xenopus eggs stimulates Src and tyrosine phosphorylation of PLCγ, increases the intracellular mass of IP3 and induces fertilization events that can be blocked by an IP3 receptor inhibitor or the calcium chelator, BAPTA [74]. PLD hydrolizes phosphatidylcholine to produce PA and choline, whose level also increases at fertilization [73]. Pharmacological inhibition of PLD was found to suppress Src and PLCγ activation, calcium release and other fertilization-induced events [74]. Molecular species analysis and mass measurements suggest that sperm activates the PLD1b isoform to elevate PA [74].
The calcium transient initiates a plethora of biochemical and cellular events in fertilized Xenopus eggs, such as exit from meiotic arrest, membrane depolarization, exocytosis of cortical granules, elevation of fertilization envelope, cortical contraction, cortical rotation, sperm decondensation, pronuclear formation, etc. The following sections of the manuscript reveal these events in more detail.
4. Fertilization-Induced Meiotic Exit
The major event of Xenopus egg activation following fertilization is calcium-induced exit from meiotic metaphase II arrest and cell cycle resumption. Fertilization triggers MPF inactivation before Mos degradation and MAPK pathway inactivation, pinpointing MPF as an early target. Calcium-dependent degradation of cyclin B occurs within 15 min of fertilization. It causes the disruption of positive feedback between MPF and CSF and Mos protein degradation within 30–40 min after fertilization [75]. The calcium/calmodulin-dependent protein kinase, CaMKII, was found to mediate the effect of calcium on cyclin degradation. Inhibition of CaMKII prevents cyclin degradation and exit from metaphase arrest after calcium addition, whereas microinjection of constitutively active CaMKII into unfertilized Xenopus eggs inactivates Cdk1 and releases the eggs from the CSF arrest in the absence of the calcium transient [76]. It was found that CaMKII conveys fertilization-induced cyclin degradation and meiotic exit in Xenopus eggs via the APC/C inhibitor, Emi2/Erp1 (Figure 1). CaMKII directly phosphorylates Emi2/Erp1 on Thr 195 to create a polo-box binding site for the polo-like kinase Plx1. Binding of Plx1 promotes the secondary phosphorylation of Emi2/Erp1 by Plx1 on Ser 33 and Ser 38 at the site of the phosphorylation-dependent degradation signal [77,78]. The signal is then recognized by the SCF1 (SKP2-cullin1-F-box protein)-E3 ubiquitin ligase complex targeting Emi2/Erp1 for 26S proteasome-mediated degradation [18,78,79,80,81]. Degradation of Emi2/Erp1 leads to APC/C activation and cyclin B ubiquitination, directing Cdk1/cyclin B complex to the 26S proteasome. Finally, proteasome-dependent dissociation of the complex, followed by cyclin B degradation, inactivates MPF [82].
In addition to targeting the protein degradation machinery, activated CaMKII inhibits Cdc25C phosphatase by direct phosphorylation on Ser 287 (Figure 1) [83]. This phosphorylation is suppressed in unfertilized M phase-arrested Xenopus eggs. Calcium-induced inhibition of Cdc25C activity in the fertilized eggs increases phosphorylation levels of Thr 14 and Tyr 15 in Cdk1, thereby inhibiting its kinase activity.
Independently of CaMKII, the fertilization-induced calcium transient activates the calcium/calmodulin-dependent serine/threonine protein phosphatase calcineurin (PP2B) (Figure 1). Calcineurin activity is essential for overcoming meiotic arrest. Inhibition or depletion of calcineurin in Xenopus egg extracts delays cyclin B destruction, Cdk1 inactivation and the global dephosphorylation of M-phase-specific phosphoproteins in response to calcium [84,85]. Furthermore, inhibition of calcineurin in unfertilized eggs prevents meiotic exit upon egg activation [85]. It was reported that calcineurin is rapidly and transiently activated immediately after calcium addition to CSF-arrested Xenopus egg extracts [85]. Then, calcineurin dephosphorylates the APC/C activator Cdc20 and the core APC/C component Apc3 (also known as Cdc27). These dephosphorylation events have been suggested to contribute to APC/C activation via a yet unknown mechanism. Previously, evidence was provided that phosphorylation of Cdc20 inhibits its ability to activate the APC/C [86]. It remains to be clarified whether APC/C activation depends on dephosphorylation of its subunits by calcineurin. Furthermore, the role of calcineurin in the meiotic activation of PP2A-B55 phosphatase requires investigation. The findings obtained using Xenopus egg extracts demonstrate that PP2A-B55 phosphatase, suppressed in metaphase II by the high activity of Gwl kinase (see Section 2), becomes activated when Cdk1 is inactivated and the CSF-arrested extracts are released into interphase [27,87].
