Abstract
About 50% of malaria infections in India are attributed to Plasmodium falciparum but relatively little is known about the genetic structure of the parasite populations. The molecular genotyping of the parasite populations by merozoite surface protein (msp1 and msp2) and glutamate-rich protein (glurp) genes identifies the existing parasite population in the regions which help in understanding the molecular mechanisms involved in the parasite's drive for survival. This study reveals the genetic profile of the parasite population in selected regions across the country with varying degree of endemicity among them. We also report the prevalence of Pfcrt mutations in this parasite population to evaluate the pattern of drug resistance development in them.
1. Introduction
Malaria parasites infect about 650 million people worldwide and P. falciparum alone leads to almost one million deaths per year making it the most virulent parasite causing malaria. It is pertinent to develop efficient means of controlling P. falciparum in areas to which malaria infections are highly endemic. Since malaria infections are endemic to India, it is necessary to characterize the parasite population and know the genotypic pattern of the circulating parasite populations [1]. The identification of multiclones in the population not only helps in assessing the parasite population dynamics but also provides insights into the existing population [2]. Previous studies have revealed high polymorphism in msp1, msp2, and glurp genes from different regions to which malaria is endemic [3]. In a country like India to which malaria is endemic, continuous molecular surveillance of the field isolates is required to know the pattern of existing and emerging drug resistance. This knowledge is the key to effective malaria control programs.
Genotyping the P. falciparum populations using antigenic genes msp1, msp2, and glurp has been known to describe allelic variability within parasite populations and also to distinguish recrudescence from new infections of P. falciparum disease [4]. Mostly these polymorphic markers (msp1 and msp2) have been used to assess the multiplicity of infection (MOI) for detecting the number of clones per isolate. Block-2 of msp1 gene, block-3 or the central repetitive domain of msp2 gene, and RII repeat region of glurp show large allelic polymorphism [5]. In msp1 gene three distinct allelic families K1, MAD20, and RO33 have been described whereas msp2 gene consists of FC27 and IC3D7 families. Several genes of P. falciparum have shown extensive genetic polymorphism in genetic analysis and therefore have been extensively used as markers to study the genetic diversity and MOI [6, 7].
Chloroquine (CQ) is the most widely used antimalarial drug but P. falciparum has established mechanisms to evade the drug pressure. It is not very clear how the CQ resistance (CQR) is implemented but point mutations in P. falciparum CQR transporter protein (Pfcrt), encoded by the pfcrt gene, have been shown to make the parasite less sensitive to CQ [8].
The purpose of the present study was to genotype the parasite population in P. falciparum infections in India using antigenic polymorphic markers msp1, msp2, and glurp and drug resistance gene, namely, Pfcrt, so as to assess the parasite population dynamics in the country.
2. Materials and Methods
2.1. Sample Collection
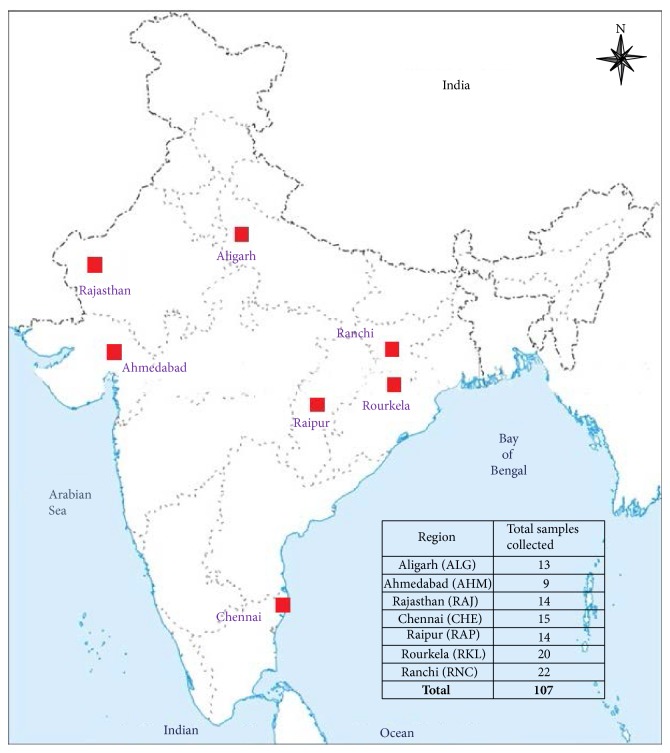
One hundred and seven symptomatic malaria blood samples were collected by finger prick method from different regions of India (Figure 1); the details are given in Table 1. Only after the ethical clearance from the institute the collection of P. falciparum infected blood samples was done. The collection of samples was carried out during the years 2009–2012.
