Abstract
Polybrominated diphenyl ethers (PBDEs) are flame retardants that have been widely used in manufacturing. They are major household and environmental contaminants that bioaccumulate. Humans are exposed primarily through dust inhalation and dietary ingestion of animal products. In animal studies, high doses of penta-brominated diphenyl ethers (penta-BDEs) in the mg/kg body weight (BW) range negatively impact brain development, behavior, memory, circulating thyroid hormone concentrations, the reproductive system and bone development. We investigated the effects of ingestion of a relatively low dose of the penta-BDE mixture DE-71 by pregnant and lactating rats on reproductive and thyroid parameters of the F1 offspring. F0 mothers received 60 μg/kg BW of DE-71 or vehicle daily by gavage from Day 1.5 of pregnancy through lactation (except the day of parturition). F1 pups were sacrificed at 21 d of age or outbred at approximately 80 d of age. Bred F1 females were sacrificed at Day 14.5 of pregnancy or at five months of age. Bred F1 males were sacrificed at five months of age. DE-71 treatment of the mothers affected the F1 females as evidenced by lower body weights at 80 d and five months of age, elevated serum T3 and T4 concentrations at Day 14.5 of pregnancy and increased thyroid gland weight and ovarian osteopontin mRNA at five months of age. Perinatal DE-71 exposure also increased testicular osteopontin mRNA in 21-day-old F1 males. Utilizing a granulosa cell in vitro model, we demonstrated that DE-71 activated the rat osteopontin gene promoter. Our results are the first to demonstrate that PBDEs increase rodent circulating T3 and T4 concentrations and gonadal osteopontin mRNA, and activate the osteopontin gene promoter. These changes may have clinical implications as others have shown associations between human exposure to PBDEs and subclinical hyperthyroidism, and overexpression of ovarian osteopontin has been associated with ovarian cancer.
Keywords: PBDE, DE-71, osteopontin, T3, T4, thyroid hormone, thyroid gland, ovary, testis
Introduction
Polybrominated diphenyl ethers (PBDEs) are synthetic compounds that have been highly utilized as flame retardants since the 1960s and are both household and environmental contaminants (see refs1–3 for reviews). They are found in plastics, paints, computers, televisions, electronics, furniture, bedding, carpets and other materials, and make up to 30% of the weight of some of these products. Because PBDEs are not covalently bound to polymers used in these products, they leach from their origin over time and disperse widely through air and run-off. They are persistent pollutants that remain in the environment for years without appreciable degradation. Human uptake of PBDEs is through inadvertent dust inhalation, absorption through skin and dietary ingestion of animal products such as fish, meat and dairy.4 Comparison of human serum samples collected in the USA in 1973 with others collected in 2003 showed an approximate 80- to 90-fold increase in serum PBDE concentrations during the 30-year period.5
In humans, specific congeners of PBDEs have been measured in breast milk, adipose tissue, placenta, brain and serum.1 Bromination of diphenyl ethers can result theoretically in 209 possible congeners of PBDEs based on the number and position of bromine groups (Figure 1) that are divided into 10 congener groups ranging from mono- to deca-BDE.3 Commercially available mixtures of PBDEs include penta-BDEs that contain predominantly the less brominated congeners, and octa- and deca-BDEs that contain predominantly the congeners with higher numbers of bromine atoms. The less brominated PBDE congeners are lighter and more common in the atmosphere than the more heavily brominated congeners.1 The less brominated congeners are also lipid soluble. They bioaccumulate in fat, increase in concentration as they move up the food chain, are resistant to metabolism and are potential toxins.1,3
Figure 1.
Generic structure of PBDEs. Bromines can be added to the 2–6 and 2′–6′ positions. Note the similarity to the structure of thyroxine
Penta-BDE mixtures (e.g. DE-71) contain the predominant congeners BDE-47, -99, -100, -153 and -154. The daily dietary intake of these five congeners in humans in a Swedish study was predicted to be approximately 51 ng/d for adults and 110 ng/d for breast-fed infants.3,6 In another study using Nordic data, daily dietary intake of PBDEs was estimated to be approximately 200–700 ng/d.3 These values are in reasonable agreement with those calculated for dietary intake in the US population.4 A study performed in the Washington, DC area calculated a daily PBDE intake by inadvertent dust ingestion of 120–6000 ng/d for young children (ages 1–4).7 In addition, measurable levels of PBDEs have also been found in human fetuses, demonstrating the ability of PBDEs to pass from the mother to the developing child in utero.8
Growing concern over PBDEs has resulted from animal studies showing negative effects of specific congeners on neural development, behavior and memory, reproductive capacity, thyroid hormone function and bone development.3 The structure of PBDEs shares similarities with 3,5,3′-triiodothyronine (T3) and thyroxine (T4) (Figure 1), and specific PBDE congeners bind thyroid hormone receptors and act as receptor agonists or antagonists.1 Other PBDE congeners act as estrogen receptor (ER) agonists9 or androgen receptor antagonists.10 The mixture DE-71 has been shown to exert estrogenic effects.11 Thus, PBDEs may work as endocrine disrupters through a variety of mechanisms.
It is of importance to conduct animal studies to evaluate the health effects of ingestion of PBDE mixtures present in the environment at doses that might be relevant to human exposure. Few studies in which PBDEs have been administered to animals used low doses that even approach what is predicted for human ingestion. Most studies have been performed with high doses (≥1 mg/kg body weight [BW]) of individual PBDE congeners, and many of these have administered PBDEs by injection. The goal of our study was to test the hypothesis that a low dose of the PBDE mixture DE-71 (60 μg/kg BW) delivered by daily oral ingestion to the F0 mother during the sensitive developmental stages of pregnancy and lactation would affect reproductive endpoints, including gene regulation and thyroid function of F1 offspring.
Materials and methods
Animals
Outbred, 58- to 65-day-old male and female rats (CD®IGS) were purchased from Charles River Laboratories, Inc (Wilmington, MA, USA). They were housed in a room with controlled lighting (lights on 07:00–19:00 h daily) and temperature (20–22°C) and given Teklad Rodent Diet 8604 (Harlan, Madison, WI, USA) and tap water ad libitum. Polysulfone cages and glass water bottles were employed. Animals were maintained and used in accordance with the standards of the Institutional Animal Care and Use Committee of the University of South Carolina.