Thus, the fertilization-induced calcium transient independently activates CaMKII and calcineurin. They both contribute to inhibition of Cdk1 activity in fertilized Xenopus eggs. Cdk1 inactivation triggers the disruption of positive feedback between MPF and CSF. Mos protein is dephosphorylated at Ser 3, a site of direct phosphorylation by Cdk1, and degraded by the N-terminal Pro2-dependent ubiquitin pathway [88]. In addition, EDEN-directed deadenylation of mos mRNA effectively suppresses Mos translation after fertilization [89,90]. Mos degradation leads to shutdown of the MAPK cascade and complete CSF inactivation. It was suggested that the calcium-dependent cysteine protease, calpain, may also play a role in fertilization-induced Mos degradation [91]. However, this idea was challenged by the finding that calpain is capable of degrading Mos in vitro only at supraphysiological concentrations [92].
Of note, the involvement of CaMKII and calcineurin in egg activation is not evolutionary conserved. Mature eggs of many invertebrate species arrest at metaphase I, and their genomes do not encode the CaMKII target protein, Erp1/Emi2. It was shown that the two phosphatases, calcineurin and PP2A, but not CaMKII, are required for normal egg activation during fertilization in ascidians [93]. On the other hand, cell cycle resumption in fertilized mammalian (mouse) eggs relies solely on CaMKII [20,94,95]. It was hypothesized that during animal evolution, the mechanism of egg activation evolved from the phosphatase-dependent process to the protein kinase-dependent one, with Xenopus being an intermediate species involving both of them [93]. Analysis of egg activation signaling pathways in various animal species is necessary to validate this hypothesis.
5. Other Effects of Calcium in Fertilized Xenopus Egg
The fertilization-induced calcium transient is responsible for several biochemical and cellular events of Xenopus egg activation. Membrane depolarization, exocytosis of cortical granules, elevation of fertilization envelope, sperm decondensation, activation-associated increase in intracellular pH and pronuclear formation were found to be inhibited or delayed in the activated eggs by prior microinjection of the calcium chelator, BAPTA [96,97]. Furthermore, BAPTA or heparin, an IP3 receptor antagonist, prevented the calcium-dependent fertilization events, such as gravitational rotation, contraction wave and cleavage furrow formation [98,99]. Further, it was shown that calcium triggers sister chromatid segregation in the extracts prepared from unfertilized Xenopus eggs, i.e., CSF-arrested extracts [100,101]. At present, the pathways leading to these calcium-mediated events are poorly understood.
It is thought that activation of protein kinase C (PKC) due to elevation of DAG and intracellular calcium is responsible for cortical granule exocytosis, cortical contraction, reformation of the nuclear envelope and sperm chromatin decondensation. Indeed, a wave of PKC activation accompanies the calcium transient after fertilization of Xenopus egg [102]. Activators of PKC are able to trigger cortical granule exocytosis, cortical contraction and cleavage furrow formation in unfertilized Xenopus eggs [103]. Furthermore, nuclear lamina disassembly of permeabilized sperm nuclei in sea urchin egg extracts was found to be a result of lamin B phosphorylation, which is reversibly inhibited by PKC-specific inhibitors, but not by inhibitors of PKA, Cdk1 or CaMKII [104]. As lamin B phosphorylation and solubilization precede chromatin decondensation, PKC inhibition also prevents sperm decondensation.
Importantly, the DAG increase at fertilization does not originate from PIP2 hydrolysis by PLCγ. It was reported that the increase in DAG mass and translocation of PKCα and PKCβ to a membrane fraction occur in Xenopus egg in about 7 min after fertilization [73]. Thus, the DAG increase at fertilization occurs later than that of IP3, and besides, it is approximately 280-times greater. Combined with the facts that choline mass also increases at fertilization and that the total choline increase is comparable to that of DAG, these data suggest that most of DAG in fertilized Xenopus eggs is produced by activated PLD, which hydrolyzes phosphatidylcholine to PA and choline, and PA is further converted to DAG [54,74,105].
Although PKC is widely involved in calcium-mediated fertilization signaling, many events of egg activation do not require its participation. For instance, the depolarizing fertilization potential, which provides the electric block to polyspermy, is directly mediated by calcium-activated Cl− channels. These channels become open due to an increase in intracellular calcium triggered by IP3-induced calcium release [43,106,107]. Furthermore, PKC activators, unlike calcium ionophores, cannot mimic fertilization-induced increase in intracellular pH, suggesting that this increase is not related to PKC-dependent phosphorylation of the Na+/H+ exchanger in the plasma membrane [108]. Presently, the mechanism of fertilization-induced calcium-mediated increase in intracellular pH remains obscure.
Further, the fertilization-induced surface contraction wave (SCW) of pigmentation change at the egg cortex, which originates at the animal pole and proceeds to the vegetal pole, does not depend on PKC and relies, most probably, on Cdk1 activity. It was found that the contraction phase requires inactivation of MPF and is blocked when MPF activity is maintained at elevated levels [109]. Thus, SCW represents a local surface response to a wave of cytoplasmic MPF inactivation. It was hypothesized that the change in pigmentation is controlled by the phosphorylation of microtubule-associated proteins by Cdk1 [110].
