Abstract
Acute psychological stress (PS) mobilizes metabolic responses that are of immediate benefit to the host, but the current medical paradigm holds that PS exacerbates systemic and cutaneous inflammatory disorders. Although the adverse consequences of PS are usually attributed to neuroimmune mechanisms, PS also stimulates an increase in endogenous glucocorticoids (GCs) that compromises permeability barrier homeostasis, stratum corneum cohesion, wound healing, and epidermal innate immunity in normal skin. Yet, if such PS-induced increases in GC were uniformly harmful, natural selection should have eliminated this component of the stress response. Hence, we hypothesized here instead that stress-induced elevations in endogenous GC could benefit, rather than aggravate, cutaneous function and reduce inflammation in three immunologically diverse mouse models of inflammatory diseases. Indeed, superimposed exogenous (motion-restricted) stress reduced, rather than aggravated inflammation and improved epidermal function in all three models, even normalizing serum IgE levels in the atopic dermatitis model. Elevations in endogenous GC accounted for these apparent benefits, because coadministration of mifepristone prevented stress-induced disease amelioration. Thus, exogenous stress can benefit rather than aggravate cutaneous inflammatory dermatoses through the anti-inflammatory activity of increased endogenous GC.
Introduction
Acute psychological stress (PS) in response to perceived external threats provokes a suite of metabolic responses that benefit the host, not only through the rapid mobilization of endogenous glucocorticoid (GC) but also through autonomic responses (reviewed in McEwen and Kalia, 2010, Nowotny et al., 2010). In contrast, it is generally accepted that excessive PS, an inevitable accompaniment of chronic illness of all types (Hansel et al., 2010, McEwen and Kalia, 2010), adversely affects outcomes in chronic inflammatory disorders, as diverse as inflammatory bowel disease (Reber et al., 2011), coronary artery disease (Hamer et al., 2010, Ahmadi et al., 2011), and inflammatory dermatoses, including atopic dermatitis (AD), psoriasis, and chronic urticaria (Rostenberg, 1960, Gupta and Gupta, 1996, Tausk and Nousari, 2001). Nevertheless, a recent body of work supports the concept that moderate amounts of PS can benefit the host, through a short-term enhancement of a variety of immune and neuroendocrine functions (Dhabhar, 2013).
The negative consequences of PS have been extensively studied in normal skin, where PS in humans (or simply stress in animals) delays wound healing (Kiecolt-Glaser et al., 1995), disrupts permeability barrier homeostasis, impairs stratum corneum (SC) cohesion (Choi et al., 2005a), and compromises epidermal innate immunity (Aberg et al., 2007). Although neuroendocrine mechanisms doubtlessly have a role (Radek and Gallo, 2007), to a large extent, these adverse cutaneous effects could be attributed to an increase in endogenous GCs, whose levels increase in proportion to the extent of stress (Denda et al., 2000, Kao et al., 2003, Choi et al., 2006). Pertinently, the link to GC could be demonstrated directly through the ability of either systemically coadministered antalarmin, an inhibitor of corticotrophin-releasing factor, or mifepristone (Ru486), a GC receptor antagonist, to normalize function in the face of ongoing stress (Denda et al., 2000, Choi et al., 2005a, Aberg et al., 2007). Accordingly, exogenous GC, whether administered systemically or topically, recapitulated all of these negative outcomes (Kao et al., 2003, Choi et al., 2006, Aberg et al., 2007). In theory then, a sustained increase in endogenous GC due to intrinsic illness–associated or superimposed stress could harm clinical outcomes in inflammatory disorders by further compromising epithelial function. Yet, one could then ask why this component of the stress response was retained during evolution, if its consequences are uniformly detrimental.
Alternatively, because of the increase in endogenous GC that accompanies PS, we hypothesized instead that superimposed PS could exert beneficial, rather than deleterious, effects in inflammatory disorders. Pertinently, supraphysiological doses of GC, whether administered systemically or topically, exert potent anti-inflammatory benefits (Gardner and Shoback, 2011); conversely, there are multiple, adverse consequences of GC deficiency in patients with Addison's disease (Gardner and Shoback, 2011). To address this issue, we examined the impact of additional exogenous stress on inflammation and epidermal function in mouse models of inflammatory skin disorders. Our results show that intervals of exogenous stress that universally compromise function in normal epidermis, paradoxically reduce inflammation and improve functional parameters in three immunologically diverse murine dermatosis models. Improvement paralleled an increase in endogenous GC, whereas blockade of GC peripheral action prevented the stress-induced improvements in inflammation and epidermal function, providing the link between the stress-induced increase in GC and improved clinical and functional outcomes. Assuming that these results will also apply to extracutaneous inflammatory disorders, these findings could explain why this component of the stress response, previously thought to be deleterious to health, has been conserved during human evolution.