Treatments and tissue collection
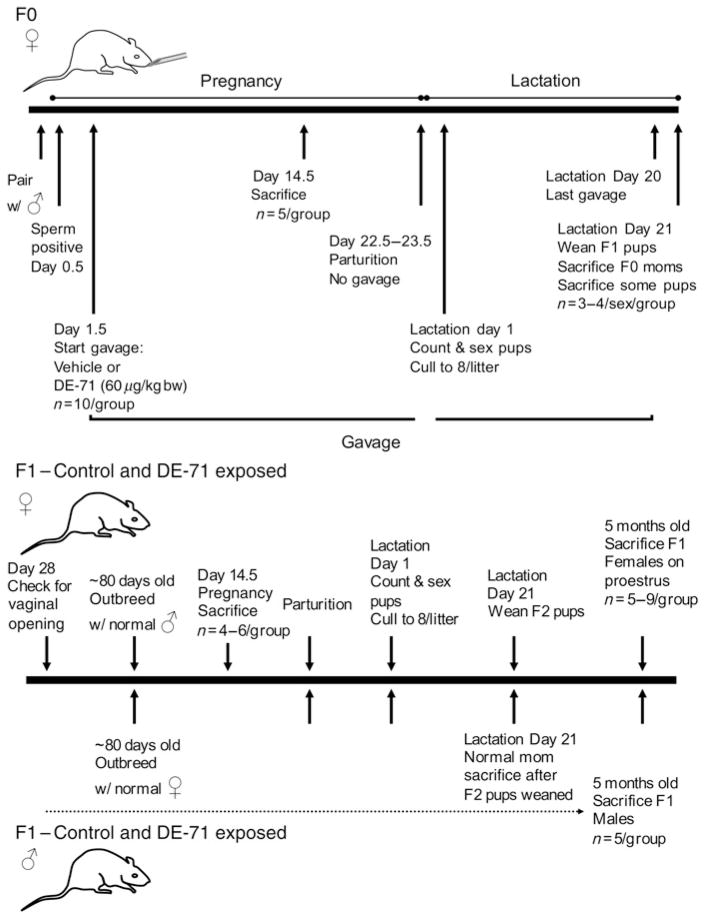
Rats were bred 14–17 d after arrival. Individual virgin female rats were placed in a cage containing a sexually inexperienced male rat at approximately 12:00 h. Vaginal lavage was performed between 09:00 and 11:00 h the following morning, and the presence of sperm as visualized by microscopic examination was designated Day 0.5 of pregnancy of the F0 generation. A total of 20 individually housed pregnant F0 rats were used. The study is diagrammed in Figure 2.
Figure 2.
The animal protocols used in this study. Only F0 mothers received control vehicle or DE-71 in vehicle by gavage during pregnancy and lactation
F0 generation pregnant rats received 60 μg/kg BW DE-71 (TBDE-71X, lot no. 05500F16P; Wellington Laboratories Inc, Guelph, Ontario, Canada) in corn oil vehicle (0.25 mL) or vehicle alone by gavage daily (1000–1200 h) from Day 1.5 of pregnancy through Day 13.5 of pregnancy (these rats were sacrificed on Day 14.5 of pregnancy) or through Day 20 of lactation (except the day of delivery, approximately Day 23 of pregnancy). The DE-71 was dissolved in toluene (HPLC grade, T290-1, lot no. 061535; Fisher Scientific, Suwanee, GA, USA; 100 μL per 100 mg DE-71) at room temperature for 15 min. Corn oil (Corn oil, pure, stripped, 405454000, lot A018245701; Acros Organics, Geel, Belgium) was then added to yield the desired final concentration. Corn oil was added to toluene not containing DE-71 for the control. The mixtures were stirred with magnetic stirring bars uncovered in a laminar flow hood to allow evaporation of the toluene. All solutions were made in glass containers. All solutions were protected from light.
Half (n = 5) of the pregnant F0 rats in each group were weighed and killed on Day 14.5 of pregnancy. These rats and all other rats in this study were sacrificed between 11:00 and 16:00 h. Because anesthetics and stress can alter serum hormone concentrations, we decapitated the animals rapidly without prior exposure to anesthetics or CO2. Serum from trunk blood was stored frozen at −70°C for subsequent radioimmunoassay of T3 and T4 concentrations. The uterus was examined and the numbers of fetuses and resorptions were recorded. Organs were collected and weighed.
The other half in each group delivered their young on approximately Day 22.5–23.5 as evidenced by the young in their cages at that time. On lactation Day 1, the litters were culled to eight pups for each dam with four of each sex when possible. On lactation Day 21, some of the F0 mothers (4 vehicle-treated and 3 DE-71-treated) and one F1 pup of each sex from each of these F0 mothers were killed by decapitation for the collection of trunk blood and organs. Tissue samples from the pups were snap frozen and stored at −70°C for subsequent RNA isolation. The remaining female F1 littermate rats from these seven mothers and the female F1 offspring of the two remaining DE-71-treated F0 mothers were examined daily for vaginal opening starting on Day 28 after birth. One of the vehicle-treated F0 mothers suffered throat damage due to gavage during lactation. This mother and her pups were not used in the postlactation studies.
Some of the F1 females and some of the male F1 rats in both control and experimental groups were bred at approximately 80 d of age with normal untreated, 70- to 80-day-old male and female rats, respectively. Some of the F1 pregnant animals (n = 4–6/group) were decapitated on Day 14.5 of pregnancy. Trunk blood was collected, the uterus was examined and organs were collected as described for F0 rats on Day 14.5 of pregnancy. Tissue samples were snap frozen and stored at −70°C for subsequent RNA isolation. The rest of the F1 pregnant rats and all of the normal pregnant rats that had been bred with F1 males delivered on approximately Day 22.5–23.5 of pregnancy. On lactation Day 1, the litters were culled to eight pups for each dam with four of each sex when possible. F1 females (n = 5–9/group) that had been bred with normal males and F1 males (n = 5/group) that had been bred with normal females were decapitated at five months of age for the collection of trunk blood, organs and tissues. Females were killed between 11:00 and 12:00 h on proestrus as determined by daily examination of vaginal smears with the light microscope. The F2 pups were not used.
Radioimmunoassay of T3 and T4
Solid-phase radioimmunoassays for total serum T3 and T4 concentrations were performed using Coat-A-Count kits (Diagnostic Products Corp., Los Angeles, CA, USA). In both assays, samples were assayed in duplicate and the results were averaged.
Immunohistochemistry
The right ovary of five-month-old F1 rats was fixed in paraformaldehyde and prepared for immunohistochemical staining with citrate unmasking as described previously.12 Tissue sections were stained for osteopontin utilizing a mouse monoclonal IgG1 primary antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA; OPN: sc-21742 diluted1:1200). Tissue sections were stained with normal mouse IgG (Santa Cruz) as a control. The secondary antibody was rabbit anti-mouse IgG1-HRP (Invitrogen, Carlbad, CA, USA; 61-0120).
Statistics
Animal and radioimmunoassay data for the control and DE-71 groups were compared by unpaired two-tailed t-tests.
Microarray screening
Total RNA was isolated using Trizol reagent (Invitrogen). The RNA from the left ovary from three five-month-old F1 rats and the left testis from three 21-day-old F1 animals were further purified using RNeasy Micro Kit (Qiagen, Valencia, CA, USA) and pooled for like treatment groups and microarray analysis was performed using Affymetrix GeneChip Rat Genome 230 2.0 Arrays. The microarray hybridizations were performed at the Medical University of South Carolina’s Proteogenomics facility (Charleston, SC, USA). Microarray files of this project can be accessed online at http://proteogenomics.musc.edu/ma/musc_madb.php?page=home&act=manage under the cRNA target record number _1175643444.646013. Hybridization signals were quantified and data analyzed for genes exhibiting more than a three-fold difference in expression between F1 tissues derived from control and DE-71 groups. Based on the findings that osteopontin mRNA was upregulated in the DE-71 group in ovaries (3.1-fold) and testes (5.7-fold), we performed the following realtime polymerase chain reaction (PCR) and osteopontin gene promoter amplification and transfection experiments.
