Abstract
Purpose of review
Obstructive sleep apnea (OSA) and hypertension are highly prevalent and treatable conditions that often coexist and both contribute to increased cardiovascular risk. The ability of continuous positive airway pressure (CPAP) to improve blood pressure in hypertensive patients with OSA is debated. This review highlights findings from recent studies that have investigated the impact of CPAP on blood pressure in patients with OSA.
Recent findings
Comparing the results of various studies is complicated by important methodological differences among them. In hypertensive patients with OSA, treatment with CPAP improves blood pressure to a smaller degree than that derived from antihypertensive medication. Patients with more severe OSA and with greater adherence to CPAP are likely to gain the most benefit from the therapy.
Summary
CPAP should be used in combination with antihypertensive medications in hypertensive patients with OSA. CPAP has additional benefits of restoring nocturnal dipping and improving arterial stiffness, thus potentially influencing cardiovascular morbidity in these high-risk patients.
Keywords: sleep apnea, blood pressure, hypertension, continuous positive airway pressure
Introduction
Obstructive sleep apnea (OSA) is common among patients with hypertension and especially resistant hypertension, yet many remain undiagnosed [1, 2]. OSA is characterized by intermittent collapse of the upper airway during sleep, resulting in repeated episodes of hypopnea or apnea. These episodes can be prevented with the use of continuous positive airway pressure (CPAP) during sleep. OSA represents a major public health burden due to an increasing prevalence of the disorder [3] and an increased risk of hypertension [4], depression [5], motor vehicle accidents [6], cardiovascular (CV) events [7, 8], and all-cause mortality [9] associated with OSA. In this review, we describe the mechanisms for hypertension in OSA and the impact of OSA on cardiovascular disease (CVD) as well as discuss salient findings from recent studies that have examined the effect of CPAP on blood pressure (BP).
Epidemiology and Clinical Features
OSA is estimated to affect 2-4% of the general adult population, typically occurring in middle-aged, overweight men [10]. Symptoms of OSA may be subtle, such as fatigue or problems with memory and concentration. OSA should be suspected in an obese patient who snores as snoring is almost universal among patients with OSA, but only half of patients who snore for the majority of the night have OSA [11]. A detailed sleep history provided by the patient should be supplemented with information from the patient's sleep partner. Additional clinical characteristics that may be indicative of OSA include headaches, excessive daytime sleepiness, and apnea or choking spells during sleep. While any of these signs or symptoms may be clues of OSA, definitive diagnosis requires nocturnal polysomnography, which also grades the severity of OSA with an apnea-hypopnea index (AHI).
Mechanisms of hypertension and CV morbidity
There is an overwhelming body of evidence establishing a clear link between hypertension and OSA [4, 12-14]. Approximately one-third to one-half of patients with hypertension have OSA [1, 2], which represents a greater proportion than that of normotensive controls and is independent of body weight [1]. However, among hypertensive patients who subsequently undergo polysomnography testing as part of a study protocol, many have evidence of OSA that was previously undiagnosed [1, 15, 16]. Although causal inference is limited in cross-sectional studies, a causal link between OSA and hypertension is supported by the “doseresponse” relationship such that the severity of OSA is independently associated with the prevalence and degree of hypertension [4, 13, 14], and the prevalence of OSA in resistant hypertension may exceed 80% [15, 16]. This link is strengthened by the temporal relationship between the two entities that is observed in longitudinal studies. Patients with mild OSA have a two-fold increased odds of subsequently developing hypertension compared to those with AHI of 0 [17]. In fact, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure lists OSA as an identifiable cause of hypertension [18]. However, the potential effect of OSA on BP is relatively small and dependent on OSA severity. For a patient with AHI of 15, BP is predicted to be 3.6/1.8 mmHg higher than a patient with the same body mass index (BMI) and AHI of 0 [14].