Results
Stress increases endogenous GC and decreases inflammation in murine inflammatory dermatosis models
To generate exogenous stress, we deployed restraint (frustration) for 18 hours (Youm et al., 2013), which results in a significant increase in endogenous GC levels in comparison with nonrestrained, control mice (e.g., Denda et al., 1998, Choi et al., 2006). Stress was administered immediately after each application of either an irritant (i.e., the phorbol ester, phorbol 12-myristate 13-acetate (TPA); irritant contact dermatitis (ICD) model) or following single versus multiple (3-10) hapten (oxazolone (Ox)) challenges to previously sensitized mice (acute allergic contact dermatitis (ACD) and chronic allergic contact dermatitis, with features of AD, respectively). We next assessed whether added exogenous stress alters the severity of inflammation in these immunologically diverse, inflammatory dermatoses models.
Irritant contact dermatitis
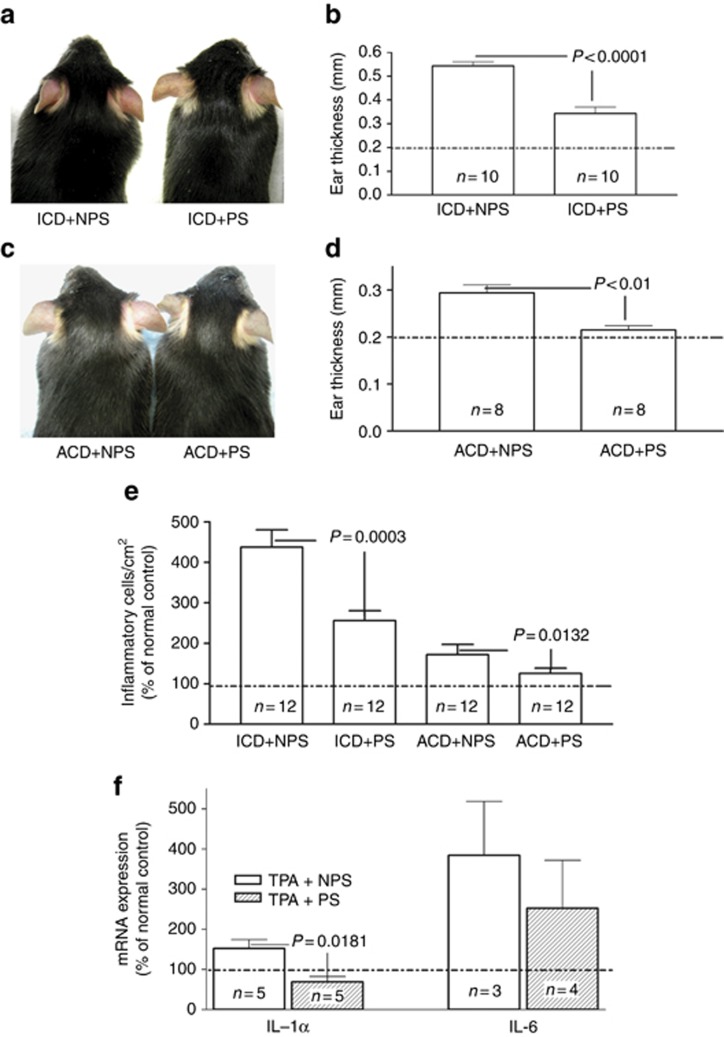
In these studies, simultaneous exposure to coadministered exogenous stress raised endogenous GC levels ≈4-fold (Supplementary Figure 1 online). Although nonstressed mice demonstrated inflammation 2 hours after a single topical application of the phorbol ester TPA to the ear, stressed mice displayed visible reductions in erythema (Figure 1a). Yet, the ability of exogenous stress to dampen inflammation in the ICD model declined in mice treated with a higher concentration of TPA, suggesting a dose–response relationship (Supplementary Figure 2 online). Moreover, the stress-induced reduction in inflammation in the ICD mice could be quantified as a significant decrease in ear thickness in comparison with untreated, nonstressed controls (Figure 1b). In addition, inflammatory cell density was reduced (Figure 1e), epidermal mRNA levels of the proinflammatory cytokines IL-1α and IL-6 declined (Figure 1f; P<0.1 for IL-6), and a reduction in inflammation was evident in parallel histologic studies after exposure to exogenous stress (Supplementary Figure 3A vs. B online). Finally, the stress-induced increase in endogenous GC accounted for reduced inflammation, because coadministered Ru486 worsened TPA-induced inflammation (see below). Together, these results demonstrate that co-provision of exogenous stress exerts potent anti-inflammatory activity in a mouse model of ICD.
Figure 1.