Realtime PCR
Total RNA was isolated from the ovaries and testes of 21-day-old and five-month-old F1 animals of both sexes and F1 rats on Day 14.5 of pregnancy using Trizol reagent (Invitrogen). Primers for rat osteopontin (spp1) and glyceraldehyde-3-phosphate dehydrogenase (gapd) were purchased from SABiosciences (Frederick, MD, USA). Three micrograms of RNA were used to generate cDNA with Reaction Ready™ First Strand cDNA Synthesis Kit (SABiosciences). Realtime PCR was performed using RT2 SYBR® Green qPCR Master Mix and 20 ng cDNA in triplicate reactions. Initial experiments were performed for the primer sets to verify that a single product of the correct size was obtained from selected samples as well as the linear range of amplification for the primer sets. Serial dilutions of purified amplicons were used to generate a standard curve. The sample Ct values obtained were used to determine the starting amount of mRNA for each target. The ratio of the spp1 amount to the gapd amount in each sample was calculated. The mean of these ratios for each treatment group was calculated and used to determine the fold change between control and DE-71 animals. RNA expression data for the control and DE-71 groups were compared by unpaired two-tailed t-test.
Osteopontin promoter amplification and transfections
The primers to amplify an 1159 bp portion of the 5′-flanking region and part of exon 1 of the rat osteopontin gene were derived from Genbank sequence accession no. AF017274 and were upstream 5′ GGGGTACCAGCACACAGCGTCT CAACCTCAAGGTTGCAG 3′ and downstream 5′ GCGAAGCTTCGCAGGAGACTGCAAAGCCAAGGATGCTG 3′ with additional Kpn1 and Hind3 sites added at the 5′-primer ends (underlined), respectively. Genomic DNA was isolated from the liver of a control rat using the QIAamp DNA Mini Kit (Qiagen). The BD Advantage-GC Genomic PCR kit (BD Biosciences Clontech, Palo Alto, CA, USA) was used for amplification. The osteopontin amplicon was cloned into a pCRII plasmid (Invitrogen) and sequenced (University of Maine DNA sequencing facility). The Kpn1–Hind3 fragment was excised from the pCRII plasmid and inserted into the same sites of the firefly luciferase vector, paLuc described elsewhere.13 Abattoir ovaries from prepubertal gilts were purchased from Carolina Pride (Greenwood, SC, USA). Primary cultures of porcine granulosa cells were cultured and transfected as previously described13 using 0.95 μg osteopontin promoter luciferase construct (OPN-Luc), 0.05 μg ptkd238Luc, a modified renilla luficerase control plasmid,14 6 μL Lipofectamine (Invitrogen), in minimal essential medium (MEM) only for five hours. Post-transfection cells were incubated in complete medium (MEM + 3% fetal calf serum + antibiotics) for approximately 18 h prior to treatment in serum-free MEM containing antibiotics. Cells were subsequently treated with vehicles (ethanol, dimethyl sulfoxide [DMSO]), T3 (in ethanol) or DE-71 (in DMSO) for 6 and 24 h. Cells were lysed and luciferase values determined using the Dual Luciferase Kit (Promega Corp., Madison, WI, USA). Data were analyzed using repeated-measures analysis of varaince followed by Tukey’s test using GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Animal and organ weights
Oral intake of DE-71 (60 μg/kg BW) daily for 13 d from Day 1.5 to 13.5 of pregnancy had no significant effects on pregnancy as evidenced by weight gain of the mothers or number of fetuses or resorptions at Day 14.5 of pregnancy (Table 1). Treatment also was without effect on maternal adrenal gland and ovarian weights (Table 1). Continuation of treatment with DE-71 for an additional 9–10 d in the other half of the pregnant F0 rats had no effect on the length of pregnancy (pups were observed on the morning of Day 22.5 for 4 vehicle-treated and 3 DE-71-treated mothers and on the morning of Day 23.5 for 1 vehicle-treated and 2 DE-71-treated rats) or number of live pups (14.0 ± 2.0 versus 14.0 ± 1.1; mean ± SEM).
Table 1.
Effects of DE-71 administered by gavage daily to F0 pregnant rats from Day 1.5 to Day 13.5 of pregnancy on their body and organ weights and numbers of fetuses and resorptions on Day 14.5*
| Parameter | Control (n = 5) | DE-71 (n = 5) |
|---|---|---|
| BW (g) Day 14.5 | 300.4 ± 5.0 | 292.8 ± 9.4 |
| BW (g) gain Day 0–Day 14.5 | 59.2 ± 4.8 | 55.4 ± 5.4 |
| Left adrenal gland (mg) | 40.8 ± 2.3 | 38.9 ± 1.5 |
| Right adrenal gland (mg) | 40.7 ± 3.7 | 35.5 ± 2.1 |
| Paired adrenals/BW (mg/kg) | 271 ± 19 | 254 ± 7 |
| Left ovary (mg) | 62.4 ± 5.7 | 69.1 ± 4.7 |
| Right ovary (mg) | 61.3 ± 4.5 | 54.8 ± 12.4 |
| Paired ovaries/BW (mg/kg) | 411 ± 27 | 419 ± 34 |
| Number of fetuses | 16.2 ± 1.5 | 14.2 ± 2.1 |
| Number of resorptions | 0.8 ± 0.4 | 1 ± 0.4 |
BW, body weight
Values are means ± SEM
The administration of DE-71 to mothers during pregnancy and lactation had no statistically significant effect on female or male F1 offspring weight or their adrenal, gonadal, kidney or liver weights at weaning, as evidenced by study of one female and one male pup from each of four controls and three DE-71-treated mothers (Table 2). This prolonged treatment with DE-71 also had no effect on vaginal opening (control 32.5 ± 0.5 d [n = 11; 2–3 pups/4 moms] versus DE-71 32.8 ± 0.4 d [n = 17; 3–4 pups/5 moms]).
Table 2.