As shown in Figure 1, there are numerous proposed mechanisms for the development of hypertension in OSA including increased sympathetic nervous system activity [19], inflammation [20], oxidative stress [21], endothelial dysfunction [22], vascular stiffness [23], hyperaldosteronism [24], and volume expansion [25, 26]. In addition, some of these mechanisms may be partially mediated or potentiated by obesity [27].
Figure 1. Proposed mechanisms contributing to blood pressure elevation in OSA.
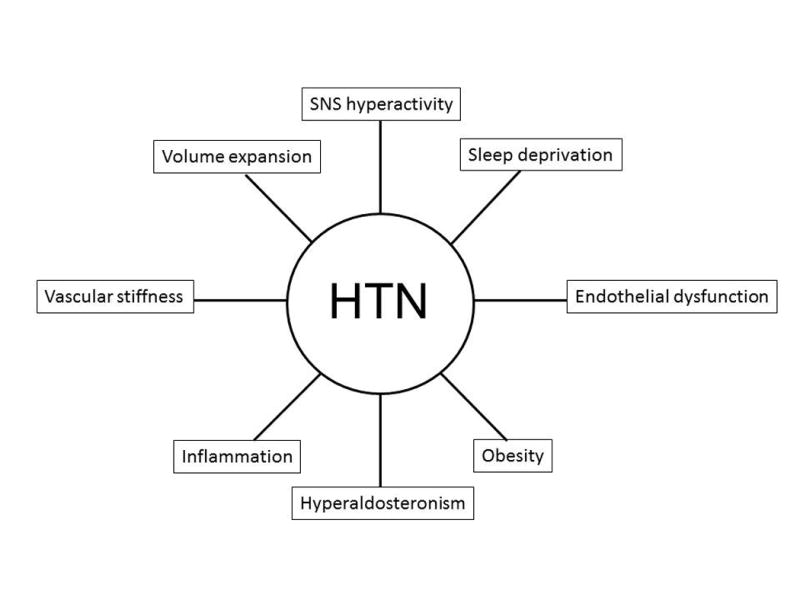
Abbreviations: HTN, hypertension; SNS, sympathetic nervous system.
Despite OSA frequently occurring in combination with traditional CV risk factors, untreated severe OSA represents an independent risk factor for CV events [7, 8]. Twenty-four hour ambulatory BP monitoring of patients with OSA commonly demonstrates the absence of nocturnal dipping [28], which is an independent CV risk factor [29]. Also, sleep-related breathing disorders are associated with both a trial and ventricular arrhythmias [30], likely mediating an increased risk of sudden cardiac death observed in these patients [31]. Finally, OSA confers a 6-fold increased risk of all-cause mortality, independent of traditional CV risk factors [9]. These risks underscore the importance of identifying and treating patients with OSA.
Treatment
Given the suboptimal control rates of patients with hypertension [32], considerable CV morbidity and mortality associated with uncontrolled hypertension [33, 34], and an extremely high prevalence of OSA in those with resistant hypertension, the identification and treatment of patients with OSA represents a critical aspect of hypertension management. Lifestyle modifications, including dietary sodium restriction, weight loss, aerobic exercise, and limiting alcohol intake, should be recommended to all hypertensive patients with OSA. Diuretics seem to be a logical agent of choice to ameliorate fluid redistribution during sleep [35■]; and a randomized trial comparing diuretics to calcium channel blocker in hypertensive patients with OSA is planned [36].
CPAP represents an OSA-targeted therapy to potentially reverse the pathophysiologic mechanisms responsible for hypertension. The acute and chronic effects of CPAP on sleep characteristics and BP were demonstrated in an uncontrolled, single-arm study of 11 patients with resistant hypertension and OSA [37]. Aside from significantly reducing AHI, 2 months of CPAP significantly reduced 24-hour BP by 10.5/5.7 mmHg (P<0.05) in the absence of medication changes or weight loss. This small case series provided proof of concept and laid important groundwork for future studies.