Similarly, Cdk1 activity also governs cortical rotation, a fertilization-induced microtubule-mediated process that translocates the egg cortex relative to the cytoplasm, specifying the orientation of the embryonic dorso-ventral axis. Cortical rotation stops at mitosis I entry, at the time of MPF activation, accompanied by a visible SCW [110,111]. Cortical rotation can be prolonged if MPF activation does not occur, while injections of MPF can provoke ectopic SCW waves that arrest cortical rotation [111]. It was further demonstrated that cortical rotation is arrested not by MPF-dependent inhibition of molecular motors, such as kinesin-related proteins and dynein, but as a result of MPF-induced microtubule depolymerization [112]. The involvement of microtubule-associated proteins, whose activity is modulated by MPF-dependent phosphorylation, was suggested [113,114,115]; however, the exact mechanism of cortical rotation and its regulation by MPF still remain to be understood.
Another example of the PKC-independent egg activation event concerns sister chromatid segregation at anaphase. This event is induced by CaMKII. A constitutively active CaMKII mutant was found to trigger anaphase in the absence of the calcium transient [101,116]. It was further shown in activated Xenopus eggs and calcium-treated CSF-arrested egg extracts that segregation of sister chromatids at the second meiotic anaphase is controlled by the APC/Cdc20-dependent pathway [117], which is suppressed in MII-arrested eggs and becomes activated in fertilized eggs (for details, see Section 2 and Section 4 of this review). CaMKII and APC/C co-localize in mitotic spindles and centrosomes of mammalian cells, suggesting their functional connections [118]. A direct target of the activated APC/C in centromeres is securin, which is degraded in late metaphase via the ubiquitin-proteasome pathway. Non-degradable Xenopus securin prevents in vitro assembled spindles from separating sister chromatids at exit from mitosis in Xenopus egg extracts [119]. Destruction of securin unleashes the large cysteine endopeptidase, separase, which is held inactive by association with securin. It was reported that removal of an inhibitory phosphate is also necessary for full separase activation [120]. Finally, separase cleaves the kleisin subunit SCC1 of the ring-shaped multi-protein complex cohesion, which is responsible for holding sister chromatids together [121].
6. Conclusions and Perspectives
The calcium signal at fertilization has been exceptionally conserved through the course of evolution. It represents an early indispensable event of fertilization-induced egg activation. So far, the research on the molecular mechanisms of fertilization has been focused on several biological species. Among them, Xenopus laevis has served as a very useful model organism for studying the fertilization-induced calcium transient. Xenopus eggs are arrested in meiotic metaphase II awaiting fertilization. The multiple interlocking loops of positive feedback, particularly that between MPF and CSF, stabilize the meiotic metaphase arrest. Fertilization-induced elevation of intracellular calcium disrupts the feedback, activates eggs and releases them from meiotic arrest. The importance of the Src-PLCγ-IP3 pathway in the generation of the calcium transient at fertilization has been established. However, the initial steps of sperm-egg interaction upstream of this pathway are obscure. Most notably, the identity of the egg membrane-associated receptor for sperm remains elusive. The main event of Xenopus egg activation at fertilization is calcium-induced exit from meiotic metaphase arrest. The calcium transient independently activates CaMKII and calcineurin in Xenopus eggs. They both contribute to Cdk1 inhibition by activating the APC/C ubiquitin ligase via different mechanisms involving Emi2/Erp1, Apc3 and Cdc20 regulators of APC/C. Cdk1 inactivation triggers disruption of the major loop of the positive feedback between MPF and CSF, resulting in meiotic exit. The emerging involvement of PP2A-B55 phosphatase in these processes requires further investigation.
In addition, calcium orchestrates a plethora of biochemical and cellular events of Xenopus egg activation, such as membrane depolarization, increase in intracellular pH, cortical granule exocytosis, cortical contraction, contraction wave, cortical rotation, reformation of the nuclear envelope, sperm chromatin decondensation, sister chromatid segregation and some others. Little is known about the calcium-dependent mechanisms underlying these steps of egg activation. Their future investigation may potentially reveal novel molecular targets of calcium in eukaryotic cells. Thus, Xenopus eggs will remain a major biological model for fertilization and basic physiological studies, due to their high biological relevance, convenience of manipulation and biochemical tractability.