Effects of DE-71 administered by gavage during pregnancy and lactation to F0 pregnant rats on body and organ weights of female and male F1 offspring on Day 21 of lactation*
| Parameter | Control (n = 4) | DE-71 (n = 3) |
|---|---|---|
| Female | ||
| BW (g) | 59.8 ± 3.1 | 62.0 ± 1.2 |
| Paired adrenals/BW (mg/kg) | 291 ± 23 | 236 ± 51 |
| Paired ovaries/BW (mg/kg) | 313 ± 49 | 431 ± 60 |
| Left kidney/BW (mg/kg) | 6019 ± 98 | 6282 ± 238 |
| Right kidney/BW (mg/kg) | 6423 ± 210 | 6404 ± 197 |
| Whole liver/BW (mg/kg) | 37,906 ± 708 | 40,071 ± 2614 |
| Male | ||
| BW (g) | 63.0 ± 2.1 | 67.3 ± 2.3 |
| Paired adrenals/BW (mg/kg) | 267 ± 23 | 282 ± 13 |
| Paired testes/BW (mg/kg) | 4326 ± 328 | 4734 ± 160 |
| Left kidney/BW (mg/kg) | 5638 ± 194 | 5873 ± 136 |
| Right kidney/BW (mg/kg) | 5836 ± 241 | 6265 ± 195 |
| Whole liver/BW (mg/kg) | 37,543 ± 1996 | 40,814 ± 1605 |
BW, body weight
Values are means ± SEM
At approximately 80 d of age, some of the female F1 rats were bred with normal males and sacrificed at Day 14.5 of pregnancy (Table 3) or at five months of age (Table 4) after delivering F2 generation pups. F1 generation pregnant rats whose F0 mothers had been treated with DE-71 were significantly lighter at the onset of pregnancy (P < 0.05) than those whose mothers had been treated with vehicle (Table 3). Inclusion of the body weights at the start of pregnancy of the F1 rats sacrificed at five months of age supported this difference (293.6 ± 8.9 g for 9 controls versus 270.3 ± 5.5 g for 15 in the DE-71 group; P < 0.03). There was a trend for these mothers to gain less weight from Day 0 to Day 14.5 of pregnancy (Table 3; P = 0.08), which was associated with a trend to have fewer fetuses (P = 0.12). Absolute organ weights or organ weights normalized for body weight were not different between the two groups except for paired ovarian weights which were significantly heavier in the DE-71 group when normalized for body weight.
Table 3.
Effects of DE-71 administered by gavage to F0 mothers during pregnancy and lactation on body and organ weights and numbers of fetuses and resorptions on Day 14.5 of pregnancy of F1 generation offspring bred with normal males†
| Parameter | Control (n = 4) | DE-71 (n = 6) |
|---|---|---|
| BW (g) Day 0 | 292.7 ± 19.5 | 258.8 ± 3.1* |
| BW (g) Day 14.5 | 365.0 ± 21.4 | 317.0 ± 3.6* |
| BW (g) gain Day 0–Day 14.5 | 72.3 ± 4.9 | 58.2 ± 4.9 |
| Paired adrenals (mg) | 92.4 ± 10.4 | 71.4 ± 6.1 |
| Paired adrenals/BW (mg/kg) | 255 ± 27 | 225 ± 20 |
| Paired ovaries (mg) | 124.6 ± 3.7 | 119.3 ± 1.7 |
| Paired ovaries/BW (mg/kg) | 341.2 ± 2.5 | 376.4 ± 10** |
| Left kidney/BW (mg/kg) | 3307 ± 88 | 3484 ± 50 |
| Right kidney/BW (mg/kg) | 3399 ± 50 | 3450 ± 55 |
| Whole liver/BW (mg/kg) | 43,260 ± 862 | 42,809 ± 684 |
| Number of fetuses | 16.3 ± 0.9 | 12.7 ± 1.5 |
| Number of resorptions | 0.8 ± 0.3 | 0.3 ± 0.3 |
BW, body weight
Values are means ± SEM
P < 0.05,
P < 0.025 compared with control group
Table 4.
Effects of DE-71 administered by gavage to F0 mothers during pregnancy and lactation on body and organ weights in five-month-old, proestrous female F1 generation offspring bred previously with normal males†
| Parameter | Control (n = 5) | DE-71 (n = 9) |
|---|---|---|
| BW (g) | 365.2 ± 11.2 | 333.4 ± 8.3* |
| Paired adrenals (mg) | 81.6 ± 4.0 | 70.5 ± 2.2** |
| Paired adrenals/BW (mg/kg) | 225 ± 14 | 212 ± 8 |
| Paired ovaries (mg) | 95.7 ± 4.9 | 97.7 ± 4.5 |
| Paired ovaries/BW (mg/kg) | 263 ± 12 | 294 ± 14 |
| Left kidney (mg) | 1276 ± 60 | 1100 ± 40* |
| Right kidney (mg) | 1274 ± 54 | 1113 ± 40* |
| Left kidney/BW (mg/kg) | 3512 ± 217 | 3299 ± 90 |
| Right kidney/BW (mg/kg) | 3507 ± 204 | 3338 ± 83 |
| Whole liver (mg) | 13,314 ± 323 | 11,933 ± 436* |
| Whole liver/BW (mg/kg) | 36,632 ± 1621 | 35,784 ± 867 |
| Thyroid gland (mg) | 14.8 ± 0.8 | 16.7 ± 0.8 |
| Thyroid gland/BW (mg/kg) | 40.4 ± 1.3 | 49.9 ± 1.7*** |
| Spleen/BW (mg/kg) | 1583 ± 164 | 1877 ± 81 |
BW, body weight
P < 0.05,
P < 0.02,
P = 0.0025 compared with control group
Values are means ± SEM
F1 generation female rats whose F0 mothers had been treated with DE-71 had successful pregnancies. However, they were significantly lighter at five months of age than those whose mothers had been treated with vehicle (Table 4). This difference in body weight was accompanied by significantly lighter adrenals, kidneys and livers (absolute weights; P < 0.05). However, these differences disappeared when the gland weights were normalized to body weight. By contrast, absolute thyroid gland weight was not decreased and showed a trend to be increased (P = 0.16) in the DE-71 group despite the decreased body weight in that group. The increase in thyroid gland weight was statistically significant (P = 0.0025) and dramatic (approximately 25% heavier in the DE-71 group) when normalized for body weight. Originally, we did not collect thyroid glands from animals. However, after preliminary radioimmunoassay data suggested differences in thyroid hormone concentrations on Day 14.5 of pregnancy in F1 animals, we subsequently collected thyroid glands from F1 female and male rats that were sacrificed at five months of age.
Some of the male F1 rats were bred with normal females at approximately 80 d of age and sacrificed at five months of age. There were no statistically significant differences in body or organ weights between the two groups (Table 5).
Table 5.
Effects of DE-71 administered by gavage to F0 mothers during pregnancy and lactation on body and organ weights in five-month-old, male F1 generation offspring bred previously with normal females*
| Parameter | Control (n = 5) | DE-71 (n = 5) |
|---|---|---|
| BW (g) | 731.0 ± 20.4 | 700.0 ± 21.3 |
| Paired adrenals/BW (mg/kg) | 84.0 ± 3.5 | 81.2 ± 6.2 |
| Paired testes/BW (mg/kg) | 5271 ± 161 | 5665 ± 214 |
| Left kidney/BW (mg/kg) | 2971 ± 108 | 3371 ± 229 |
| Right kidney/BW (mg/kg) | 2900 ± 80 | 3255 ± 215 |
| Whole liver/BW (mg/kg) | 38,055 ± 1549 | 37,809 ± 3264 |
| Thyroid gland/BW (mg/kg) | 34.6 ± 1.7 | 35.7 ± 2.7 |
| Spleen/BW (mg/kg) | 1308 ± 68 | 1440 ± 99 |
BW, body weight
Values are means ± SEM
Serum T3 and T4 concentrations
Total serum T3 and T4 concentrations were approximately 50% and 25% greater, respectively, in F1 Day 14.5 pregnant rats whose mothers had been treated with DE-71 compared with those whose mothers had been treated with vehicle (Table 6).