Stress reduces cutaneous inflammation in irritant and allergic contact dermatitis models. (a, b) To induce irritant contact dermatitis (ICD), both ears of one group of C57BL/J mice were treated topically with 20 μl of 0.03% phorbol 12-myristate 13-acetate (TPA). The ear thickness was measured with a digital caliper (Mitutoyo, Tokyo, Japan) 18 hours after oxazolone (Ox) or TPA application, and the results are presented in panel d. Frustration (action-restricted) stress was administered as described in Methods. (c, d) For acute allergic contact dermatitis (ACD), 5% Ox was applied topically once to the flank of C57BL/J mice. One week later, the ears of one group C57BL/J mice were topically treated with a single dose of 0.5% Ox. After TPA or Ox treatments, parallel groups of mice were kept individually in 5 × 11 × 4 cm box for 18 hours, to induce frustration (restraint model) (Youm et al., 2013). The two-tailed Student's t-test was used to determine significant differences.
Acute allergic contact dermatitis model
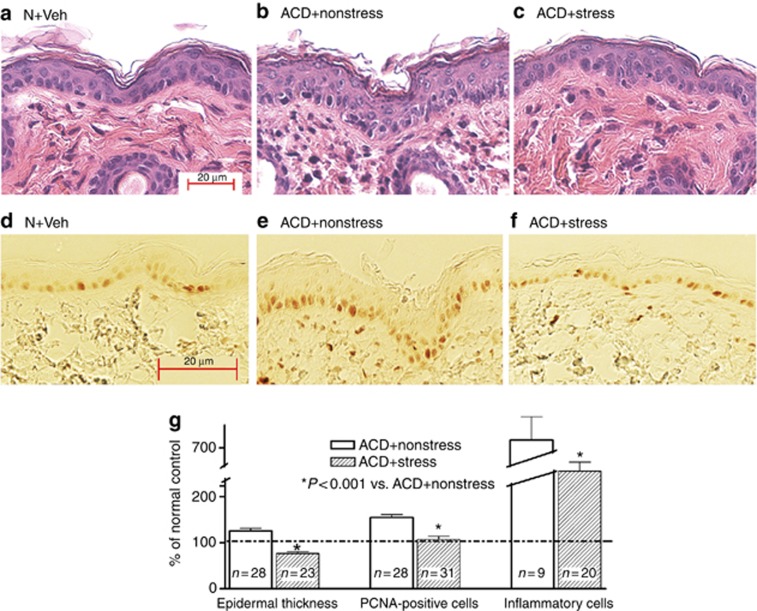
We next evaluated the impact of exogenous stress in an ACD mouse model in which either the hapten, Ox, or vehicle alone was applied once to opposing ears of previously sensitized mice, under either stressed or nonstressed conditions. Exogenous stress again markedly reduced visible erythema and scale in Ox-challenged ears (Figure 1c), changes that were paralleled by significant reductions in ear thickness (Figure 1d) and inflammatory cell infiltrations (Figures 1e and 2g), as well as histologic evidence of a marked reduction in dermal inflammation (Figure 2a–c and Supplementary Figure 3C and D online). The stress-induced reductions in epidermal hyperplasia were mirrored by a normalization of epidermal hyperplasia and epidermal thickness, further quantified as a decreased density of proliferating cell nuclear antigen (PCNA)-positive basal cells (Figure 2d–g). Together, these results show that the imposition of exogenous stress displays potent anti-inflammatory activity in a mouse model of ACD.
Figure 2.
Stress reduces epidermal hyperplasia and inflammation in acute allergic contact dermatitis (ACD) mice. (a–c) Representative hematoxylin and eosin sections of normal or ACD mice, with or without superimposed stress. (d–f) Proliferating cell nuclear antigen (PCNA) immunostaining in parallel sections (see a–c). (g) Quantification of epidermal hyperplasia (thickness) of nucleated cell layers, PCNA+cells (PNA+cells/unit length of basal layer), and inflammatory cell density/mm2. Bar=20 μm.
Atopic dermatitis model
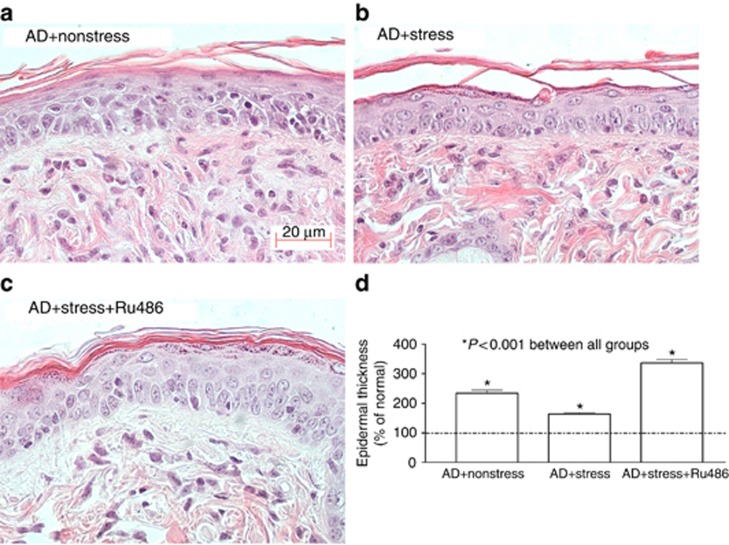
We next assessed the impact of exogenous stress in a repeatedly hapten-challenged, chronic contact dermatitis model that exhibits multiple features of human AD, including the following: (i) elevated serum IgE levels; (ii) epidermal hyperplasia; (iii) an inflammatory infiltrate enriched in eosinophils and mast cells; and (iv) a prominent permeability barrier abnormality (Man et al., 2008). Superimposed stress markedly reduced epidermal hyperplasia and dermal inflammation in these AD mice (Figure 3a vs. b), and quantitative studies confirmed the stress-induced decrease in epidermal hyperplasia (Figure 3d). Finally, concurrent stress significantly reduced serum IgE levels in the AD model (Supplementary Figure 4 online). Thus, exogenous stress exerts potent anti-inflammatory activity in a repeatedly hapten-challenged mouse model, with features of AD.