Figure 1.
Map of India showing different regions from where malaria symptomatic samples were collected. The table specifies the number of samples collected from each region.
Table 1.
Summary of the P. falciparum isolates collected from various regions across India.
| S. number | Region | Total samples collected | Samples positive for P. falciparum by microscopy and RDT | P. falciparum monoinfections by PCR |
|---|---|---|---|---|
| 1 | Aligarh (ALG) | 13 | 13 | 13 |
| 2 | Ahmedabad (AHM) | 9 | 9 | 9 |
| 3 | Rajasthan (RAJ) | 14 | 4 | 4 |
| 4 | Chennai (CHE) | 15 | 5 | 5 |
| 5 | Raipur (RAP) | 14 | 14 | 14 |
| 6 | Rourkela (RKL) | 20 | 20 | 13 |
| 7 | Ranchi (RNC) | 22 | 22 | 22 |
|
| ||||
| Total | 107 | 87 | 80 | |
2.2. P. falciparum Detection and Diagnosis
Microscopy and rapid diagnostic tests (RDT) were used for the first line of P. falciparum malaria diagnosis and bloodspots of infected samples were made on Whatman (number 3) filter paper for further molecular studies. The microscopically confirmed P. falciparum samples were then subjected to genotyping by different polymorphic markers.
2.3. DNA Isolation and PCR Analysis
Genomic DNA of P. falciparum positive samples was isolated by QIAamp DNA Blood Mini Kit (Qiagen Inc.) according to manufacturer's protocol. Mixed infections of P. vivax and P. falciparum were confirmed by nested PCR assay of 18srRNA primers [9].
2.4. Molecular Genotyping
The single P. falciparum infections after the species specific PCR were only considered for further molecular analysis. The repetitive polymorphic regions in different allelic families of msp1 (block 2), msp2 (block 3), and region II of glurp genes were amplified by PCR. These alleles have conserved regions flanked by repeat sequences of variable regions [5]. The primary PCR amplification of msp1, msp2, and glurp genes comprised an initial step of 95°C for five minutes followed by 30 cycles of 95°C for 1 minute, 58°C for 2 minutes, 72°C for 2 minutes, and a final extension of 72°C for 5 minutes. The nested PCR cycling parameters for glurp were the same as the primary reaction but for msp1 and msp2 the annealing temperature was 61°C in the nested PCR. The PCR products for msp1, msp2, and glurp were separated on 1.5% ethidium bromide stained agarose gels for visualization under UV illumination.
The point mutations in the Pfcrt gene were typed to study the polymorphisms in the drug resistance gene. In Pfcrt gene of P. falciparum the primers amplifieda fragment of 450 bp which carries SNPs from 72–76 and 97 positions. Both primary and nested PCR amplifications consisted of an initial denaturation of 94°C for five minutes with 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 1.5 minutes, and a final extension of 72°C for 7 minutes. Restriction fragment length polymorphism (RFLP) was carried out for the amplified region of pfcrt gene by enzyme Apo I (New England BioLabs Inc.) for the detection of SNPs as described previously with slight modifications [1, 10].
3. Results
A total of 87 samples were microscopically confirmed out of which 80 were found to be positive for P. falciparum monoinfections by PCR assay. Out of these 80 P. falciparum monoinfections, 67 samples were successfully analyzed by PCR for msp1, 71 samples for msp2, and 51 samples for glurp loci in the isolates.