Table 6.
Total serum T3 (ng/dL) and T4 (μg/dL × 10) concentrations in control and DE-71-exposed F0 rats and F1 offspring†
| Animal group | T3 Control | T3 DE-71 | T4 Control | T4 DE-71 |
|---|---|---|---|---|
| F0 mother Day 14.5 pregnancy | 61.4 ± 5.1 n = 5 | 62.4 ± 4.7 n = 5 | 28.5 ± 2.5 n = 5 | 25.6 ± 5.7 n = 5 |
| F0 mother Day 21 lactation | 70.3 ± 6.9 n = 4 | 55.3 ± 12.4 n = 3 | 40.3 ± 1.8 n = 4 | 46.7 ± 6.6 n = 3 |
| F1 female Day 21 | 81.7 ± 11.1 n = 4 | 89.7 ± 2.2 n = 3 | 39.5 ± 3.6 n = 4 | 32.7 ± 1.3 n = 3 |
| F1 male Day 21 | 94.8 ± 5.1 n = 4 | 91.3 ± 3.7 n = 3 | 40.3 ± 3.6 n = 4 | 40.3 ± 3.5 n = 3 |
| F1 mother Day 14.5 pregnancy | 57.0 ± 4.9 n = 4 | 84.8 ± 3.0** n = 4‡ | 30.5 ± 0.6 n = 4 | 38.0 ± 2.1* n = 4‡ |
| F1 female 5 months | 89.2 ± 3.6 n = 5 | 83.6 ± 4.9 n = 9 | 36.2 ± 3.0 n = 5 | 37.1 ± 2.2 n = 9 |
| F1 male 5 months | 83.6 ± 3.4 n = 5 | 75.4 ± 4.8 n = 5 | 45.6 ± 5.2 n = 5 | 51.6 ± 2.4 n = 5 |
BW, body weight
Values are means ± SEM
There was insufficient serum collected to assay T3 and T4 in two of the six rats in this group
P = 0.013,
P = 0.003 compared to control group
Osteopontin mRNA and transfections
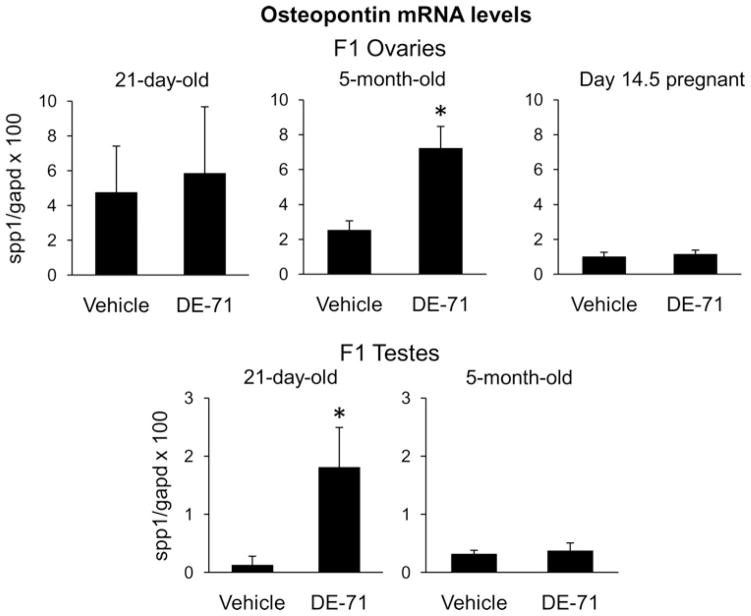
Realtime PCR was conducted using ovaries and testes from 21-day-old and five-month-old F1 animals and ovaries from F1 females at Day 14.5 of their pregnancy. Osteopontin mRNA was significantly elevated (P < 0.05) in the ovaries of five-month-old F1 rats and the testes of 21-day-old F1 rats that had been exposed to DE-71 perinatally compared with the tissues of F1 rats that had been exposed to vehicle perinatally by 2.9- and 14.9-fold, respectively (Figure 3). These findings are consistent with the preliminary microarray findings that revealed a 3.1-fold and 5.7-fold increase, respectively, in the ovaries of five-month-old F1 rats and the testes of 21-day-old F1 rats that had been exposed to DE-71 perinatally. Osteopontin mRNA levels were not significantly different between control and DE-71 groups for the F1 ovaries from 21-day-old rats, F1 ovaries collected at Day 14.5 of their pregnancy or F1 testes from five-month-old animals.
Figure 3.
Osteopontin (spp1) mRNA levels normalized to glyceraldehyde-3-phosphate dehydrogenase (gapd) mRNA levels (×100) in F1 rats that had been exposed perinatally to vehicle or DE-71 in vehicle. Bars represent mean plus SEM for 3–8 rats per group. *P < 0.05
Because the osteopontin mRNA was regulated in DE-71-exposed F1 ovaries and testes at specific developmental stages, we hypothesized that the rat osteopontin gene promoter would be activated by the PBDE mixture. Thus, a section of the rat promoter was amplified and cloned into a luciferase reporter construct for use in transfection studies. In preliminary studies, the osteopontin promoter construct was transfected in the clonal COS-1 cells and treated with vitamin D3 (100 nmol/L), a known activator of the rat promoter,15 T3 (10–100 nmol/L) and DE-71 (0.01–50 μg/mL). Basal levels of osteopontin promoter activity were strong but only vitamin D3 was able to further increase luficerase activity (data not shown). As immunohistochemical studies (not shown) showed a strong signal for osteopontin in the granulosa cells of five-month-old rats of both groups, we utilized a heterologous primary porcine granulosa cell model for testing the effects of DE-71 on osteopontin promoter activation. Using the porcine granulosa cell model, DE-71 at 50 μg/mL significantly increased transactivation of the rat osteopontin promoter construct, P < 0.05, whereas the promoterless luciferase vector (paLuc) showed no regulation by treatments (Figure 4). The raw basal luciferase activity of the OPN-Luc construct was several hundred folds greater than the promoterless construct in granulosa (not shown).
Figure 4.
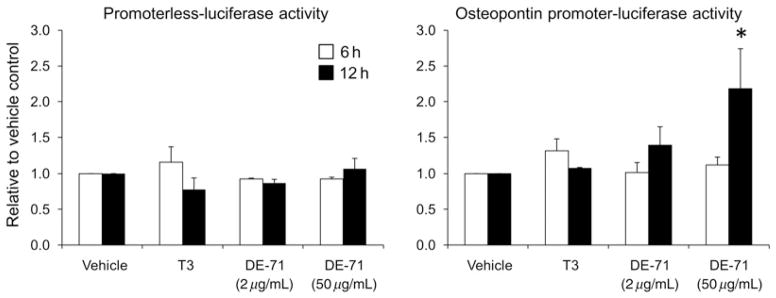
Effects of T3 (10 nmol/L) and DE-71 (2 and 50 μg/mL) on relative activity of the promoterless luciferase (paLuc) and osteopontin gene promoter-luciferase (OPN-Luc) constructs plotted as fold change with respect to vehicle control (activity = 1.0). Bars represent mean plus SEM. *P < 0.05 compared with vehicle control for OPN-Luc. For paLuc n = 2 experiments. For OPN-Luc n = 5 separate experiments except n = 4 for DE-71 (2 μg/mL)
Discussion
There are two important points to consider based on the results of the present study when assessing biological effects of PBDEs. First, oral ingestion of a mixture of PBDEs that contained the prevalent environmental congeners to mother rats during pregnancy and lactation at a dose that may be relevant to human exposure had effects on body weight and serum thyroid hormone concentrations of the female offspring and on osteopontin mRNA in the gonads of both female and male offspring. Second, consumption of these amounts of PBDEs had no statistically significant effect on a multitude of other parameters indicative of normal general health or reproductive system function that were compromised in animals exposed to greater amounts of PBDEs in other studies.