Figure 3.
Psychological stress reduces inflammation and epidermal hyperplasia in an atopic dermatitis (AD) model. (a, b) Changes in epidermal hyperplasia and inflammation in nonstressed versus stressed animals (quantitative data are in d). (c, d) Reversal of benefits of stress following coadministered mifeprostone (Ru486). One-way analysis of variance with Tukey's correction was used to determine significant differences. Bar=20 μm.
Stress improves epidermal function in AD mice
Not only does permeability barrier dysfunction “drive” inflammation, but chronic inflammation in AD further alters epidermal barrier function in AD (see Elias and Steinhoff, 2008, Elias et al., 2008, Cork et al., 2009, Irvine et al., 2011). Hence, we next assessed whether exogenous stress improves epidermal function in parallel with reduced inflammation in the AD model. Although both barrier function and SC hydration deteriorated in AD mice not subjected to exogenous stress, both of these functions normalized after the imposition of concurrent stress (Figure 4a and Table 1). Thus, despite the known propensity for stress to compromise multiple epidermal functions in normal skin (Denda et al., 2000, Kao et al., 2003, Choi et al., 2005a, 2006, Aberg et al., 2007), simultaneous exposure to exogenous stress improves two key epidermal functions in an inflammatory dermatosis model with features of AD.
Figure 4.
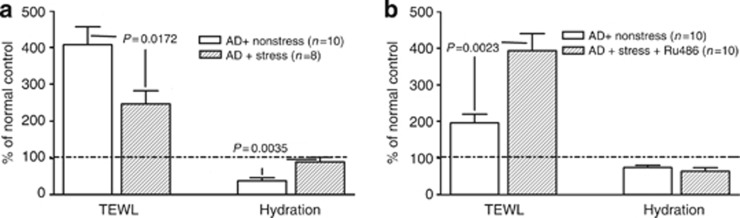
Co-administered stress improves epidermal function in the atopic dermatitis (AD) model. (a) Stress improves barrier function and stratum corneum hydration in AD mice. (b) Coadministration of mifeprostone (Ru486) worsens barrier function, but it does not change hydration in AD mice (note: differences in basal transepidermal water loss (TEWL) values in a and b reflect two different cohorts of mice).
Table 1. Influence of exogenous stress on cutaneous function in an AD model.
| TEWL | SC hydration | |
|---|---|---|
| AD+nonstressed (n=10) | 14.2±1.6* | 39.5±3.5 |
| AD+stress+vehicle (n=10) | 11.8±1.3* | 46.4±4.2 |
| AD+stress+Ru486 (n=10) | 28.4±3.0 | 34.1±5.1 |
| Significance | *P<0.001 vs. Ru486 | NS |
Abbreviations: AD, atopic dermatitis; NS, nonsignificant; SC, stratum corneum; TEWL, transepidermal water loss.
Stress-induced improvements in inflammation and epidermal function can be attributed to increased endogenous GC
If exogenous stress reduces inflammation and improves epidermal function through an increase in endogenous GC, one would predict that blockade of GC peripheral action should counteract the beneficial effects of stress in AD mice. Hence, we next coadministered intraperitoneal mifepristone (Ru486) to ICD and AD mice, with or without superimposed stress. As predicted, coadministered mifepristone aggravated epidermal hyperplasia and inflammation in ICD and AD mice, exposed to exogenous stress (Figure 3c and d, Supplementary Figure 5A and B online, and Table 1). Finally, barrier function (but not SC hydration) worsened when mifepristone was coadministered with exogenous stress (Figure 4b and Table 1). Together, these results show that exogenous stress improves inflammation and epidermal barrier function through an increase in the peripheral action of endogenous GC.