Acknowledgments
This review was written by the support, in part, of the Research Fund for Foreign Visiting Professor from Kobe University (to Alexander A. Tokmakov) and the Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (25,440,023 to Alexander A. Tokmakov).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Masui Y., Markert C.L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 2.Mueller P.R., Coleman T.R., Kumagai A., Dunphy W.G. Myt1: A membrane-associated inhibitory kinase that phosphorylates Ccd2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 3.Murakami M.S., vande Woude G.F. Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development. 1998;125:237–248. doi: 10.1242/dev.125.2.237. [DOI] [PubMed] [Google Scholar]
- 4.Nakajo N., Yoshitome S., Iwashita J., Iida M., Uto K., Ueno S., Okamoto K., Sagata N. Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev. 2000;14:328–338. [PMC free article] [PubMed] [Google Scholar]
- 5.Duckworth B.C., Weaver J.S., Ruderman J.V. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA. 2002;99:16794–16799. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagata N., Watanabe N., vande Woude G.F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh Y., Masuyama N., Dell K., Shirakabe K., Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J. Biol. Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh Y., Nishida E. The MAP kinase cascade: Its role in Xenopus oocytes, eggs and embryos. Prog. Cell Cycle Res. 1995;1:287–297. doi: 10.1007/978-1-4615-1809-9_23. [DOI] [PubMed] [Google Scholar]
- 9.Haccard O., Lewellyn A., Hartley R.S., Erikson E., Maller J.L. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev. Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- 10.Castro A., Peter M., Magnaghi-Jaulin L., Vigneron S., Galas S., Lorca T., Labbé J.C. Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell. 2001;12:2660–2671. doi: 10.1091/mbc.12.9.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard E.L., Charlesworth A., Welk J., MacNicol A.M. The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol. Cell. Biol. 1999;19:1990–1999. doi: 10.1128/mcb.19.3.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer A., Gavin A.C., Nebreda A.R. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller P.R., Coleman T.R., Dunphy W.G. Cell cycle regulation of a Xenopus wee1-like kinase. Mol. Biol. Cell. 1995;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue D., Ohe M., Kanemori Y., Nobui T., Sagata N. A direct link of the Mos–MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature. 2007;446:1100–1104. doi: 10.1038/nature05688. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T., Ohsumi K., Kishimoto T. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature. 2007;446:1096–1099. doi: 10.1038/nature05696. [DOI] [PubMed] [Google Scholar]
- 16.Schwab M.S., Roberts B.T., Gross S.D., Tunquist B.J., Taieb F.E., Lewellyn A.L., Maller J.L. Bub1 is activated by the protein kinase p90 (Rsk) during Xenopus oocyte maturation. Curr. Biol. 2001;11:141–150. doi: 10.1016/S0960-9822(01)00045-8. [DOI] [PubMed] [Google Scholar]
- 17.Tung J.J., Padmanabhan K., Hansen D.V., Richter J.D., Jackson P.K. Translational unmasking of Emi2 directs cytostatic factor arrest in meiosis II. Cell Cycle. 2007;6:725–731. doi: 10.4161/cc.6.6.3936. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt A., Duncan P.I., Rauh N.R., Sauer G., Fry A.M., Nigg E.A., Mayer T.U. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 2005;19:502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung J.J., Hansen D.V., Ban K.H., Loktev A.V., Summers M.K., Adler J.R., 3rd, Jackson P.K. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoji S., Yoshida N., Amanai M., Ohgishi M., Fukui T., Fujimoto S., Nakano Y., Kajikawa E., Perry A.C. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrieu A., Brassac T., Galas S., Fisher D., Labbé J.C., Dorée M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- 22.Gavin A.C., Ni Ainle A., Chierici E., Jones M., Nebreda A.R. A p90(rsk) mutant constitutively interacting with MAP kinase uncouples MAP kinase from p34(cdc2)/cyclin B activation in Xenopus oocytes. Mol. Biol. Cell. 1999;10:2971–2986. doi: 10.1091/mbc.10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumagai A., Dunphy W.G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 24.Qian Y.W., Erikson E., Maller J.L. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998;282:1701–1704. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- 25.De Moor C.H., Richter J.D. The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol. 1997;17:6419–6426. doi: 10.1128/mcb.17.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matten W.T., Copeland T.D., Ahn N.G., Vande Woude G.F. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev. Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 27.Mochida S., Ikeo S., Gannon J., Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J., Zhao Y., Li Z., Galas S., Goldberg M.L. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Castilho P.V., Williams B.C., Mochida S., Zhao Y., Goldberg M.L. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell. 2009;20:4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigneron S., Brioudes E., Burgess A., Labbé J.C., Lorca T., Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009;28:2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mochida S., Maslen S.L., Skehel M., Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 32.Gharbi-Ayachi A., Labbé J.C., Burgess A., Vigneron S., Strub J.M., Brioudes E., Van-Dorsselaer A., Castro A., Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 33.Hara M., Abe Y., Tanaka T., Yamamoto T., Okumura E., Kishimoto T. Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat. Commun. 2012;3:1059. doi: 10.1038/ncomms2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupré A., Buffin E., Roustan C., Nairn A.C., Jessus C., Haccard O. The phosphorylation of ARPP19 by Greatwall renders the auto-amplification of MPF independently of PKA in Xenopus oocytes. J. Cell Sci. 2013;126:3916–3926. doi: 10.1242/jcs.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitaker M. Control of meiotic arrest. Rev. Reprod. 1996;1:127–135. doi: 10.1530/ror.0.0010127. [DOI] [PubMed] [Google Scholar]
- 36.Stricker S.A. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 37.Saunders C.M., Larman M.G., Parrington J., Cox L.J., Royse J., Blayney L.M., Swann K., Lai F.A. PLCζ: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 38.Swann K., Saunders C.M., Rogers N.T., Lai F.A. PLCζ: A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin. Cell Dev. Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Swann K., Lai F.A. PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell Calcium. 2013;53:55–62. doi: 10.1016/j.ceca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 40.McGinnis L.K., Carroll D.J., Kinsey W.H. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol. Reprod. Dev. 2011;78:831–845. doi: 10.1002/mrd.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinsey W.H. Intersecting roles of protein tyrosine kinase and calcium signaling during fertilization. Cell Calcium. 2013;53:32–40. doi: 10.1016/j.ceca.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busa W.B., Nuccitelli R. An elevated free cytosolic Ca2+ wave follows fertilization in eggs of the frog, Xenopus laevis. J. Cell Biol. 1985;100:1325–1329. doi: 10.1083/jcb.100.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuccitelli R., Yim D.L., Smart T. The sperm-induced Ca2+ wave following fertilization of the Xenopus egg requires the production of Ins(1,4,5)P3. Dev. Biol. 1993;158:200–212. doi: 10.1006/dbio.1993.1179. [DOI] [PubMed] [Google Scholar]
- 44.Sakakibara K., Sato K., Yoshino K., Oshiro N., Hirahara S., Mahbub Hasan A.K., Iwasaki T., Ueda Y., Iwao Y., Yonezawa K., et al. Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J. Biol. Chem. 2005;280:15029–15037. doi: 10.1074/jbc.M410538200. [DOI] [PubMed] [Google Scholar]
- 45.Mahbub Hasan A.K., Hashimoto A., Maekawa Y., Matsumoto T., Kushima S., Ijiri T.W., Fukami Y., Sato K. The egg membrane microdomain-associated uroplakin III-Src system becomes functional during oocyte maturation and is required for bidirectional gamete signaling at fertilization in Xenopus laevis. Development. 2014;141:1705–1714. doi: 10.1242/dev.105510. [DOI] [PubMed] [Google Scholar]
- 46.Kline D., Kopf G.S., Muncy L.F., Jaffe L.A. Evidence for the involvement of a pertussis toxin-insensitive G-protein in egg activation of the frog, Xenopus laevis. Dev. Biol. 1991;143:218–229. doi: 10.1016/0012-1606(91)90072-B. [DOI] [PubMed] [Google Scholar]
- 47.Jaffe L.A. First messengers at fertilization. J. Reprod. Fertil. Suppl. 1990;42:107–116. [PubMed] [Google Scholar]
- 48.Runft L.L., Watras J., Jaffe L.A. Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCgamma or G(q)-mediated activation of PLCβ. Dev. Biol. 1999;214:399–411. doi: 10.1006/dbio.1999.9415. [DOI] [PubMed] [Google Scholar]
- 49.Hepler J.R., Kozasa T., Smrcka A.V., Simon M.I., Rhee S.G., Sternweis P.C., Gilman A.G. Purification from Sf9 cells and characterization of recombinant Gqα and G11α: Activation of purified phospholipase C isozymes by Gα subunits. J. Biol. Chem. 1993;268:14367–14375. [PubMed] [Google Scholar]
- 50.Singer W.D., Miller R.T., Sternweis P.C. Purification and characterization of the α subunit of G13. J. Biol. Chem. 1994;269:19796–19802. [PubMed] [Google Scholar]
- 51.Kozasa T., Gilman A.G. Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits: Characterization of α12 and inhibition of adenylyl cyclase by αz. J. Biol. Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 52.Ciapa B., Chiri S. Egg activation: Upstream of the fertilization calcium signal. Biol. Cell. 2000;92:215–233. doi: 10.1016/S0248-4900(00)01065-0. [DOI] [PubMed] [Google Scholar]