It is of importance to study the biological effects of individual PBDE congeners found in the environment as well as their mixtures which may reflect different effects due to interactions of the individual congeners. We used the commercial penta-BDE mixture DE-71, which is comprised primarily of PBDE-47, -99, -100, -153 and -154 and were reported to be the five most frequently found PBDE conge-ners in human breast milk.3 Utilizing a fat content of 14% of body weight as used by Talsness et al.,16 the daily 60 μg/kg BW dose of DE-71 we used represents approximately 10% less than the highest Σ of PBDE-47, -99, -100, -153 and -154 concentrations (462 μg/kg fat) reported in human breast adipose tissue.17 The dose we employed is the lowest dose reported in the literature and this is the first study with PBDEs that exposes offspring in utero from early pregnancy through lactation.
The presence of the predominant PBDE congeners that make up DE-71 in breast milk3 and the ability of PBDEs to pass from the mother to the developing child in utero8 emphasize the potential for PBDEs to affect numerous processes during critical times during development. Changes in the F1 generation whose mothers had been treated with DE-71 during pregnancy and lactation could be due to PBDE actions exerted during development, actions exerted by retained PBDEs after transfer during pregnancy and milk ingestion or to epigenetic changes caused by the transferred PBDEs at any time after the onset of exposure. It has been demonstrated that PBDE-99 was still present in rat blood and adipose tissue of adult offspring whose mothers had been injected subcutaneously with PBDE-99 (1 or 10 mg/kg/BW) daily from pregnancy Day 10–18.18
Under the conditions of this study, we found no evidence that DE-71 adversely affected the F0 mother, pregnancy, lactation or growth of the F1 pups during lactation. Ingestion of DE-71 daily for 13 d from Day 1.5 to 13.5 of pregnancy had no significant effects on pregnancy as evidenced by weight gain of the mothers, their adrenal and ovarian weights or number of resorptions at Day 14.5 of pregnancy. Continuation of daily DE-71 treatment in additional mothers did not affect the day of parturition or the number of pups. Continuation of daily DE-71 treatment of the mothers throughout lactation was without significant effect on pup body or organ weights at the end of lactation. These parameters do not appear to be susceptible to DE-71. In a similar study conducted by Kodavanti et al.,19 daily administration of DE-71 by gavage at higher doses (28.3-to 510-fold) than those employed in the present study to mothers from pregnancy Day 6 through weaning had no effect on maternal body weight during pregnancy and lactation, length of pregnancy, number of pups or weight of pups through weaning. In studies that administered a single dose of 60 or 300 μg/kg BW of PBDE-99 by gavage on Day 6 of pregnancy, it was suggested that this PBDE congener increased the rate of resorptions by a small amount based on the limits considered normal for controls, but statistics were not employed.16
By contrast, growth postweaning was compromised in the present study in F1 females whose mothers were treated with DE-71, as evidenced by lower mean body weights at 80 d and five months of age in the DE-71 group. This effect was sex specific as DE-71 had no effect on body weight gain in F1 males. Most interestingly, Kodavanti et al.19 observed this sex difference using higher doses of DE-71 during pregnancy and lactation. Lower body weights in the F1 females, but not in F1 males, in the DE-71 groups were evident at days 35–60 after birth. The reason for this sex difference is unclear, but the results of the present study indicate that it was not associated with abnormal growth of several organs, as evidenced by their weights when expressed on a body weight basis.
Under the conditions of the present study, DE-71 had no significant effects on the timing of vaginal opening and the success of F1 females mating and having a successful pregnancy with a normal number of pups. However, there was a statistically significant increase in ovarian weight when paired ovarian weights were expressed on a body weight basis in F1 rats on pregnancy Day 14.5. Adult female F1 offspring of pregnant rats in another study receiving 1 or 10 mg/kg BW/d of PBDE-99 intravenous from pregnancy Day 10 to 18 also exhibited increased ovarian weights and other changes in the female reproductive system.18 These offspring had increased uterine IGF-I and ER α and β mRNAs at the lower 1 mg/kg BW dose and lower progesterone receptor mRNA at both prenatal doses. These changes in uterine mRNAs occurred without change in uterine weights, suggesting that specific mRNAs may be more sensitive markers of endocrine disruption than gross morphological changes.18
Two developmental studies reported effects of PBDE-99 on the female reproductive system. Adult female F1 offspring of rat dams receiving a single dose of 60 or 300 μg/kg BW of PBDE-99 by gavage on Day 6 of pregnancy exhibited histological changes in the uterus and vagina and ultrastructural changes in the ovaries of F1 offspring.16 Daily subcutaneous injection of higher doses of PBDE-99 for a longer period of time during pregnancy (1 or 10 mg/kg BW on Days 10–18) caused a delay in vaginal opening and a decrease in the number of ovarian follicles as observed at seven months of age.20 A delay in vaginal opening was also observed in a postnatal study after daily administration of DE-71 by gavage starting on postnatal Day 22 at high doses that caused liver enlargement.21 Administration of high doses of DE-71 by gavage during pregnancy and lactation also impaired rat mammary gland development as evidenced at weaning.19 The delay in vaginal opening, but not necessarily the other effects of PBDEs on the female reproductive system, appears to be evident only when high doses of PBDEs were employed that often were accompanied by hepatomegaly.
With the exception of the effects on DE-71 on osteopontin mRNA in the testes of 21-day-old F1 males, observed effects of treatment of F0 mothers with DE-71 in the present study were limited to females. Other effects of PBDEs on the male reproductive system were observed by others when higher doses of DE-71 were employed. Kodavanti et al.,19 using a very high daily dose of DE-71 (30,600 μg/kg BW) during pregnancy and lactation, observed DE-71 to cause a significant delay in preputial separation in male pups, which represents a delay in the onset of puberty,22 but no effects on testes or male accessory reproductive organ weights or serum testosterone concentrations at 60 d of age. In another study, a decrease in spermiogenesis was observed in male offspring of dams exposed to a single injection of 60 or 300 μg/kg BW PBDE-99 on gestation Day 6.23 Additional effects of administration of DE-71 by gavage on the male reproductive system were observed when DE-71 was administered at very high doses that significantly increased liver weight.10,21 Daily gavage of male rats with a very high dose of DE-71 (60,000 μg/kg BW) for 31 d from Days 23–53 after birth delayed preputial separation and decreased weight of the some of the male accessory reproductive organs.21 Daily gavage of eight-week-old male rats for four weeks with DE-71 (270–200,000 μg/kg BW) caused a dose-dependent decrease in male accessory organ weights and sperm head deformities.24 With the possible exception of the sperm head deformities, these effects are likely due to PBDE inhibition of androgen binding10 and the immediate presence of high PBDE congeners in blood. Administration of low doses of DE-71 as used in the present study or high doses as used in the investigations of Kodavanti et al.19 during pregnancy and lactation did not affect male accessory organ weights in the adult F1 offspring. This lack of effect is likely due to most of the DE-71 remaining in the males being stored in lipid with blood PBDE congeners being at too low a concentration to inhibit androgen binding significantly. Kodavanti et al.19 reported blood to have the lowest of all tissue and fluid concentrations of PBDEs examined on postnatal Day 22 after administration of DE-71 during pregnancy and lactation.