Discussion
Acute PS mobilizes glucose reserves and promotes insulin resistance as part of a highly conserved, acute response to perceived external threats that provides net benefits to the host (Nowotny et al., 2010). In contrast, sustained PS reportedly provokes and exacerbates both cutaneous and extracutaneous inflammatory disorders (Hansel et al., 2010, McEwen and Kalia, 2010). Skin disorders with epidermal functional abnormalities, such as psoriasis and AD, seem particularly susceptible to PS (Rostenberg, 1960, Gupta and Gupta, 1996, Tausk and Nousari, 2001). According to the current paradigm, the pathophysiologic basis of PS can be explained by abnormalities in immune and neuroendocrine mechanisms, which amplify inflammation and pruritus in these disorders (e.g., O'Sullivan et al., 1998, Radek and Gallo, 2007). Yet, studies in rodent models show that the deleterious effects of stress, at least in normal skin, are largely mediated by increased endogenous GC levels, based upon studies with the nonspecific GC receptor antagonist, mifepristone (Ru486), and also supported by the fact that the administration of exogenous GC mirrors the stress-induced abnormalities (Denda et al., 2000, Kao et al., 2003, Choi et al., 2005b, 2006).
An elevation in endogenous GC, explained by altered GC production and/or peripheral activation, often accompanies chronic illness. Although such sustained increases in GC are presumed to pose a further threat to the host (Boonen et al., 2013, Gomez-Sanchez, 2013), our studies suggest instead that increases in illness-associated or exogenous stress could be beneficial. We showed here that a stress-induced increase in circulating GC ameliorates cutaneous inflammation. As GC can also be produced within various cell types in the skin, it remains possible that skin-specific changes in GC could also have contributed to the apparent benefits of stress. Accordingly, recent studies have shown that failure to mount a sufficient cortisol response under stressful conditions can trigger allergic inflammation and exacerbate disease in AD patients (Buske-Kirschbaum et al., 2002, 2003, 2007; Yehuda and Seckl, 2011). Moreover, HPA axis hyporesponsiveness has been linked to increased severity of the full range of atopic inflammatory disorders (Buske-Kirschbaum et al., 2010), further linked to decreased production of proinflammatory cytokines, such as IL-1 and tumor necrosis factor-α (Chesnokova and Melmed, 2002), as well as other neuroendocrine mediators (Chen and Miller, 2007).
In light of the apparent benefits of exogenous stress in the dermatosis models, it becomes more difficult to defend the prevailing hypothesis—i.e., that PS exacerbates chronic inflammatory disorders. The apparently paradoxical nature of our results likely can be explained as follows: PS clearly compromises epidermal function, including innate immune status, in otherwise normal human (Altemus, et al., 2001, Garg, et al., 2001) and rodent (Denda, et al., 1998, 2000, Choi, et al., 2005a, Aberg, et al., 2007) skin. These negative effects in normal skin appear to be offset by the anti-inflammatory benefits of exogenous stress demonstrated in inflamed skin. Indeed, superimposition of exogenous stress reduced inflammation in the ICD, ACD, and AD models, conditions whose immunophenotypes differ widely. We show here further that the benefits of exogenous stress can be attributed to elevated endogenous GC, as coadministration of mifepristone, an antagonist of the GCr, reversed the apparent benefits of stress in the ICD and AD models. Stress, similar to GC, can be anti-inflammatory by a number of related mechanisms (Fantuzzi and Ghezzi, 1993, Schleimer, 1993, Campisi et al., 2002, Baschant and Tuckermann, 2010, Marshall, 2011). For example, it is well accepted that an appropriate responsiveness of the HPA axis and a subsequent release of GC may be essential to control overshooting of the immune response (Elenkov and Chrousos, 2006). Yet, as the anti-inflammatory benefits of stress also correlated with a parallel improvement in epidermal function in the AD model, improved barrier function could contribute to reduced cutaneous inflammation by several downstream mechanisms—e.g., downregulation of the cytokine cascade (Elias and Steinhoff, 2008, Elias et al., 2008, Elias, 2010). These potent anti-inflammatory effects, which require hours to days, rather than minutes, of stress, outweigh the negative impact of elevated GC on cutaneous homeostasis in otherwise normal skin.
In contrast, PS likely triggers inflammation in a setting of normal skin that is predisposed to inflammation, such as in patients predisposed to develop AD or psoriasis (Buske-Kirschbaum et al., 2001, Wright et al., 2005). Similarly, PS is thought to trigger extracutaneous inflammatory disorders, such as allergic asthma (Chen and Miller, 2007), systemic lupus erythematosus (Pawlak et al., 2003), and inflammatory bowel disease (Furlan et al., 2006), perhaps also by compromising epithelial barrier function, cell–cell cohesion, proliferation, and/or differentiation in these extracutaneous epithelia. In these epithelia, as in the skin, PS could compromise not only barrier function but also innate immunity, likely facilitating bacterial colonization, translocation, and invasion by pathogens, e.g., from the intestinal lumen into the colonic mucosa (Ibid).