- 53.Sato K., Tokmakov A.A., Fukami Y. Fertilization signalling and protein-tyrosine kinases. Comp. Biochem. Physiol. B. 2000;126:129–148. doi: 10.1016/S0305-0491(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 54.Sato K., Fukami Y., Stith B.J. Signal transduction pathways leading to Ca2+ release in a vertebrate model system: Lessons from Xenopus eggs. Semin. Cell Dev. Biol. 2006;17:285–292. doi: 10.1016/j.semcdb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Tokmakov A.A., Iwasaki T., Sato K., Fukami Y. Analysis of signal transduction in cell-free extracts and rafts of Xenopus eggs. Methods. 2010;51:177–182. doi: 10.1016/j.ymeth.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Sato K., Iwao Y., Fujimura T., Tamaki I., Ogawa K., Iwasaki T., Tokmakov A.A., Hatano O., Fukami Y. Evidence for the involvement of a Src-related tyrosine kinase in Xenopus egg activation. Dev. Biol. 1999;209:308–320. doi: 10.1006/dbio.1999.9255. [DOI] [PubMed] [Google Scholar]
- 57.Sato K., Tokmakov A.A., Iwasaki T., Fukami Y. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev. Biol. 2000;224:453–469. doi: 10.1006/dbio.2000.9782. [DOI] [PubMed] [Google Scholar]
- 58.Tokmakov A.A., Sato K.I., Iwasaki T., Fukami Y. Src kinase induces calcium release in Xenopus egg extracts via PLCγ and IP3-dependent mechanism. Cell Calcium. 2002;32:11–20. doi: 10.1016/S0143-4160(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 59.Mammadova G., Iwasaki T., Tokmakov A.A., Fukami Y., Sato K. Evidence that phosphatidylinositol 3-kinase is involved in sperm-induced tyrosine kinase signaling in Xenopus egg fertilization. BMC Dev. Biol. 2009;9:68. doi: 10.1186/1471-213X-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tokmakov A., Iwasaki T., Itakura S., Sato K., Shirouzu M., Fukami Y., Yokoyama S. Regulation of Src kinase activity during Xenopus oocyte maturation. Dev. Biol. 2005;278:289–300. doi: 10.1016/j.ydbio.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Sato K., Aoto M., Mori K., Akasofu S., Tokmakov A.A., Sahara S., Fukami Y. Purification and characterization of a Src-related p57 protein–tyrosine kinase from Xenopus oocytes. Isolation of an inactive form of the enzyme and its activation and translocation upon fertilization. J. Biol. Chem. 1996;271:13250–13257. doi: 10.1074/jbc.271.22.13250. [DOI] [PubMed] [Google Scholar]
- 62.Sato K., Tokmakov A.A., He C.L., Kurokawa M., Iwasaki T., Shirouzu M., Fissore R.A., Yokoyama S., Fukami Y. Reconstitution of Src-dependent phospholipase Cγ phosphorylation and transient calcium release by using membrane rafts and cell-free extracts from Xenopus eggs. J. Biol. Chem. 2003;278:38413–38420. doi: 10.1074/jbc.M302617200. [DOI] [PubMed] [Google Scholar]
- 63.Mehlmann L.M, Carpenter G., Rhee S.G., Jaffe L.A. SH2 domain-mediated activation of phospholipase Cgamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev. Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- 64.Stith B.J., Goalstone M., Silva S., Jaynes C. Inositol 1,4,5-trisphosphate mass changes from fertilization through first cleavage in Xenopus laevis. Mol. Biol. Cell. 199;4:435–443. doi: 10.1091/mbc.4.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dupont G., Goldbeter A. Properties of intracellular Ca2+ waves generated by a model based on Ca2+-induced Ca2+ release. Biophys. J. 1994;67:2191–2204. doi: 10.1016/S0006-3495(94)80705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner J., Li Y.X., Pearson J., Keizer J. Simulation of the fertilization Ca2+ wave in Xenopus laevis eggs. Biophys. J. 1998;75:2088–2097. doi: 10.1016/S0006-3495(98)77651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner J., Fall C.P., Hong F., Sims C.E., Allbritton N.L., Fontanilla R.A., Moraru I.I., Loew L.M., Nuccitelli R. A wave of IP3 production accompanies the fertilization Ca2+ wave in the egg of the frog, Xenopus laevis: Theoretical and experimental support. Cell Calcium. 2004;35:433–447. doi: 10.1016/j.ceca.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Machaca K., Haun S. Store-operated calcium entry inactivates at the germinal vesicle breakdown stage of Xenopus meiosis. J. Biol. Chem. 2000;275:38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Jouni W., Jang B., Haun S., Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Dev. Biol. 2005;288:514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 70.Yu F., Sun L., Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc. Natl. Acad. Sci. USA. 2009;106:17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun L., Yu F., Ullah A., Hubrack S., Daalis A., Jung P., Machaca K. Endoplasmic reticulum remodeling tunes IP3-dependent Ca2+ release sensitivity. PLoS One. 2011;6:e27928. doi: 10.1371/journal.pone.0027928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ullah A., Jung P., Ullah G., Machaca K. The role of IP3 receptor channel clustering in Ca2+ wave propagation during oocyte maturation. Prog. Mol. Biol. Transl. Sci. 2014;123:83–101. doi: 10.1016/B978-0-12-397897-4.00006-1. [DOI] [PubMed] [Google Scholar]