Numerous randomized controlled trials (RCTs) have demonstrated BP improvement with the use of CPAP [38-40], but this benefit has not been universal [41-43]. In 2007, a meta-analysis was published that pooled results from 12 RCTs conducted in patients with OSA [44]. Investigators reported a 1.69 mmHg incremental improvement in 24-hour mean BP attributed to CPAP. However, many of these studies were limited by short follow-up and small sample sizes, and the percentage of hypertensive patients that comprised the study cohorts ranged from 0-100%. Other potential explanations for conflicting results include differences in study design (e.g. how investigators handled patients with sub-optimal adherence, which patients served as controls), study populations (e.g. baseline BP, OSA severity), number of hours of CPAP use, co-interventions (e.g. use of antihypertensive medications), or outcome measures (e.g. ambulatory or office BP). A subsequent meta-analysis was published in 2012 [45], which pooled 28 RCTs (including 11 of the 12 trials included in the aforementioned study). This updated meta-analysis was also hampered by study heterogeneity. Nevertheless, investigators reported a statistically significant BP reduction with CPAP with weighted mean reductions in daytime and nocturnal BP of 2.58/2.01 mmHg and 4.09/1.85 mmHg, respectively. Since the latter meta-analysis was published, additional studies have been published and are described below.
Martínez-García et al. [46■■] recently conducted a large, multicenter RCT among 194 patients in Spain with resistant hypertension and moderate to severe OSA. All antihypertensive medications were continued at their entry dosages; and after 12 weeks, those randomized to CPAP demonstrated a 3 mmHg greater reduction in 24-hour mean BP compared to those in the control group (P=0.02). CPAP had the additional benefit of restoring nocturnal dipping. The CPAP group had a significantly greater percentage of dippers at the end of the study (35.9% CPAP vs. 21.6% control; adjusted odds ratio 2.4; P=0.02); and CPAP reduced the percentage of risers (i.e. patients whose BP increases during sleep—a group at particular high risk for CV events [29]) compared to baseline, while the percentage of risers remained unchanged in the control group. The number of hours of CPAP use was correlated with the degree of BP improvement, which explains why the above differences were further amplified when CPAP adherence was considered in a per-protocol analysis.
Pedrosa et al. [47■■] conducted a similar but longer RCT to evaluate the effect of CPAP on BP control among patients in Brazil with resistant hypertension and moderate to severe OSA. Patients were randomized to 6 months of CPAP plus medical therapy vs. the control arm of medical therapy alone, and medications were not adjusted during the trial. Of 40 patients who underwent randomization, 35 were followed for the full 6 months. As opposed to a BP rise in the control group, patients in the CPAP group demonstrated a daytime BP reduction of 6.5/4.5 mmHg during the study, while nocturnal BP increased slightly in both groups. Remarkably, this daytime BP reduction occurred despite higher baseline BMI and waist circumference in the intervention arm, and BMI did not change in either group during the course of the study. The lack of a beneficial effect of CPAP on nocturnal BP is both counterintuitive and contrary to findings from most other studies. Nevertheless, the studies by Martínez-García et al. and Pedrosa et al. support the use of CPAP even in patients with resistant hypertension and moderate-severe OSA.