The apparent benefits of PS through the anti-inflammatory activity of increased endogenous GC production could provide benefits in combating the sequelae of traumatic injury and infectious challenges. Thus, conservation of stress reactivity within the HPA axis, with the capability to increase endogenous GC production, could have equipped humans with an endogenous anti-inflammatory mechanism in eras before the availability of exogenous GC as anti-inflammatory therapy. Yet, although preservation of HPA axis reactivity likely benefitted humans by dampening inflammation during illness, a more prolonged increase in PS can decrease HPA axis reactivity, resulting in a reduction in endogenous GC levels (Reber et al., 2007). Thus, the potential benefits of PS through elevated endogenous GC on epithelial barrier function, inflammation, and innate immunity likely accrue only during periods of established disease/inflammation, and predominantly in earlier stages of an inflammatory response. We propose that a prolonged increase in endogenous GC production, induced by a variety of different, subacute-to-chronic stressors (i.e., trauma, surgery, infections, and chronic inflammation), correlates with the benefits of exogenous GC in the management of autoimmune and inflammatory diseases, as well as following life-threatening trauma (e.g., cranioencephalic or spinal cord injury). Together, these studies support the concept that enhanced GC in response to PS could have an important role in modulating inflammation and preserving health in humans.
Materials and Methods
Materials
Six- to eight-week-old female hairless mice (hr/hr) and C57BL/J male mice were purchased from Charles River Laboratories (Wilmington, MA) and fed a standard mouse diet (Ralston-Purina, St Louis, MO) and water ad libitum. TPA, 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (Ox), and acetone were purchased from Sigma Chemical (St Louis, MO). Biotinylated anti-PCNA antibody was from CalTag Laboratories (Burlingame, CA). ABC-peroxidase kit was purchased from Vector Laboratories (Burlingame, CA).
Experimental protocols and disease models
All animal procedures were approved by the Animal Studies Subcommittee of the San Francisco Veterans Affairs Medical Center and performed in accordance with their guidelines. As a model of ICD, the ears of C57BL/J mice were treated tropically with either 20 μl of TPA (0.03%) or ethanol vehicle alone to the opposite ear. Two hours after applications, we assessed changes in ear thickness and histologic evidence of inflammation as we have described (Sheu et al., 1998, Fowler et al., 2003). As a model of ACD, mice were sensitized with a single application of the hapten, 3% Ox, and housed in regular cages thereafter. One week later, mouse ears were challenged with 20 μl of 0.5% Ox. After either TPA treatments or a second Ox challenge (0.5%), separate cohorts of mice were kept individually in a 5 × 11 × 4 cm box for 18 hours, a model that induces sustained stress. Changes in ear thickness were measured with a digital caliper (Mitutoyo, Tokyo, Japan) 18 hours after TPA or Ox applications. For the chronic contact dermatitis with features of AD model, three groups of hairless mice were sensitized by topical application of 10 μl of 3% Ox, as above. One week later, both groups of Ox-treated mice were treated topically with 60 μl of 0.05% Ox in ethanol once daily for 4 days. Immediately after each Ox application, one group of mice was kept in regular cages and another two groups of mice were stressed by individual housing in 5 × 11 × 4 cm boxes for 18 hours daily for 4 days. An additional cohort of similarly treated stressed mice was given a single intraperitoneal dose of mifepristone (Ru486; 170 μg) in corn oil. Stressed mice injected with corn oil alone served as controls. At the end of the treatment period, transepidermal water loss, SC hydration, and skin surface pH were assessed with respective probes connected to a Tewameter (MPA5, Courage & Khazaka, Cologne, Germany), as described previously (Man et al., 2008, Hatano et al., 2011). Skin samples for PCNA, hematoxylin and eosin staining, and electron microscopy were taken immediately after functional determinations.
Serum IgE and GC measurements
Blood samples were collected from AD mice, with or without superimposed stress. Serum IgE concentrations were determined with a mouse IgE ELISA quantification kit from Bethyl Laboratories (Montgomery, TX), by following instructions provided by the manufacturer.
Immunohistochemistry
For the determination of epidermal proliferation, a previously described method was used (Man, et al., 2008). Briefly, 5-μm paraffin sections were incubated with an antibody against proliferating cell nuclear antigen (Ki67) (CalTag Laboratories) overnight at 4 °C. Immunostaining was detected by the ABC peroxidase method. Sections were visualized with a Zeiss Microscope (Jena, Germany), and digital images were taken with Axio Vision software 3.1 (Carl Zeiss Vision, Munich, Germany).
