Abstract
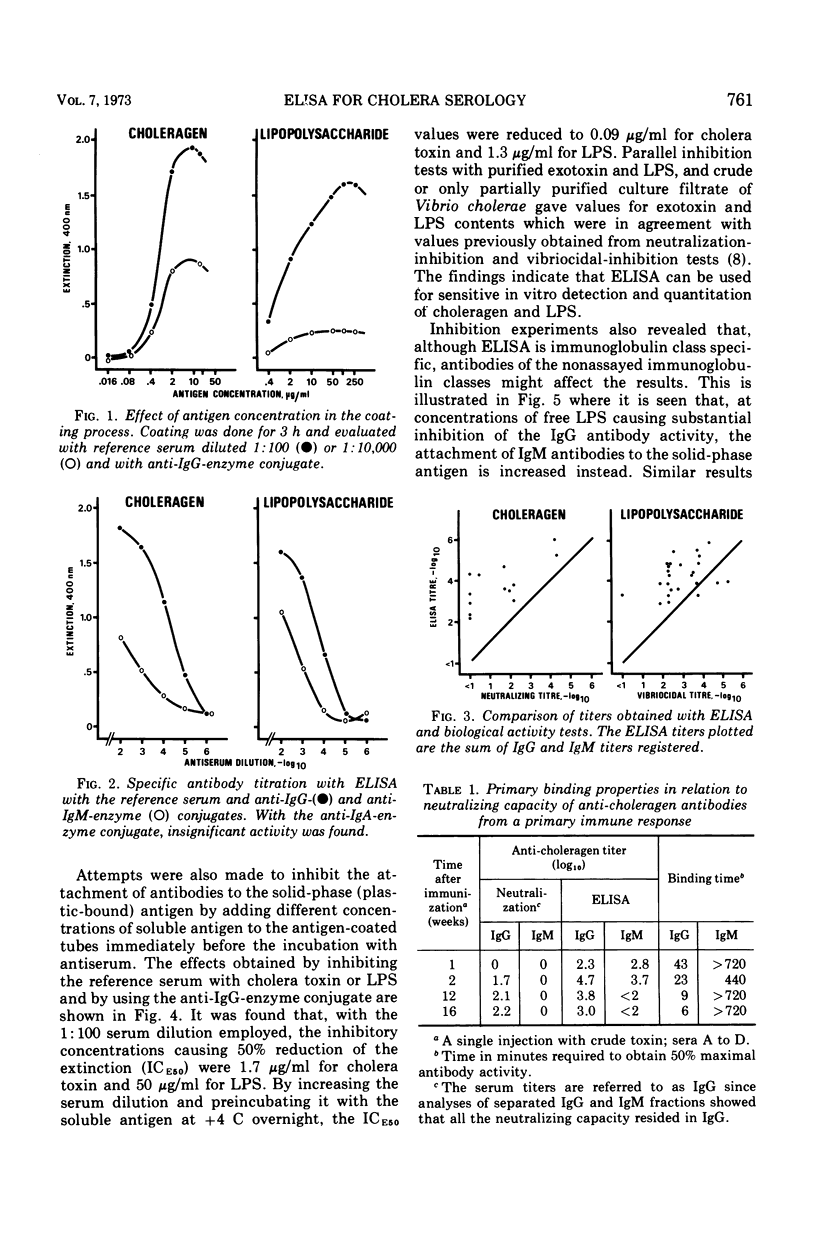
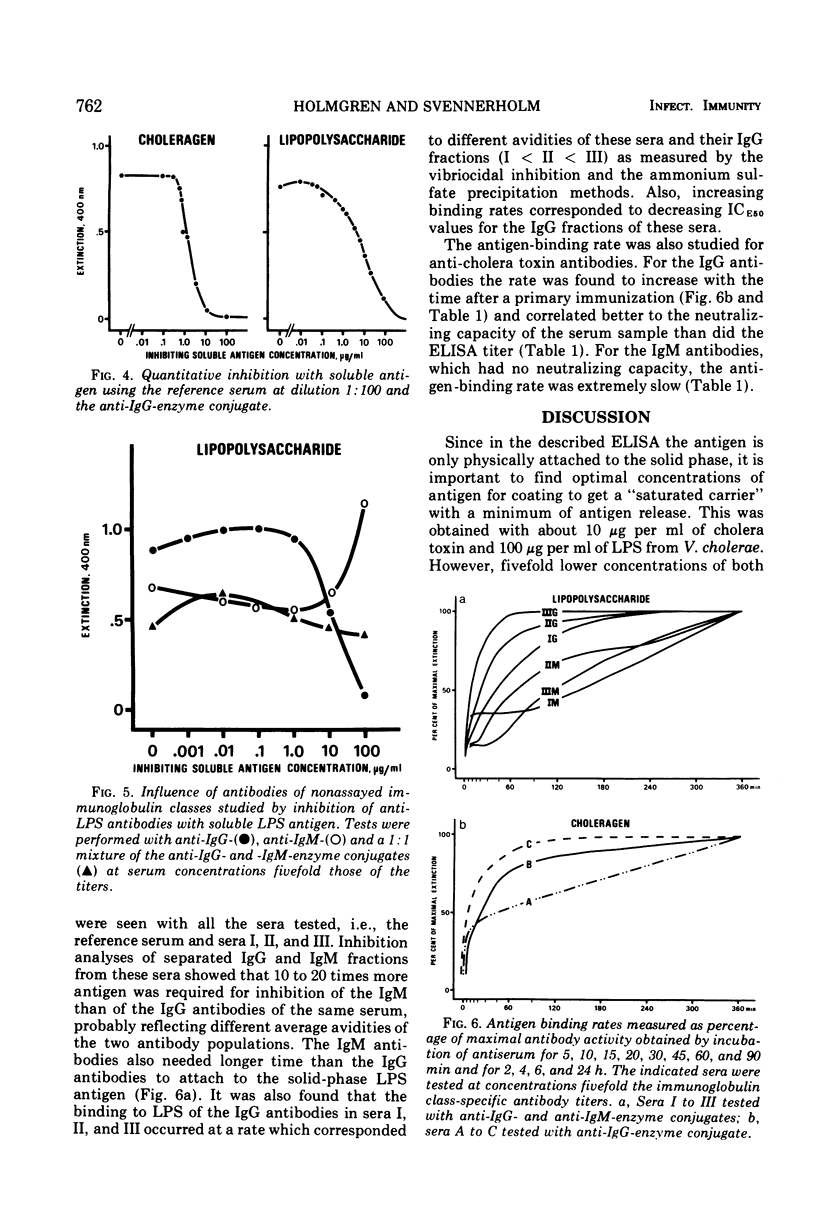
The enzyme-linked immunosorbent assay (ELISA) principle of Engvall and Perlmann, which employs antigen-coated tubes and enzyme-labeled anti-immunoglobulins, was elaborated for use in cholera serology. Immunoglobulin class-specific determinations of primary binding titers of antibodies to cholera exotoxin and endotoxin were more sensitive than were neutralization and vibriocidal tests. It was notable that rather high ELISA titers of immunoglobulin M (IgM) antibodies to exotoxin, which lacked neutralizing capacity were registered, whereas corresponding levels of immunoglobulin G (IgG) antibodies were effectively neutralizing. A modified technique permitting measurement of the binding rate of antibody to the solid-phase antigen was introduced as a possible tool for antibody avidity estimation. Such measurements indicated a wide avidity difference between the IgG and the IgM anti-exotoxin antibodies, which could explain their different neutralizing capacities. The observation that increasing binding rate of the IgG antibodies during the course of the primary immune response could compensate for decreasing antibody amount with regard to neutralizing capacity of serum also indicated the importance of antibody avidity for toxin neutralization. Inhibition with soluble antigen permitted quantitative determination of antigen; levels of exotoxin 0.09 μg/ml and of endotoxin to 1.3 μg/ml were measured.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Production of highly purified choleragen and choleragenoid. J Infect Dis. 1970 May;121(Suppl):63+–63+. doi: 10.1093/infdis/121.supplement.s63. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Ouchterlony O. Immunochemical Studies of Two Cholera Toxin-Containing Standard Culture Filtrate Preparations of Vibrio cholerae. Infect Immun. 1971 Jun;3(6):747–755. doi: 10.1128/iai.3.6.747-755.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. A radial diffusion agar plaque technique for estimation of the avidity of bactericidal antibodies against Vibrio cholerae. Scand J Immunol. 1972;1(4):345–350. doi: 10.1111/j.1365-3083.1972.tb03300.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ouchterlony O. Experimental studies on cholera immunization. 1. The response of neutralizing and vibriocidal antibodies in rabbits after immunization with culture filtrate material from V. cholerae. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):489–496. [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ouchterlony O. Quantitation of vibriocidal antibodies using agar plague techniques. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):708–714. [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]