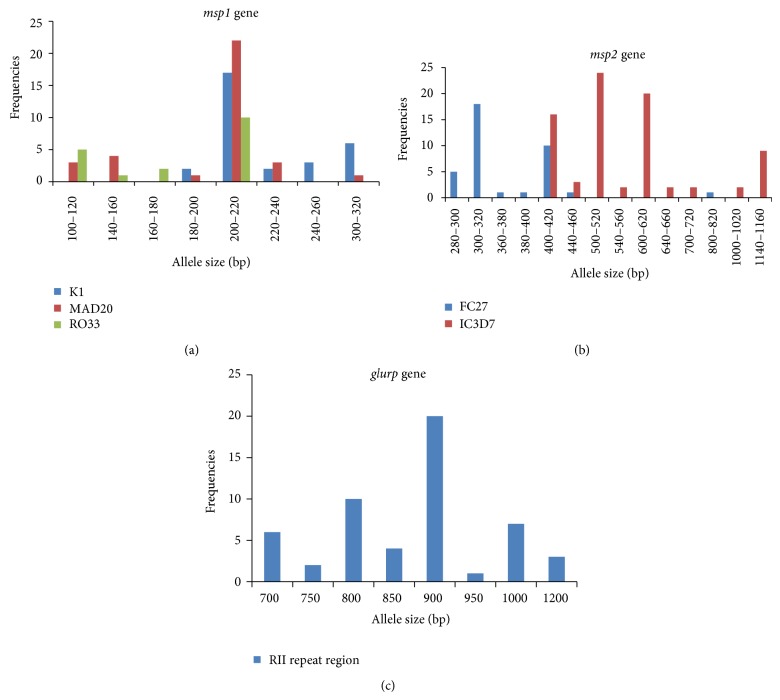
After the PCR assay, the classification of the alleles was done according to the number and size of fragments and the allelic family (Figure 2). The msp1 gene block-1 amplification for K1 allelic family was positive in 29 samples (36.25%) with five different allelic sizes ranging from 180 to 300 bp among which 200 bp allelic fragment was predominant. The other family MAD20 in msp1 was found in 32 samples (40.0%) depicting seven different allelic sizes within 100–300 bp and 200 bp was found to be the predominant allele size in this group. The RO33 family was detected in 17 samples (21.25%) having four distinct alleles with fragment sizes of 100–200 bp and its predominant allele was found to be 200 bp fragment (Figure 3(a)). The msp2 amplification for FC27 family was positive in 37 isolates (46.25%) having seven different allele types ranging from 280 to 800 bp predominated by 300 bp fragment size. The IC3D7 allelic family was amplified in 45 isolates (56.25%) demarcated by nine different alleles spanning between 400 and 1150 bp fragments where 500 bp fragment was in majority (Figure 3(b)). For glurp 51 samples (63.75%) were found to be positive for RII repeat region producing eight different sizes ranging between 700 and 1200 bp, among which 900 bp allele was found to be predominant (25.0%) (Figure 3(c)). The number of genotypes for each marker is shown in Tables 2 and 3.
Figure 2.
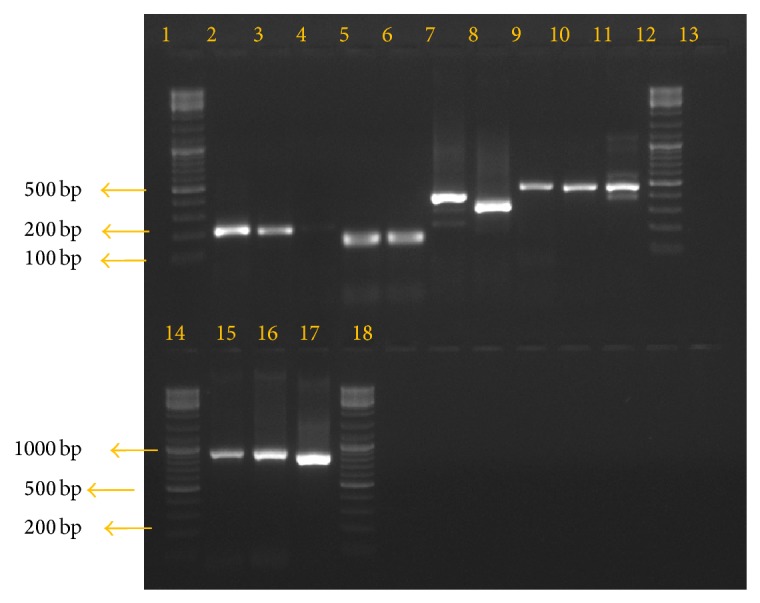
Gel picture showing the amplification of P. falciparum msp1, msp2, and glurp genes. Lane 2 shows msp1 allelic family K1, lanes 3 and 4 show allelic family MAD20, lanes 5 and 6 show allelic family RO33, lanes 7 and 8 show msp2 allelic family FC27, lanes 9, 10, and 11 show family IC3D7, lanes 15, 16, and 17 show glurp gene amplification, lane 13 is the negative control, and lanes 1, 12, 14, and 18 show 100 bp DNA ladder.