- 73.Stith B.J., Woronoff K., Espinoza R., Smart T. sn-1,2-Diacylglycerol and choline increase after fertilization in Xenopus laevis. Mol. Biol. Cell. 1997;8:755–765. doi: 10.1091/mbc.8.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bates R.C., Fees C.P., Holland W.L., Winger C.C., Batbayar K., Ancar R., Bergren T., Petcoff D., Stith B.J. Activation of Src and release of intracellular calcium by phosphatidic acid during Xenopus laevis fertilization. Dev. Biol. 2014;386:165–180. doi: 10.1016/j.ydbio.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe N., Hunt T., Ikawa Y., Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991;352:247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- 76.Lorca T., Cruzalegui F.H., Fesquet D., Cavadore J.C., Méry J., Means A., Dorée M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- 77.Hansen D.V., Tung J.J., Jackson P.K. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc. Natl. Acad. Sci. USA. 2006;103:608–613. doi: 10.1073/pnas.0509549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rauh N.R., Schmidt A., Bormann J., Nigg E.A., Mayer T.U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt A., Rauh N.R., Nigg E.A., Mayer T.U. Cytostatic factor: An activity that puts the cell cycle on hold. J. Cell Sci. 2006;119:1213–1218. doi: 10.1242/jcs.02919. [DOI] [PubMed] [Google Scholar]
- 80.Wu J.Q., Kornbluth S. Across the meiotic divide-CSF activity in the post-Emi2/XErp1 era. J. Cell Sci. 2008;121:3509–3514. doi: 10.1242/jcs.036855. [DOI] [PubMed] [Google Scholar]
- 81.Liu J., Maller J.L. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 82.Nishiyama A., Tachibana K., Igarashi Y., Yasuda H., Tanahashi N., Tanaka K., Ohsumi K., Kishimoto T. A nonproteolytic function of the proteasome is required for the dissociation of Cdc2 and cyclin B at the end of M phase. Genes Dev. 2000;14:2344–2357. doi: 10.1101/gad.823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hutchins J.R., Dikovskaya D., Clarke P.R. Regulation of Cdc2/cyclin B activation in Xenopus egg extracts via inhibitory phosphorylation of Cdc25C phosphatase by Ca2+/calmodulin-dependent protein [corrected] kinase II. Mol. Biol. Cell. 2003;14:4003–4014. doi: 10.1091/mbc.E03-02-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mochida S., Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- 85.Nishiyama T., Yoshizaki N., Kishimoto T., Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;449:341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]
- 86.Chung E., Chen R.H. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- 87.Hunt T. On the regulation of protein phosphatase 2A and its role in controlling entry into and exit from mitosis. Adv. Biol. Regul. 2013;53:173–178. doi: 10.1016/j.jbior.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 88.Nishizawa M., Furuno N., Okazaki K., Tanaka H., Ogawa Y., Sagata N. Degradation of Mos by the N-terminal proline (Pro2)-dependent ubiquitin pathway on fertilization of Xenopus eggs: Possible significance of natural selection for Pro2 in Mos. EMBO J. 1993;12:4021–4027. doi: 10.1002/j.1460-2075.1993.tb06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paillard L., Omilli F., Legagneux V., Bassez T., Maniey D., Osborne H.B. EDEN and EDEN-BP, a cis element and an associated factor that mediate sequence-specific mRNA deadenylation in Xenopus embryos. EMBO J. 1998;17:278–287. doi: 10.1093/emboj/17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ueno S., Sagata N. Requirement for both EDEN and AUUUA motifs in translational arrest of Mos mRNA upon fertilization of Xenopus eggs. Dev. Biol. 2002;250:156–167. doi: 10.1006/dbio.2002.0787. [DOI] [PubMed] [Google Scholar]
- 91.Watanabe N., Vande Woude G.F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989;342:505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- 92.Lorca T., Galas S., Fesquet D., Devault A., Cavadore J.C., Dorée M. Degradation of the proto-oncogene product p39mos is not necessary for cyclin proteolysis and exit from meiotic metaphase: Requirement for a Ca2+-calmodulin dependent event. EMBO J. 1991;10:2087–2093. doi: 10.1002/j.1460-2075.1991.tb07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levasseur M., Dumollard R., Chambon J.P., Hebras C., Sinclair M., Whitaker M., McDougall A. Release from meiotic arrest in ascidian eggs requires the activity of two phosphatases but not CaMKII. Development. 2013;140:4583–4593. doi: 10.1242/dev.096578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madgwick S., Levasseur M., Jones K.T. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J. Cell Sci. 2005;118:3849–3859. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki T., Suzuki E., Yoshida N., Kubo A., Li H., Okuda E., Amanai M., Perry A.C. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development. 2010;137:3281–3291. doi: 10.1242/dev.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kline D. Calcium-dependent events at fertilization of the frog egg: Injection of a calcium buffer blocks ion channel opening, exocytosis, and formation of pronuclei. Dev. Biol. 1988;126:346–361. doi: 10.1016/0012-1606(88)90145-5. [DOI] [PubMed] [Google Scholar]
- 97.Grandin N., Charbonneau M. The increase in intracellular pH associated with Xenopus egg activation is a Ca2+-dependent wave. J. Cell Sci. 1992;101:55–67. doi: 10.1242/jcs.101.1.55. [DOI] [PubMed] [Google Scholar]
- 98.Bement W.M., Capco D.G. Protein kinase C acts downstream of calcium at entry into the first mitotic interphase of Xenopus laevis. Cell Regul. 1990;1:315–326. doi: 10.1091/mbc.1.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stith B.J., Espinoza R., Roberts D., Smart T. Sperm increase inositol 1,4,5-trisphosphate mass in Xenopus laevis eggs preinjected with calcium buffers or heparin. Dev. Biol. 1994;165:206–215. doi: 10.1006/dbio.1994.1247. [DOI] [PubMed] [Google Scholar]