In a prospective observational study that reinforced the BP-lowering effect of CPAP, Kartali et al. [48■■] recruited 38 hypertensive patients with severe OSA to investigate the effect of CPAP on arterial stiffness, which is associated with OSA [23] and represents an independent CV risk factor [49]. Patients in the study were relatively free of other comorbidities, were not receiving antihypertensive medication, and were adherent to CPAP (≥5 hours per night of use). After 3 months of CPAP, 24-hour mean BP was significantly lowered from 141.5/87.8 to 133.5/83.0 mmHg without antihypertensive medication. Arterial stiffness was measured by pulse wave velocity (PWV) at baseline and at 3 months in patients with OSA and in normotensive controls without OSA. At baseline, PWV was higher in those with OSA than in controls (8.8 vs 7.2 m/s, P=0.003). However, at 3 months, PWV was lowered from 8.8 to 7.4 m/s in the CPAP group, such that PWV did not significantly differ between groups. In the absence of a suitable comparator group, it is unclear whether the improvement in PWV was derived from BP reduction, vascular effects of OSA and its treatment, or a combination of these. Supporting a BP-independent mechanism are that reduction in BP was not correlated with reduction in PWV in this study and that a prior study demonstrated that CPAP reduced PWV without affecting BP [50]. As the study by Kartali et al. was not an RCT and generalizability may be limited by the unique study cohort, its findings should be considered hypothesis-generating, and additional studies are needed to fully appreciate the effects of CPAP on arterial stiffness. A recent double-blind RCT was performed in 43 patients with OSA and compared CPAP to sham CPAP [51]. Though CPAP had no effect on PWV or endothelial function, participants in the trial were predominantly normotensive, and CPAP was used for a mean of only 3 hours nightly. Thus, these results are likely not generalizable to hypertensive patients with OSA as the latter patients would presumably gain more benefit and show greater change in vascular parameters from CPAP than their normotensive counterparts; and in fact, CPAP improved endothelial function and measures of vascular stiffness in prior studies that included patients with more severe OSA or hypertension [23, 52, 53]. These studies highlight the potential direct role that OSA exerts on vascular structure and function in promoting hypertension and increased CV risk as well as the amelioration of these effects with CPAP.
Another study that deserves mention is a recent prospective observational study of 91 hypertensive patients with moderate-severe OSA, which failed to demonstrate an incremental improvement in BP when CPAP-treated patients were compared to controls [54■]. Patients who were offered but refused CPAP were allocated, not randomized, to the control group—a common method utilized in similar observational studies. After a mean follow-up of 3.1 years, both groups had a marked and statistically significant reduction in BP compared to baseline; but BP reduction did not differ between groups. BP decreased from 145/95 to 133/85 mmHg in the control group and from 148/96 to 133/84 mmHg in the CPAP group. CPAP did not reduce the number of antihypertensive medications used; and on the contrary, there was a small but statistically significant increase in the number of antihypertensive medications used that did not differ between groups. Medication doses were also increased during the follow-up period; but again, there was no difference between groups. Though patients tended to be adherent to CPAP with mean use of 5.9 hours nightly, adherence was assessed by patient self-report, which tends to be an overestimate [55]. Though this long-term study failed to show additional benefit of CPAP in lowering BP beyond the use of antihypertensive medications, the remarkable BP reduction in the control group warrants further attention. This BP improvement of 12/10 mmHg in the control group greatly exceeds that seen in other studies and occurred in the absence of weight loss or clinically meaningful increase in the number of antihypertensive medications.
A common theme of studies in patients with OSA is that many either refuse CPAP or have suboptimal adherence, thus limiting clinical effectiveness. An alternative to CPAP is a mandibular advancement device (MAD), which is designed to reposition the tongue and/or lower jaw to increase the airway lumen. The efficacy of MAD was tested in a short-term randomized crossover trial that compared the two therapies, each being used for 1 month, in a group of Australian patients with OSA [56■■]. Patients preferred and were more adherent to MAD, but CPAP-treated patients had greater improvement in AHI. In the 42% of patients who were hypertensive at baseline, there was a statistically significant reduction from baseline in 24-hour BP of ∼3/2 mmHg that did not differ between treatment groups. This study demonstrated the non-inferiority of MAD in terms of BP change as well as certain advantages of this therapy. While potentially promising, studies of longer duration with larger cohorts of hypertensive patients are necessary to fully understand the role of MAD in the treatment of OSA.
In summary, the BP reduction seen with CPAP is modest, necessitating its use adjunctively with antihypertensive medications. Baseline OSA severity, baseline BP, and duration of CPAP use are likely important contributing factors that influence the therapy's clinical effectiveness such that a more robust BP response to CPAP is expected in adherent patients with more severe hypertension. Given that mean CPAP use in clinical trials tends to be only 4-5 hours nightly, leaving a considerable portion of sleep untreated, we believe the full impact of CPAP may be underappreciated; and the lack of substantial BP reduction with CPAP should not temper enthusiasm for its use as there are many potential benefits to CPAP (see Table 1). Most importantly, multiple studies demonstrate a reduction of CV risk with CPAP use [7, 62].