The present study is, to our knowledge, the first to report PBDEs to cause an increase in serum thyroid hormone concentrations in rodents. Administration of daily DE-71 treatment of the mothers throughout pregnancy and lactation was without significant effect on serum T3 and T4 concentrations in the F0 mother or F1 pups. However, it did exert effects on the thyroid system of F1 female rats when adult. Both serum T3 and T4 concentrations were markedly increased in the F1 mothers on Day 14.5 of pregnancy and thyroid gland weight was markedly elevated at five months of age in the F1 females. By contrast, there are developmental,25–27 peripubertal21,27,28 and adult29,30 studies in rodents employing much higher doses of DE-71 or its congeners than we used that report PBDEs to decrease serum T4 concentrations25–27,29,30 or serum T3 and T4 concentrations.21,28 When liver weights were measured, these effects were associated with PBDE regimens that increased liver weights.21,25–27 PBDEs share structural similarity to thyroid hormones (Figure 1). High levels of PDBEs have been shown to alter thyroid hormone levels and clearance and can compete for thyroid hormone serum binding protein transthyretin.1,3 Some forms of PBDEs can bind to the alpha and beta forms of the thyroid hormone receptor and may act as thyroid agonists or antagonists depending on the specific congener.1 Our data suggest that during pregnancy there is an exaggerated release or reduced clearance of thyroid hormones in F1 females exposed to low doses of DE-71 via their mothers during the perinatal period. This alteration in thyroid hormone may affect the development of the F2 generation in utero. The decreases in circulating thyroid hormone concentrations observed by others employing higher doses may be due to a different mechanism(s) or reflect toxicity.
Reports that administration of high doses of DE-71 to mothers during the perinatal period reduce serum thyroid hormone concentration in the mother and pups are of particular concern as thyroid hormone is essential for brain development. Perinatal exposure to PBDE-99 has been linked to abnormal brain development,31,32 and these effects could be due to low circulating thyroid hormone concentrations.
To date there have been few studies that have looked at the association of PBDEs with any human disease. The recent study by Harley et al.33 reported an association between PBDEs and endocrine status and reproduction. Our observations that DE-71 caused increased serum T3 and T4 concentrations have similarities with these human studies in which elevations in PBDE-47, -99, -100 and -153 concentrations in serum were correlated positively with longer time to pregnancy.33 In this population, PBDE exposure was associated with subclinical hyperthyroidism.
Perinatal exposure to penta-BDEs caused changes in specific gonadal gene expression in the absence of changes in gonadal weights. In our preliminary microarray work, osteopontin mRNA was the only gene increased more than three-fold in both the immature testes and adult ovary by DE-71 exposure. DE-71 had time-dependent effects on gonadal osteopontin mRNA levels that differed by sex. Osteopontin mRNA was significantly elevated in 21-day-old but not five-month-old F1 males and in five-month-old but not 21-day-old or Day 14.5 pregnant F1 females. The effect in 21-day-old males could be due to the immediate presence of PBDE congeners at elevated levels in blood. The effects in five-month-old females at a time when circulating PBDE congeners would be expected to be lower may be due to PBDE actions exerted during development, actions exerted by retained PBDEs after transfer during pregnancy and milk ingestion or to epigenetic changes caused by the transferred PBDEs.
Osteopontin is an extracellular calcium-binding matrix protein synthesized in a variety of tissues. Its synthesis is stimulated by vitamin and by estrogen or progesterone.34–36 Osteopontin is involved in a variety of processes that include bone remodeling, inflammation, cell adhesion and migration and prevention of apoptosis.37 Remodeling, cell adhesion and apoptosis are ongoing processes in the gonads; yet a direct function for osteopontin in gonadal tissue has not been determined. However, osteopontin mRNA increases in the granulosa and theca cells of maturing ovarian follicles as they enlarge, suggesting a role in antral follicle growth.38 Noteworthy, osteopontin overexpression is associated with ovarian cancer in the human ovary.39
Using a heterologous granulosa cell model, we demonstrated that the PBDE mixture DE-71 activated the rat osteopontin gene promoter construct. This effect supports the view that DE-71 likely acts at the promoter to increase ovarian osteopontin transcription and potentially ovarian local osteopontin concentrations. Our data did not support T3 as a regulator of the osteopontin promoter in this cell model; however, there is evidence that T3 in certain cellular contexts can activate the murine osteopontin promoter through its vitamin D response element.40 In addition, ER α and ER-related α proteins can activate the murine promoter acting through a steroidogenic factor 1 response element,41,42 inferring that some PBDE congeners with estrogenic activity could potentially act through the similar element in the rat promoter. A detailed analysis of the promoter for PBDE responsive regions will be the subject of future studies.
In summary, we conducted a study in rats that analyzed the effects of ingestion of a low dose of a PBDE mixture at a dose that might be relevant to human exposure on aspects of reproduction and thyroid hormone concentrations in the F1 offspring. DE-71 was without apparent effect on basic reproductive processes. However, two effects of this low-dose exposure administered to F0 mothers that were manifested in adult F1 females relate to clinical conditions. First, in contrast to previous studies using rodents that reported higher doses of PBDEs to suppress circulating thyroid hormone concentrations, our study demonstrates for the first time that low-dose PBDEs increase both serum T3 and T4 concentrations. Such findings are relevant to studies in humans that have shown PBDE exposure to be associated with subclinical hyperthyroidism. Second, our study demonstrates that PBDEs cause changes in gonadal osteopontin gene expression as well as its gene promoter activation. The potential relationships between PBDEs and hyperthyroidism and PBDEs, osteopontin and ovarian cancer warrant further investigation.
Acknowledgments
We thank William McAmis and Roopa Varadarajan for excellent technical assistance. This work was supported by grants from the NIH (MD00233) and the University of South Carolina.
Footnotes
Author contributions: HAL, CAB and GLM conceived and designed the study; HAL and CAB performed the animal experiments; CAB performed the radioimmunoassays; HAL and YYH performed the molecular biology experiments; HAL and CAB analyzed the data and wrote the manuscript.