Figure 3.
Genetic diversity of P. falciparum by msp1, msp2, and glurp genes. PCR amplification was represented by groups where PCR product size differed by 20 bp. (a) Genetic diversity by msp1 gene. Blue bars denote the K1 allelic family, size ranging from 180 to 300 bp; red bars denote the MAD20 allelic family, size ranging from 100 to 220 bp; and green bars denote the RO33 allelic family, size ranging from 100 to 200 bp. (b) Genetic diversity by msp2 gene. Blue bars denote the FC27 allelic family, size ranging from 280 to 800 bp and the red bars denote the IC3D7 allelic family, size ranging from 400 to 1150 bp. (c) Genetic diversity by glurp gene. Blue bars denote the RII repeat region of glurp gene, size ranging from 700 to 1200 bp.
Table 2.
Region-wise genetic diversity of P. falciparum by msp1, msp2, and glurp.
| S. number | Region | Number of samples (n) | PCR assay | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| msp1 | msp2 | glurp | ||||||||
| K1 | MAD20 | RO33 | Total | FC27 | IC3D7 | Total | ||||
| 1 | Aligarh | 13 | 2 | 7 | 3 | 12 | 9 | 5 | 14 | 10 |
| 2 | Ahmedabad | 9 | 1 | 2 | 4 | 7 | 7 | 5 | 12 | 8 |
| 3 | Rajasthan | 4 | 1 | 2 | 2 | 5 | 3 | 1 | 4 | 3 |
| 4 | Chennai | 5 | 2 | 4 | 3 | 9 | 3 | 1 | 4 | 3 |
| 5 | Raipur | 14 | 8 | 5 | 2 | 15 | 9 | 8 | 17 | 12 |
| 6 | Rourkela | 13 | 9 | 3 | 3 | 15 | 5 | 8 | 13 | 11 |
| 7 | Ranchi | 22 | 6 | 9 | 0 | 15 | 1 | 17 | 18 | 4 |
Table 3.
Summary of all the isolates positive for each allelic family for msp1, msp2, and glurp along with the number of alleles and the range of sizes of alleles.
| Loci | Number of samples positive by PCR | Number of distinct alleles | Sizes of alleles (bp) |
|---|---|---|---|
| MSP-1 | |||
| K1 | 29 | 5 | 180–300 |
| MAD20 | 32 | 7 | 100–300 |
| RO33 | 17 | 4 | 100–200 |
| MSP-2 | |||
| FC27 | 37 | 7 | 280–800 |
| IC3D7 | 45 | 9 | 400–1150 |
| GLURP | 51 | 8 | 700–1200 |
It was seen that 46.25% of the isolates studied were multiclonal in nature with two or more alleles present in msp1, msp2, and glurp genes. Thirteen multiple alleles were seen in msp1 (16.25%), 28 were found in msp2 (35%), and two multiple alleles were seen in glurp (2.5%) genes showing more genotypic variation in msp2 than in msp1 and glurp. There was only one isolate from Rourkela, positive for all the allelic families of msp1 and msp2. There were 21 distinct haplotype patterns observed among the parasite population and the prominent genotype pattern was MAD20 for msp1 and FC27 for msp2.