Table 1. The potential effects of CPAP.
Conclusion
OSA has a strong link with hypertension and likely has a causal influence on BP. Treatment with CPAP reverses some of the pathophysiologic mechanisms responsible for hypertension and has a modest effect on lowering BP. While a small change in BP may be relatively unimpressive at the individual patient level, this change could have substantial implications for health outcomes at the population level. CPAP has additional advantages of restoring nocturnal dipping, improving arterial stiffness, and reducing CV risk. As patients with OSA report decreased quality of life and are at increased risk of CV events and death, an OSA targeted therapy with CPAP should be an integral part of treatment to reduce the morbidity burden associated with OSA.
Key Points.
The overall BP response to treatment with CPAP is modest.
The more severe the hypertension and OSA, the more robust the BP response to CPAP can be expected.
The potential of CPAP to improve arterial stiffness and restore normal dipping patterns may have far-reaching consequences on reduction of CV risk.
Acknowledgments
This work was supported by the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health award F32DK098914 [MD].
Grant support: National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health (NIDDK/NIH) award F32DK098914 [MD]
Footnotes
Disclosures: The authors report no conflicts of interest.
References
- 1.Fletcher EC, DeBehnke RD, Lovoi MS, Gorin AB. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med. 1985;103:190–195. doi: 10.7326/0003-4819-103-2-190. [DOI] [PubMed] [Google Scholar]
- 2.Drager LF, Genta PR, Pedrosa RP, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 doi: 10.1093/aje/kws342. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 6.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–956. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 7.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 8.Yumino D, Tsurumi Y, Takagi A, et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99:26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 10.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 11.Ferini-Strambi L, Zucconi M, Castronovo V, et al. Snoring & sleep apnea: a population study in Italian women. Sleep. 1999;22:859–864. [PubMed] [Google Scholar]
- 12.Worsnop CJ, Naughton MT, Barter CE, et al. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med. 1998;157:111–115. doi: 10.1164/ajrccm.157.1.9609063. [DOI] [PubMed] [Google Scholar]
- 13.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 14.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 15.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, et al. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–459. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 16.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Narkiewicz K, van de Borne PJ, Cooley RL, et al. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 20.Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9:1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 22.Carlson JT, Rångemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens. 1996;14:577–584. doi: 10.1097/00004872-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Buchner NJ, Quack I, Stegbauer J, et al. Treatment of obstructive sleep apnea reduces arterial stiffness. Sleep Breath. 2012;16:123–133. doi: 10.1007/s11325-010-0465-x. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125:112–117. doi: 10.1378/chest.125.1.112. [DOI] [PubMed] [Google Scholar]
- 25.Kita H, Ohi M, Chin K, et al. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7:199–207. doi: 10.1046/j.1365-2869.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 26.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 27.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 28.Ancoli-Israel S, Stepnowsky C, Dimsdale J, et al. The effect of race and sleep-disordered breathing on nocturnal BP "dipping": analysis in an older population. Chest. 2002;122:1148–1155. doi: 10.1378/chest.122.4.1148. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Dov IZ, Kark JD, Ben-Ishay D, et al. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49:1235–1241. doi: 10.1161/HYPERTENSIONAHA.107.087262. [DOI] [PubMed] [Google Scholar]
- 30.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 33.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 34.Lawes CM, Vander Hoorn S, Rodgers A, International Society of Hypertension Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 35■.Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32:673–680. doi: 10.1097/HJH.0000000000000047. This small, single-arm study demonstrated reductions in neck circumference, AHI, and BP with intensified diuretic therapy, indicating the role of fluid redistribution during sleep in the pathogenesis of OSA. [DOI] [PubMed] [Google Scholar]