Quantitative PCR for mRNA expression
Total epidermal RNA was isolated from haired mouse treated for 9 days as described above using TRIzol Reagent (Invitrogen Ambion RNA catalog number 15596026) according to the manufacturer's description. First-strand cDNA was synthesized from 1 μg of total RNA with the Transcriptor First-Strand cDNA Synthesis Kit (Roche, Indianapolis, IN; catalog number 04897030001) in a total volumn of 20 μl. The real-time PCR contained 60 ng of reversed-transcribed cDNA, 300 nM each of forward and reverse primers, and 10 μl of 2 × SensiMix SYBr Hi-ROX Master mix (Bioline, Stockholm, Sweden; catalog number QT605-05) in a final volume of 20 μl in 384-well plates using the ABI 7900 HT Real-time PCR System. Quantification was performed by the comparative threshold cycle method with mouse glyceraldehyde-3-phosphate dehydrogenase used for normalization. The primer sequences for tumor necrosis factor-α were 5′-GCCTCTTCTCATTCCTGCTTG-3′ (forward) and 5′-CTGATGAGAGGGAGGCCATT-3′ (reverse). The primer sequences for IL-6 were 5′-GAGGATACCACTCCCAACAGACC-3′ (forward) and 5′-AAGTGCATCATCGTTGTTCATACA-3′ (reverse). The primer sequences for mouse glyceraldehyde-3-phosphate dehydrogenase were 5′-AAGGTCATCCCAGAGCTGAA-3′ (forward) and 5′-ATGTAGGCCATGAGGTCCAC-3′ (reverse). Relative expression of the mRNAs compared with mRNA in normal control mice was calculated. Data are expressed as percentage of control (setting normal control as 100%).
Inflammatory cell quantifications
The number of inflammatory cells infiltrating the ear was counted on printed photographs at every 5-cm segment of ear sections stained with hematoxylin and eosin and captured at × 20. The data are presented as the mean of all areas counted per cm2 (mean±SEM).
Quantification of epidermal thickness, proliferation, and inflammatory cell density
Thickness of the epidermal nucleated cell layers was measured on × 100 micrographs taken every 2 cm along the epidermis from biopsies from normal untreated and repeated Ox-treated mice, with or without additional stress (n=10 sites from each group). The data presented depict the mean of all measured points ±SEM. The number of PCNA-positive cells was counted on × 100 micrographs at 1-cm frequencies along the epidermis and presented as the mean of all PCNA-positive cells/cm ±SEM. The density of inflammatory cells in the dermis was counted at 4-cm frequencies along the overlying epidermis, in an area between the basement membrane and 4 cm below the basement membrane, and presented as the mean of inflammatory cells/cm2 ±SEM.
Statistics
GraphPad Prism 4 software was used for all statistical analyses. A two-tailed Student's t-test was used to determine significant differences for comparisons between two groups. A one-way analysis of variance, with Tukey's correction, was used to determine significant differences, when three or more groups were compared.
Acknowledgments
These studies were supported by the San Francisco Veterans Affairs Medical Center and National Institutes of Health (NIH) grant AR019098. Ms Joan Wakefield provided superb editorial assistance. These contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the NIH.
Glossary
- ACD
acute allergic contact dermatitis
- AD
atopic dermatitis
- GC
glucocorticoid
- ICD
irritant contact dermatitis
- Ox
oxazolone
- PCNA
proliferating cell nuclear antigen
- PS
psychological stress
- SC
stratum corneum
- TPA
phorbol 12-myristate 13-acetate
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
This article is dedicated in memory of Ray H. Rosenman, MD (1920–2013), an eminent cardiologist and expert on psychological stress. Dr Rosenman first described the deleterious consequences of the type A personality for humans.
Supplementary Material
References
- Aberg KM, Radek KA, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108:29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- Altemus M, Rao B, Dhabhar FS, et al. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001;117:309–317. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477–1488. doi: 10.1056/NEJMoa1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Ebrecht M, Hellhammer DH. Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain Behav Immun. 2010;24:1347–1353. doi: 10.1016/j.bbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Geiben A, Hellhammer D. Psychobiological aspects of atopic dermatitis: an overview. Psychother Psychosom. 2001;70:6–16. doi: 10.1159/000056219. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Geiben A, Hollig H, et al. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. 2002;87:4245–4251. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Krieger S, Wilkes C, et al. Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. J Clin Endocrinol Metab. 2007;92:3429–3435. doi: 10.1210/jc.2006-2223. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, von Auer K, Krieger S, et al. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease. Psychosom Med. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Acute stress decreases inflammation at the site of infection. A role for nitric oxide. Physiol Behav. 2002;77:291–299. doi: 10.1016/s0031-9384(02)00861-2. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21:993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Melmed S. Minireview: Neuro-immuno-endocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology. 2002;143:1571–1574. doi: 10.1210/endo.143.5.8861. [DOI] [PubMed] [Google Scholar]
- Choi EH, Brown BE, Crumrine D, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005;124:587–595. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- Choi EH, Demerjian M, Crumrine D, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1657–R1662. doi: 10.1152/ajpregu.00010.2006. [DOI] [PubMed] [Google Scholar]