MOI is calculated as the number of genotypes for a gene divided by the number of isolates with positive PCR amplification. For each region studied MOI of both msp1 and msp2 was calculated whose results are shown in Figure 4.
Figure 4.
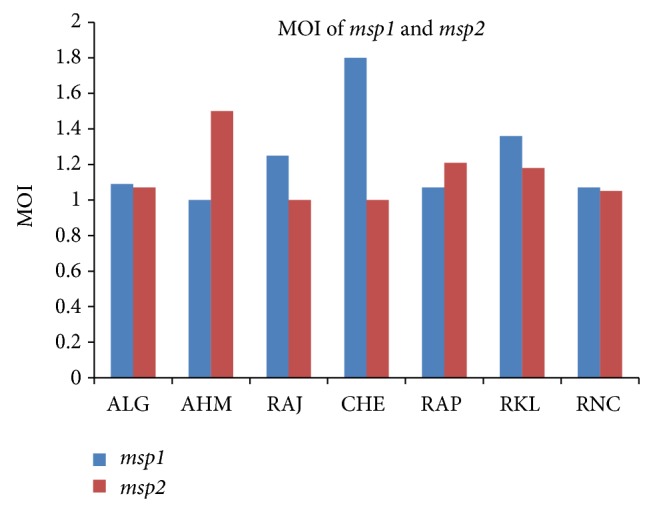
Graph showing comparison of MOI for msp1 and msp2 genes in different regions of India. ALG: Aligarh, AHM: Ahmedabad, RAJ: Rajasthan, CHE: Chennai, RAP: Raipur, RKL: Rourkela, and RNC: Ranchi.
A fragment of 450 bp in pfcrt gene was amplified for 80 P. falciparum isolates out of which 68 samples showed successful amplification. Restriction sites for Apo I enzyme are seen at codon 76 where K76T mutation was observed in 29.4% of the isolates.
4. Discussion
Though, for parasite genotyping, msp1, msp2, and glurp genes have been the recommended molecular markers for several drug efficacy studies, still the parasite population genetic profile has not been assessed systematically in a country to which malaria is endemic like India [6, 11, 12]. The field isolates in the present study have been genotyped using the polymorphic regions of these three genes in order to compare the diversity and the existing allelic frequencies.
The msp1 and msp2 showed 16 each, and glurp markers showed eight allelic families in the studied parasite population. These findings indicate that, for detection of MOI, msp1 served as a better marker as MOI for msp1 was higher in comparison to msp2. However it is noticeable that glurp did not indicate the existing genetic pattern with lower frequency of diverse alleles [13]. This study shows the complex diversity existing in the P. falciparum field isolates in areas of the country where malaria is endemic. The diversity seen in these isolates is due to the complexity present in the parasite population.
Previously it has been reported that a higher MOI was found in severe infections as compared to the mild type infections in studies from Uganda [14]. These findings were in tandem with studies from India, which showed a strong association between multiple genotype infections, significantly high MOI, and severity of P. falciparum malaria [15, 16]. Findings from Yemen show a regional variation with a higher MOI in isolates from foothills/coastland areas as compared to those from the highlands [17]. Another study from India showed a high proportion of multiclonal isolates and high MOI in regions to which P. falciparum is highly endemic [18]. Findings on the genetic pattern of these polymorphic genes msp1, msp2, and glurp of field isolates are similar to the other studies reported elsewhere [3, 4]. Studying the dynamics of multiclone infections and MOI in relation to the host immunity, disease prevalence, genetic structure, and geographical distribution would allow us to know more about the virulence of P. falciparum.
We were able to identify 21 distinct genetic patterns among the parasite population indicative of the fact that a considerable amount of gene flow is ongoing between the different regions with P. falciparum infections. It would be interesting and of importance to link the genotypic pattern of the parasites with the clinical phenotype. The recent data of msp1, msp2, and glurp markers for drug efficacy studies are highly important in areas where malaria is endemic for understanding the treatment criteria [19]. These highly polymorphic surface proteins are vaccine candidate genes and monitoring these genes to understand their genetic diversity and structure would help us develop vaccines with cross-protection against a range of antigenic variants [13, 20].