- 100.Holloway S.L., Glotzer M., King R.W., Murray A.W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-V. [DOI] [PubMed] [Google Scholar]
- 101.Morin N., Abrieu A., Lorca T., Martin F., Dorée M. The proteolysis-dependent metaphase to anaphase transition: Calcium/calmodulin-dependent protein kinase II mediates onset of anaphase in extracts prepared from unfertilized Xenopus eggs. EMBO J. 1994;13:4343–4352. doi: 10.1002/j.1460-2075.1994.tb06754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larabell C.A., Rowning B.A., Moon R.T. A PKC wave follows the calcium wave after activation of Xenopus eggs. Differentiation. 2004;72:41–47. doi: 10.1111/j.1432-0436.2004.07201005.x. [DOI] [PubMed] [Google Scholar]
- 103.Bement W.M., Capco D.G. Activators of protein kinase C trigger cortical granule exocytosis, cortical contraction, and cleavage furrow formation in Xenopus laevis oocytes and eggs. J. Cell Biol. 1989;108:885–892. doi: 10.1083/jcb.108.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Collas P., Thompson L., Fields A.P., Poccia D.L., Courvalin J.C. Protein kinase C-mediated interphase lamin B phosphorylation and solubilization. J. Biol. Chem. 1997;272:21274–21280. doi: 10.1074/jbc.272.34.21274. [DOI] [PubMed] [Google Scholar]
- 105.Exton J.H. Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 106.Barish M.E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Glahn D., Nuccitelli R. Voltage-clamp study of the activation currents and fast block to polyspermy in the egg of Xenopus laevis. Dev. Growth Differ. 2003;45:187–197. doi: 10.1034/j.1600-0854.2004.00684.x. [DOI] [PubMed] [Google Scholar]
- 108.Grandin N., Charbonneau M. Intracellular pH and intracellular free calcium responses to protein kinase C activators and inhibitors in Xenopus eggs. Development. 1991;112:461–470. doi: 10.1242/dev.112.2.461. [DOI] [PubMed] [Google Scholar]
- 109.Rankin S., Kirschner M.W. The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr. Biol. 1997;7:451–454. doi: 10.1016/S0960-9822(06)00192-8. [DOI] [PubMed] [Google Scholar]
- 110.Pérez-Mongiovi D., Chang P., Houliston E. A propagated wave of MPF activation accompanies surface contraction waves at first mitosis in Xenopus. J. Cell Sci. 1998;111:385–393. doi: 10.1242/jcs.111.3.385. [DOI] [PubMed] [Google Scholar]
- 111.Pérez-Mongiovi D., Beckhelling C., Chang P., Ford C.C., Houliston E. Nuclei and microtubule asters stimulate maturation/M phase promoting factor (MPF) activation in Xenopus eggs and egg cytoplasmic extracts. J. Cell Biol. 2000;150:963–974. doi: 10.1083/jcb.150.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marrari Y., Clarke E.J., Rouvière C., Houliston E. Analysis of microtubule movement on isolated Xenopus egg cortices provides evidence that the cortical rotation involves dynein as well as Kinesin Related Proteins and is regulated by local microtubule polymerisation. Dev. Biol. 2003;257:55–70. doi: 10.1016/S0012-1606(03)00057-5. [DOI] [PubMed] [Google Scholar]
- 113.Shiina N., Moriguchi T., Ohta K., Gotoh Y., Nishida E. Regulation of a major microtubule-associated protein by MPF and MAP kinase. EMBO J. 1992;11:3977–3984. doi: 10.1002/j.1460-2075.1992.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Andersen S.S., Buendia B., Domínguez J.E., Sawyer A., Karsenti E. Effect on microtubule dynamics of XMAP230, a microtubule-associated protein present in Xenopus laevis eggs and dividing cells. J. Cell Biol. 1994;127:1289–1299. doi: 10.1083/jcb.127.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vasquez R.J., Gard D.L., Cassimeris L. Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil. Cytoskelet. 1999;43:310–321. doi: 10.1002/(SICI)1097-0169(1999)43:4<310::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 116.Lorca T., Castro A., Martinez A.M., Vigneron S., Morin N., Sigrist S., Lehner C., Dorée M., Labbé J.C. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 1998;17:3565–3575. doi: 10.1093/emboj/17.13.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peter M., Castro A., Lorca T., Le Peuch C., Magnaghi-Jaulin L., Dorée M., Labbé J.C. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat. Cell Biol. 2001;3:83–87. doi: 10.1038/35050607. [DOI] [PubMed] [Google Scholar]
- 118.Ohta Y., Ohba T., Miyamoto E. Ca2+/calmodulin-dependent protein kinase II: Localization in the interphase nucleus and the mitotic apparatus of mammalian cells. Proc. Natl. Acad. Sci. USA. 1990;87:5341–5345. doi: 10.1073/pnas.87.14.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zou H., McGarry T.J., Bernal T., Kirschner M.W. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 120.Stemmann O., Zou H., Gerber S.A., Gygi S.P., Kirschner M.W. Dual inhibition of sister chromatid separation at metaphase. Cell. 2001;107:715–726. doi: 10.1016/S0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- 121.Waizenegger I.C., Hauf S., Meinke A., Peters J.M. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399–410. doi: 10.1016/S0092-8674(00)00132-X. [DOI] [PubMed] [Google Scholar]