References
- 1.Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res. 2003;1:281–90. doi: 10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003;29:841–53. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 3.Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schecter A, Päpke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ Health Perspect. 2006;114:1515–20. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schecter A, Päpke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- 6.Darnerud PO, Atuma S, Aune M, Bjerselius R, Glynn A, Grawe KP, Becker W. Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, e.g. DDT) based on Swedish market basket data. Food Chem Toxicol. 2006;44:1597–606. doi: 10.1016/j.fct.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ Sci Technol. 2005;39:925–31. doi: 10.1021/es0486824. [DOI] [PubMed] [Google Scholar]
- 8.Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. Polybrominated diphenyl ether (PBDE) levels in livers of U.S, human fetuses and newborns. J Toxicol Environ Health A. 2007;70:1–6. doi: 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- 9.Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der BB, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharmacol. 2005;207:78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Mercado-Feliciano M, Bigsby RM. The polybrominated diphenyl ether mixture DE-71 is mildly estrogenic. Environ Health Perspect. 2008;116:605–11. doi: 10.1289/ehp.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillio-Meina C, Hui YY, LaVoie HA. GATA-4 and GATA-6 transcription factors: expression, immunohistochemical localization, and possible function in the porcine ovary. Biol Reprod. 2003;68:412–22. doi: 10.1095/biolreprod.102.009092. [DOI] [PubMed] [Google Scholar]
- 13.LaVoie HA, Garmey JC, Day RN, Veldhuis JD. Concerted regulation of the low density lipoprotein receptor gene expression by FSH and IGF-I in porcine granulosa cells: promoter activation, mRNA stability, and sterol feedback. Endocrinology. 1999;140:178–86. doi: 10.1210/endo.140.1.6439. [DOI] [PubMed] [Google Scholar]
- 14.Ho CK, Strauss JF., III Activation of the control reporter plasmids pRL-TK and pRL-SV40 by multiple GATA transcription factors can lead to aberrant normalization of transfection efficiency. BMC Biotechnol. 2004;4:10. doi: 10.1186/1472-6750-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridall AL, Daane EL, Dickinson DP, Butler WT. Characterization of the rat osteopontin gene. Evidence for two vitamin D response elements. Ann N Y Acad Sci. 1995;760:59–66. doi: 10.1111/j.1749-6632.1995.tb44620.x. [DOI] [PubMed] [Google Scholar]
- 16.Talsness CE, Shakibaei M, Kuriyama SN, Grande SW, Sterner-Kock A, Schnitker P, de Souza C, Grote K, Chahoud I. Ultrastructural changes observed in rat ovaries following in utero and lactational exposure to low doses of a polybrominated flame retardant. Toxicol Lett. 2005;157:189–202. doi: 10.1016/j.toxlet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 17.She J, Petreas M, Winkler J, Visita P, McKinney M, Kopec D. PBDEs in the San Francisco Bay Area: measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46:697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 18.Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220:104–16. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Kodavanti PR, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, Rayner JL, Kannan K, Birnbaum LS. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- 20.Lilienthal H, Hack A, Roth-Harer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2,4,4,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78:144–55. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- 22.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 23.Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low dose PBDE 99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113:149–54. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Ven LTM, van de Kuil T, Verhoef A, Leonards PE, Slob W, Cantón RF, Germer S, Hamers T, Visser TJ, Litens S, Håkansson H, Fery Y, Schrenk D, van den Berg M, Piersma AH, Vos JG. A 28-day oral dose toxicity study enhanced to detect endocrine effects of a purified technical pentabromodiphenyl ether (pentaBDE) mixture in Wistar rats. Toxicology. 2008;245:109–22. doi: 10.1016/j.tox.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Ellis-Hutchings RG, Cherr GN, Hanna LA, Keen CL. Polybrominated diphenyl ether (PBDE)-induced alterations in vitamin A and thyroid hormone concentrations in the rat during lactation and early postnatal development. Toxicol Appl Pharmacol. 2006;215:135–45. doi: 10.1016/j.taap.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Skarman E, Darnerud PO, Öhrvik H, Oskarsson A. Reduced thyroxine levels in mice perinatally exposed to polybrominated diphenyl ethers. Environ Toxicol Pharmacol. 2005;19:273–81. doi: 10.1016/j.etap.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Bondy GS, Gaertner D, Cherry W, Maclellan E, Coady L, Arnold DL, Doucet J, Rowsell PR. Brominated diphenyl ether (BDE) levels in liver, adipose, and milk from adult and juvenile rats exposed by gavage to the DE-71 technical mixture. Environ Toxicol. 2010 doi: 10.1002/tox.20603. In press. [DOI] [PubMed] [Google Scholar]
- 28.Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- 29.Fowles JR, Fairbrother A, Baecher-Steppan L, Kerkvliet NI. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology. 1994;86:49–61. doi: 10.1016/0300-483x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 30.Darnerud PO, Aune M, Larsson L, Hallgren S. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere. 2007;67:S386–92. doi: 10.1016/j.chemosphere.2006.05.133. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–8. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology. 2002;23:375–84. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 33.Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White FJ, Burghardt RC, Hu J, Joyce MM, Spencer TE, Johnson GA. Secreted phosphoprotein 1 (osteopontin) is expressed by stromal macrophages in cyclic and pregnant endometrium of mice, but is induced by estrogen in luminal epithelium during conceptus attachment for implantation. Reproduction. 2006;132:919–29. doi: 10.1530/REP-06-0068. [DOI] [PubMed] [Google Scholar]
- 35.Apparao KB, Illera MJ, Beyler SA, Olson GE, Osteen KG, Corjay MH, Boggess K, Lessey BA. Regulated expression of osteopontin in the peri-implantation rabbit uterus. Biol Reprod. 2003;68:1484–90. doi: 10.1095/biolreprod.101.001347. [DOI] [PubMed] [Google Scholar]
- 36.White FJ, Ross JW, Joyce MM, Geisert RD, Burghardt RC, Johnson GA. Steroid regulation of cell specific secreted phosphoprotein 1 (osteopontin) expression in the pregnant porcine uterus. Biol Reprod. 2005;73:1294–301. doi: 10.1095/biolreprod.105.045153. [DOI] [PubMed] [Google Scholar]
- 37.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner MK, Schmidt M, Savenkova MI, Sadler-Riggleman I, Nilsson EE. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev. 2008;75:1457–2. doi: 10.1002/mrd.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih I-M, Davidson B. Pathogenesis of ovarian cancer: clues from selected overexpressed genes. Future Oncol. 2009;5:1641–57. doi: 10.2217/fon.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrader M, Muller KM, Carlberg C. Specificity and flexibility of vitamin D signaling. Modulation of the activation of natural vitamin D response elements by thyroid hormone. J Biol Chem. 1994;269:5501–4. [PubMed] [Google Scholar]
- 41.Vanacker JM, Pettersson K, Gustafsson JA, Laudet V. Transcriptional targets shared by estrogen receptor- related receptors (ERRs) and estrogen receptor (ER) alpha, but not by ERbeta. EMBO J. 1999;18:4270–9. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanacker JM, Delmarre C, Guo X, Laudet V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell Growth Differ. 1998;9:1007–14. [PubMed] [Google Scholar]