The studied drug resistance gene indicates that extensive mapping of this gene should be carried out in more areas in which malaria is endemic. The resistant mutants are the result of drug pressure and hence control programs should be devised in such a manner that they help in reducing the drug pressure and enhance the activity of antimalarials. This study indicates the high complexity and the diversity existing in the P. falciparum population in several areas of the country where malaria is endemic. The MOI was higher in msp1 in this reported study, suggesting that the intensity of malaria transmission in these regions is high. This multiplicity of infection has definite implications not only in the drug resistant parasite but also in the outcome of the disease treatment. The diversity (MOI) in the parasite population was seen more in the rural areas like Rourkela and Ranchi than in urban areas like Ahmedabad and Aligarh. This difference in the genetic diversity could be attributed to the topographical and climatic changes in the environmental factors. This kind of study in addition to other molecular methods should be undertaken for monitoring the existing and emerging drug resistance patterns in malaria disease.
Acknowledgments
The authors thank the Indian Council of Medical Research (ICMR) for providing them with grants to carry out their study (75/05/92-ECD II). They would also like to acknowledge the constant encouragement for research from the Director, NIMR. The authors would like to sincerely thank the study participants. This paper bears the NIMR Publication Screening Committee Approval no. 041/2014. Purva Gupta is a senior research fellow of ICMR.
Conflict of Interests
The authors declare that they have no competing interests regarding the publication of this paper.
References
- 1.Abdel-Muhsin A. A., Mackinnon M. J., Awadalla P., Ali E., Suleiman S., Ahmed S., Walliker D., Babiker H. A. Local differentiation in Plasmodium falciparum drug resistance genes in Sudan. Parasitology. 2003;126(5):391–400. doi: 10.1017/S0031182003003020. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz U., Kobbe R., Danquah I., et al. Multiplicity of Plasmodium falciparum infection following intermittent preventive treatment in infants. Malaria Journal. 2010;9(1, article 244) doi: 10.1186/1475-2875-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mwingira F., Nkwengulila G., Schoepflin S., Sumari D., Beck H.-P., Snounou G., Felger I., Olliaro P., Mugittu K. Plasmodium falciparum msp1, msp2 and glurp allele frequency and diversity in sub-Saharan Africa. Malaria Journal. 2011;10, article 79 doi: 10.1186/1475-2875-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosi P., Lanteri C. A., Tyner S. D., Se Y., Lon C., Spring M., Char M., Sea D., Sriwichai S., Surasri S., Wongarunkochakorn S., Pidtana K., Walsh D. S., Fukuda M. M., Manning J., Saunders D. L., Bethell D. Evaluation of parasite subpopulations and genetic diversity of the msp1, msp2 and glurp genes during and following artesunate monotherapy treatment of Plasmodium falciparum malaria in Western Cambodia. Malaria Journal. 2013;12(1, article 403) doi: 10.1186/1475-2875-12-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snounou G., Zhu X., Siripoon N., Jarra W., Thaithong S., Brown K. N., Viriyakosol S. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93(4):369–374. doi: 10.1016/S0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- 6.Akter J., Thriemer K., Khan W. A., Sullivan D. J., Noedl H., Haque R. Genotyping of Plasmodium falciparum using antigenic polymorphic markers and to study anti-malarial drug resistance markers in malaria endemic areas of Bangladesh. Malaria Journal. 2012;11, article 386 doi: 10.1186/1475-2875-11-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Congpuong K., Sukaram R., Prompan Y., Dornae A. Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders. Asian Pacific Journal of Tropical Biomedicine. 2014;4(8):598–602. doi: 10.12980/APJTB.4.2014APJTB-2014-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittra P., Vinayak S., Chandawat H., et al. Progressive increase in point mutations associated with chloroquine resistance in Plasmodium falciparum isolates from India. The Journal of Infectious Diseases. 2006;193(9):1304–1312. doi: 10.1086/502979. [DOI] [PubMed] [Google Scholar]