- Choi EH, Man MQ, Wang F, et al. Is endogenous glycerol a determinant of stratum corneum hydration in humans. J Invest Dermatol. 2005;125:288–293. doi: 10.1111/j.0022-202X.2005.23799.x. [DOI] [PubMed] [Google Scholar]
- Cork MJ, Danby SG, Vasilopoulos Y, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T, Elias PM, et al. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R367–R372. doi: 10.1152/ajpregu.2000.278.2.R367. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T, Hosoi J, et al. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br J Dermatol. 1998;138:780–785. doi: 10.1046/j.1365-2133.1998.02213.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Psychological stress and immunoprotection versus immunopathology in the skin. Clin Dermatol. 2013;31:18–30. doi: 10.1016/j.clindermatol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress system—organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–267. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Ann Dermatol. 2010;22:245–254. doi: 10.5021/ad.2010.22.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G, Ghezzi P. Glucocorticoids as cytokine inhibitors: role in neuroendocrine control and therapy of inflammatory diseases. Mediators Inflamm. 1993;2:263–270. doi: 10.1155/S0962935193000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AJ, Sheu MY, Schmuth M, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- Furlan R, Ardizzone S, Palazzolo L, et al. Sympathetic overactivity in active ulcerative colitis: effects of clonidine. Am J Physiol Regul Integr Comp Physiol. 2006;290:R224–R232. doi: 10.1152/ajpregu.00442.2005. [DOI] [PubMed] [Google Scholar]
- Gardner DG, Shoback D. Greenspan's Basic and Clinical Endocrinology. McGraw Hill: New York; 2011. p. 880. [Google Scholar]
- Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE. Adrenal dysfunction in critically ill patients. N Engl J Med. 2013;368:1547–1549. doi: 10.1056/NEJMe1302305. [DOI] [PubMed] [Google Scholar]
- Gupta MA, Gupta AK. Psychodermatology: an update. J Am Acad Dermatol. 1996;34:1030–1046. doi: 10.1016/s0190-9622(96)90284-4. [DOI] [PubMed] [Google Scholar]
- Hamer M, O'Donnell K, Lahiri A, et al. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. Eur Heart J. 2010;31:424–429. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- Hansel A, Hong S, Camara RJ, et al. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Hatano Y, Elias PM, Crumrine D, et al. Efficacy of combined peroxisome proliferator-activated receptor-alpha ligand and glucocorticoid therapy in a murine model of atopic dermatitis. J Invest Dermatol. 2011;131:1845–1852. doi: 10.1038/jid.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Kao JS, Fluhr JW, Man MQ, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, et al. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Man MQ, Hatano Y, Lee SH, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GD., Jr The adverse effects of psychological stress on immunoregulatory balance: applications to human inflammatory diseases. Immunol Allergy Clin North Am. 2011;31:133–140. doi: 10.1016/j.iac.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59 (Suppl 1:S9–S15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Nowotny B, Cavka M, Herder C, et al. Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Horm Metab Res. 2010;42:746–753. doi: 10.1055/s-0030-1261924. [DOI] [PubMed] [Google Scholar]
- O'Sullivan RL, Lipper G, Lerner EA. The neuro-immuno-cutaneous-endocrine network: relationship of mind and skin. Arch Dermatol. 1998;134:1431–1435. doi: 10.1001/archderm.134.11.1431. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Witte T, Heiken H, et al. Flares in patients with systemic lupus erythematosus are associated with daily psychological stress. Psychother Psychosom. 2003;72:159–165. doi: 10.1159/000069735. [DOI] [PubMed] [Google Scholar]
- Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Reber SO, Birkeneder L, Veenema AH, et al. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148:670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- Reber SO, Peters S, Slattery DA, et al. Mucosal immunosuppression and epithelial barrier defects are key events in murine psychosocial stress-induced colitis. Brain Behav Immun. 2011;25:1153–1161. doi: 10.1016/j.bbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Rostenberg A., Jr The role of psychogenic factors in skin diseases. Arch Dermatol. 1960;81:81–86. doi: 10.1001/archderm.1960.03730010085010. [DOI] [PubMed] [Google Scholar]
- Schleimer RP. An overview of glucocorticoid anti-inflammatory actions. Eur J Clin Pharmacol. 1993;45 (Suppl 1:S3–S7. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- Sheu HM, Lee JY, Kuo KW, et al. Permeability barrier abnormality of hairless mouse epidermis after topical corticosteroid: characterization of stratum corneum lipids by ruthenium tetroxide staining and high-performance thin-layer chromatography. J Dermatol. 1998;25:281–289. doi: 10.1111/j.1346-8138.1998.tb02399.x. [DOI] [PubMed] [Google Scholar]
- Tausk FA, Nousari H. Stress and the skin. Arch Dermatol. 2001;137:78–82. doi: 10.1001/archderm.137.1.78. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Seckl J. Minireview: Stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 2011;152:4496–4503. doi: 10.1210/en.2011-1218. [DOI] [PubMed] [Google Scholar]
- Youm JK, Park K, Uchida Y, et al. Local blockade of glucocorticoid activation reverses stress- and glucocorticoid-induced delays in cutaneous wound healing. Wound Repair Regen. 2013;21:715–722. doi: 10.1111/wrr.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.