- 36.Cichelero FT, Martinez D, Fuchs SC, et al. The effect of antihypertensive agents on sleep apnea: protocol for a randomized controlled trial. Trials. 2014;15:1. doi: 10.1186/1745-6215-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 38.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 39.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 40.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–845. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 41.Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134:1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 42.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 43.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129:1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 44.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 45.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–96. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46■■.Martínez-García MA, Capote F, Campos-Rodríguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415. doi: 10.1001/jama.2013.281250. This large, multicenter RCT in patients with resistant hypertension showed BP improvement and restoration of nocturnal dipping with CPAP. [DOI] [PubMed] [Google Scholar]
- 47■■.Pedrosa RP, Drager LF, de Paula LK, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487–1494. doi: 10.1378/chest.13-0085. This six-month-long RCT demonstrated that CPAP reduced only daytime BP. [DOI] [PubMed] [Google Scholar]
- 48■ ■.Kartali N, Daskalopoulou E, Geleris P, et al. The effect of continuous positive airway pressure therapy on blood pressure and arterial stiffness in hypertensive patients with obstructive sleep apnea. Sleep Breath. 2013 doi: 10.1007/s11325-013-0926-0. [Epub ahead of print]. CPAP reduced BP and vascular stiffness in this prospective cohort study. [DOI] [PubMed] [Google Scholar]
- 49.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 50.Kitahara Y, Hattori N, Yokoyama A, et al. Effect of CPAP on brachial-ankle pulse wave velocity in patients with OSAHS: an open-labelled study. Respir Med. 2006;100:2160–2169. doi: 10.1016/j.rmed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Jones A, Vennelle M, Connell M, et al. Arterial stiffness and endothelial function in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med. 2013;14:428–432. doi: 10.1016/j.sleep.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Cross MD, Mills NL, Al-Abri M, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomised controlled trial. Thorax. 2008;63:578–583. doi: 10.1136/thx.2007.081877. [DOI] [PubMed] [Google Scholar]
- 53.Drager LF, Bortolotto LA, Figueiredo AC, et al. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 54■.Kasiakogias A, Tsioufis C, Thomopoulos C, et al. Effects of continuous positive airway pressure on blood pressure in hypertensive patients with obstructive sleep apnea: a 3-year follow-up. J Hypertens. 2013;31:352–360. doi: 10.1097/HJH.0b013e32835bdcda. A prospective observational study that reported substantial BP reductions in both CPAP-treated and control groups that did not differ between groups. [DOI] [PubMed] [Google Scholar]
- 55.Rauscher H, Formanek D, Popp W, Zwick H. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest. 1993;103:1675–1680. doi: 10.1378/chest.103.6.1675. [DOI] [PubMed] [Google Scholar]
- 56■■.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–887. doi: 10.1164/rccm.201212-2223OC. This RCT demonstrated the efficacy of MAD, a therapy that patients preferred over CPAP. [DOI] [PubMed] [Google Scholar]
- 57.Siccoli MM, Pepperell JC, Kohler M, et al. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31:1551–1558. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hedner J, Darpö B, Ejnell H, et al. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J. 1995;8:222–229. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 59.Xie X, Pan L, Ren D, et al. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med. 2013;14:1139–1150. doi: 10.1016/j.sleep.2013.07.006. Epub 2013 Aug 14. [DOI] [PubMed] [Google Scholar]
- 60.Saarelainen S, Hasan J, Siitonen S, Seppälä E. Effect of nasal CPAP treatment on plasma volume, aldosterone and 24-h blood pressure in obstructive sleep apnoea. J Sleep Res. 1996;5:181–185. doi: 10.1046/j.1365-2869.1996.t01-1-00007.x. [DOI] [PubMed] [Google Scholar]
- 61.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–681. doi: 10.1378/chest.11-0615. Epub 2011 Aug 11. [DOI] [PubMed] [Google Scholar]
- 62.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 63.Kohler M, Craig S, Pepperell JC, et al. CPAP improves endothelial function in patients with minimally symptomatic OSA: results from a subset study of the MOSAIC trial. Chest. 2013;144:896–902. doi: 10.1378/chest.13-0179. [DOI] [PubMed] [Google Scholar]