- 9.Gupta B., Gupta P., Sharma A., Singh V., Dash A. P., Das A. High proportion of mixed-species Plasmodium infections in India revealed by PCR diagnostic assay. Tropical Medicine and International Health. 2010;15(7):819–824. doi: 10.1111/j.1365-3156.2010.02549.x. [DOI] [PubMed] [Google Scholar]
- 10.Andriantsoanirina V., Ratsimbasoa A., Bouchier C., Jahevitra M., Rabearimanana S., Radrianjafy R., Andrianaranjaka V., Randriantsoa T., Rason M. A., Tichit M., Rabarijaona L. P., Mercereau-Puijalon O., Durand R., Ménard D. Plasmodium falciparum drug resistance in Madagascar: facing the spread of unusual pfdhfr and pfmdr-1 haplotypes and the decrease of dihydroartemisinin susceptibility. Antimicrobial Agents and Chemotherapy. 2009;53(11):4588–4597. doi: 10.1128/AAC.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olasehinde G. I., Yah C. S., Singh R., Olusola O. O., Ajayi A. A., Valecha N., Abolaji A. O., Adeyeba A. O. Genetic diversity of Plasmodium falciparum field isolates from south western Nigeria. African Health Sciences. 2012;12(3):355–361. doi: 10.4314/ahs.v12i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atroosh W. M., Al-Mekhlafi H. M., Mahdy M. A., Saif-Ali R., Al-Mekhlafi A. M., Surin J. Genetic diversity of Plasmodium falciparum isolates from Pahang, Malaysia based on MSP-1 and MSP-2 genes. Parasites and Vectors. 2011;4(1, article 233) doi: 10.1186/1756-3305-4-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt-Riccio L. R., Perce-da-Silva D. D. S., Lima-Junior J. D. C., Theisen M., Santos F., Daniel-Ribeiro C. T., de Oliveira-Ferreira J., Banic D. M. Genetic polymorphisms in the glutamate-rich protein of Plasmodium falciparum field isolates from a malaria-endemic area of Brazil. Memorias do Instituto Oswaldo Cruz. 2013;108(4):523–528. doi: 10.1590/0074-0276108042013022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiwuwa M. S., Ribacke U., Moll K., Byarugaba J., Lundblom K., Färnert A., Fred K., Wahlgren M. Genetic diversity of Plasmodium falciparum infections in mild and severe malaria of children from Kampala, Uganda. Parasitology Research. 2013;112(4):1691–1700. doi: 10.1007/s00436-013-3325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rout R., Mohapatra B. N., Kar S. K., Ranjit M. Genetic complexity and transmissibility of Plasmodium falciparum parasites causing severe malaria in Central-East coast India. Tropical Biomedicine. 2009;26(2):165–172. [PubMed] [Google Scholar]
- 16.Ranjit M. R., Das A., Das B. P., Das B. N., Dash B. P., Chhotray G. P. Distribution of Plasmodium falciparum genotypes in clinically mild and severe malaria cases in Orissa, India. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99(5):389–395. doi: 10.1016/j.trstmh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Al-abd N. M., Mahdy M. A. K., Al-Mekhlafi A. M. Q., Snounou G., Abdul-Majid N. B., Al-Mekhlafi H. M., Fong M. Y. The suitability of P. falciparum merozoite surface proteins 1 and 2 as genetic markers for in vivo drug trials in Yemen. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0067853.e67853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi H., Valecha N., Verma A., Kaul A., Mallick P. K., Shalini S., Prajapati S. K., Sharma S. K., Dev V., Biswas S., Nanda N., Malhotra M. S., Subbarao S. K., Dash A. P. Genetic structure of Plasmodium falciparum field isolates in eastern and north-eastern India. Malaria Journal. 2007;6, article 60 doi: 10.1186/1475-2875-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattamanchi A., Kyabayinze D., Hubbard A., Rosenthal P. J., Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp . American Journal of Tropical Medicine and Hygiene. 2003;68(2):133–139. [PubMed] [Google Scholar]
- 20.Bharti P. K., Shukla M. M., Sharma Y. D., Singh N. Genetic diversity in the block 2 region of the merozoite surface protein-1 of Plasmodium falciparum in central India. Malaria Journal. 2012;11, article 78 doi: 10.1186/1475-2875-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]